Abstract
Objective
Obstructive sleep apnea causes intermittent hypoxia (IH) and is associated with increased cardiovascular mortality. This increased risk may be attributable to more extensive or unstable atherosclerotic plaques in subjects with OSA. We studied the effect of chronic IH in atherosclerosis-prone mice.
Methods and Results
Apolipoprotein E-deficient (ApoE−/−) mice fed a high cholesterol diet were exposed to 4 or 12 weeks of IH and compared to intermittent air-exposed controls. At 4 weeks, IH increased plaque size in the aortic sinus and the descending aorta. At 12 weeks, atherosclerosis progressed in all groups, but more rapidly in the descending aorta of IH-exposed animals. Plaque composition was similar between IH and controls. Between 4 and 12 weeks, there were progressive increases in blood pressure, with relatively stable increases in serum lipids and arterial stiffness.
Conclusions
IH accelerates atherosclerotic plaque growth in ApoE−/− mice without affecting plaque composition. The mechanisms may include non-additive increases in serum lipids, and cumulative increases in blood pressure.
Keywords: atherosclerosis, intermittent hypoxia, obstructive sleep apnea
INTRODUCTION
Atherosclerosis is the leading cause of death in the western world, despite advances in our understanding of the disease and its complications. Even with lipid lowering therapies and angiography, 38% of deaths in North America are attributable to cardiovascular disease1, 2, indicating incomplete control of the evolution of atherosclerosis. Indeed, therapies for atherosclerosis developed out of the notion that myocardial infarctions occur when vascular lipid accumulate to the point of lumen occlusion. It is now recognized that plaque rupture is the hallmark of myocardial infarction, and stability of lesions does not necessarily correlate with their size2, 3. Therefore, efforts have shifted to characterizing and controlling plaque vulnerability. In this study, we apply these current concepts to atherosclerosis in the setting of obstructive sleep apnea (OSA).
OSA is a disease affecting 4–24% of men and 2–9% of women in the United States4. Moderate to severe OSA significantly increases the risk of all-cause and cardiovascular mortality 5, 6. Carotid intima-media thickness7, blood pressure8, cholesterol9, 10 and oxidative stress11 are increased in OSA and are decreased by continuous positive airway pressure (CPAP) treatment. While provocative, these studies rely on surrogate markers of atherosclerosis, leading to two potential limitations. First, surrogates have imperfect predictive value, and are influenced by confounding variables inherent to OSA populations. Second, these studies cannot assess the effects of OSA on the progression and composition of advanced lesions.
To overcome the first limitation, we assessed atherosclerosis in a murine model of OSA. Upper airway closures in OSA cause repetitive oxygen desaturations, termed intermittent hypoxia (IH). Exposure of C57BL/6J mice on an atherogenic diet to 12 weeks of IH induced fatty streaks in the descending aorta12. However, the burden of disease was insufficient to analyze lesion composition. We now attempt to overcome this second limitation by using atherosclerosis-prone apolipoprotein E-deficient (ApoE−/−) mice. ApoE is the principal ligand of circulating lipoprotein remnant uptake. Its absence leads to marked hypercholesterolemia and rapid atherosclerotic plaque development13.
In this study, ApoE−/− mice were fed high-fat, high cholesterol diets and exposed to IH or intermittent air (IA) control for 4 or 12 weeks, after which lesions, vascular parameters, and lipids were assessed. Because IH-exposed mice exhibited decreased food intake and growth, an additional IA weight-matched control group (IA-WM) was included. We hypothesized that IH would accelerate advanced atherosclerosis in ApoE−/− mice, leading to larger and more unstable lesions.
METHODS
Experimental Animals
Forty-eight 10-week-old male, ApoE−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). At age 13 weeks, mice started a high-fat, high cholesterol diet (4 kcal/g, 15.8% fat, and 1.25% cholesterol; TD#94059, Harlan Teklad, Madison, WI) and simultaneously began IH or IA exposure. In another control group, (IA-WM) food was rationed daily to approximate the weekly growth curves for IH-exposed mice. For surgical procedures, anesthesia was maintained with 2% isoflurane. The study was approved by the Johns Hopkins University Animal Care and Use Committee.
Intermittent Hypoxia
A gas control delivery system was designed to regulate the flow of air, nitrogen, and oxygen into cages housing mice. During each period of IH, the fractional inspired O2 (FiO2) was reduced from 20.9% to ~6.5% over 30 seconds and re-oxygenated to 20.9% in the subsequent 30 second period. Exposures lasted from 09:00 to 21:00 daily to coincide with mouse sleep cycles. Intermittent air (IA) control was delivered at identical flow rates with a constant FiO2 of 20.9%. Oxygen level was measured with a ProOx 110 analyzer (Biospherix, Redfield, NY) and pulse oximetry with a neck cuff (Starr Life Sciences Corp, Oakmont, PA).
Blood Pressure and Pulse Wave Velocity (PWV)
Blood pressure was obtained by tail cuff (CODA2, Kent Scientific, Torrington, CT). PWV was determined as described by Hartley et al.14. Mice were anesthetized with 1.5% isoflurane and placed supine. An EKG-triggered, 2-mm, 10-MHz Doppler probe was used to measure blood flow at the thoracic and abdominal aorta. PWV was calculated by the thoracic-abdominal distance divided by the time difference between pulse arrivals.
Atherosclerosis Analysis
Aortas spanning the aortic arch to the iliac bifurcation were cleaned of adventitia, fixed in formalin, cut longitudinally, stained with Sudan IV, pinned on a wax surface and examined at low power as described by Tangirala15. For cross-sectional analysis, the heart was removed and ventricles transected perpendicular to the aortic outflow tract. The upper heart was fixed in 10% formalin for 12 hours and embedded in paraffin. Serial sections, 7 μm thick, were cut for a distance of ~500 μm spanning the aortic cusps to the proximal ascending aorta. Every 5 sections was stained with hematoxylin & eosin (H&E), and photographed on an Olympus BX41 microscope. Area from 5 serial sections was averaged using Image-J software (NIH, Bethesda, MD).
Plaque Composition
Five sections per aorta were used for each type of stain. Necrotic regions were delineated by unstained H&E regions. Fibrosis was estimated by %area of plaque positive for Masson’s trichrome stain. Macrophages were identified with AIA-31240 Ab at 1:1500 dilution (Accurate Chemical and Scientific, Westbury, NY). Smooth muscle was detected with anti-actin 1A4 Ab, 1:20 dilution (Sigma-Aldrich, St Louis, MO). Endothelium was stained with anti-CD34 Ab, 1:100 dilution, (Abcam, Cambridge, MA) and anti-Von-Willebrand Factor Ab A-0082, 1:100 dilution, (DAKO, Denmark). Nitrotyrosine was detected with ab5411 Ab (Millipore, Billerica, MA), with peroxynitrite incubation as a positive control prior to staining. Primary antibodies were followed by incubation with biotinylated goat anti-rabbit IgG Ab or goat anti-rat IgG Ab. Primary Ab was omitted for negative controls. Secondary Ab was detected with horseradish peroxidase-conjugated biotin-streptavidin complexes and developed with diaminobenzidine tetrahydrochloride. All files were analyzed in blinded fashion using encoded filenames with Photoshop (Adobe Systems, San Jose, CA) software. Some regions of indeterminate composition (<5%) were not scored.
Serum Lipids and Oxidative Stress
Total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), free fatty acids (FFA), and triglycerides (TG) were measured by colorimetric assays (Wako Diagnostics, Richmond, VA). For oxidized LDL (oxLDL), lipids were precipitated from serum with manganese citrate-heparin16. Thiobarbituric acid reactive substances were assayed in the solubilized pellet (Zeptometrix, Buffalo, NY).
Statistical Analysis
All values are reported as means ± SEM. Statistical significance for all comparisons was determined by two-way ANOVA with Bonferroni posttests. A P value of <0.05 was considered significant. All analyses were performed blinded to the IA or IH status of the mice by using ear tag numbers or encoded filenames as identifiers.
RESULTS
Oxygen profiles, weight, food intake, glucose, hematocrit
Mice exposed to IH had a peak SaO2 of 98.7±0.3% which fell to 67±2.1% and exhibited a mean SaO2 of 89.5 ± 0.4% and a median of 95.4% (See supplemental data, Figure 1). Control IA and IA-WM mice maintained a mean SaO2 of 97.9±0.5%. Weight of mice is shown in supplemental data, Figure 2. IH attenuated weight gain versus IA at both the 4 week (gain of 0.3% versus 8.6% initial weight, p<0.05) and 12 week (gain of 2.7% versus 11.0% initial weight, p<0.01) time point. IA-WM mice achieved similar weight as IH mice at each weekly time point. Growth trends paralleled food consumption, which was lower in IH (3.18 g/day) and IA-WM (3.07 g/day) mice than IA controls (3.69 g/day) throughout the exposure period. Fasting blood glucose was similar in all groups, and values were within 80–120 mg/dL. IH caused an increase in hematocrit at both 4 weeks (IH: 44.7±1.2% vs. control: 38.6±1.0% p<0.005) and 12 weeks (IH: 40.6±0.9% vs. control: 37.6±1.0%, p<0.01).
Blood pressure and vascular function
Blood pressure and PWV were significantly affected by IH at both time points (Table 1). At 4 weeks, IH caused a rise in systolic blood pressure and PWV. At 12 weeks, IH caused a rise in diastolic blood pressure, increasing mean arterial blood pressure. PWV remained elevated but did not increase further at 12 weeks. Blood pressures obtained under anesthesia were not different between groups during assessment of PWV. To account for potential hypertension effects, β-index was calculated for each animal by the formula: (2.11 × PWV2/non-anesthestized diastolic blood pressure) without significantly affecting the comparative results (data not shown). IA-WM controls did not differ from IA controls in terms of blood pressure (Mean arterial BP at 4 wk: 77.1±2.9; 12 wk: 75±3.1 mmHg), or PWV (4 wk: 3.1±0.1, 12 wk: 3.0±0.1 m/s).
Table 1.
Blood pressure (BP) and pulse wave velocity (PWV) of ApoE−/− mice exposed to intermittent air control (IA) or intermittent hypoxia (IH). IH induced an early rise in systolic BP and a later rise in diastolic BP. Aortic PWV increased after 4 weeks and remained elevated at 12 weeks.
| 4 weeks | 12 weeks | |||||
|---|---|---|---|---|---|---|
| IA | IH | IA | IH | 4 vs. 12 week IA P value | 4 vs. 12 week IH P value | |
| Systolic BP (mmHg) | 95±2.0 | 102±2.5* | 100±4.4 | 115±5.4 | NS | <0.05 |
| Diastolic BP (mmHg) | 67±1.9 | 70±2.9 | 68±2.6 | 82±4.6* | NS | <0.05 |
| Mean BP (mmHg) | 76±1.8 | 80±2.6 | 78±3.1 | 93±4.8* | NS | <0.05 |
| Heart Rate (BPM) | 680±14 | 650±26 | 689±5 | 686±13 | NS | NS |
| Aortic PWV (m/s) | 3.2±0.1 | 3.5±0.1* | 3.1±0.1 | 3.5±0.1* | NS | NS |
Abbreviations: NS = not statistically significant (p>0.05).
P<0.05 for the difference between IH and IA. n = 8 per group. All values are expressed as mean ± SEM.
Cholesterol profiles
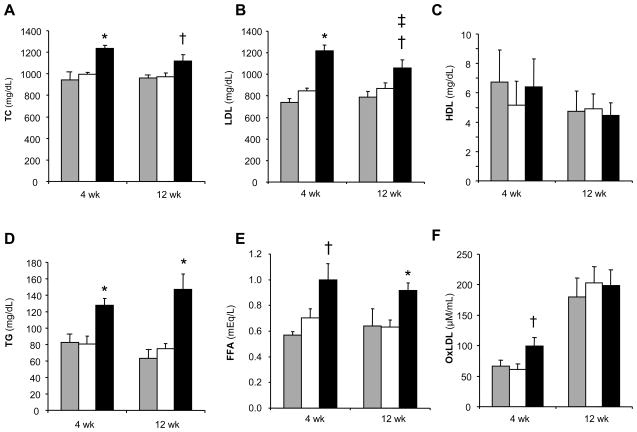
IH increased TC, LDL-C, TG, and FFA as compared to either IA or IA-WM controls (Figure 1). Interestingly, the effect of IH was slightly stronger at 4 weeks than at 12 weeks in the LDL fraction. Oxidized LDL (oxLDL) was increased by IH at 4 weeks. However, when normalized to total LDL-C, IH did not alter the proportion of circulating oxLDL at either time point.
Figure 1.
Serum cholesterol levels in ApoE−/− mice exposed to intermittent air control (IA, open bars), IA with weight matching (IA-WM, shaded bars), or intermittent hypoxia (IH, solid bars). Fasting levels of (A) total cholesterol, (B) low-density lipoprotein cholesterol, (C) high-density lipoprotein cholesterol, (D) triglycerides, (E) free fatty acids, and (F) oxidized LDL are shown. *P<0.001, †P<0.05 for IH vs. (IA or IA-WM); ‡P<0.05 for IH at 4 vs. 12 weeks. n=8 per group. Data represent mean ± SEM.
Atherosclerosis
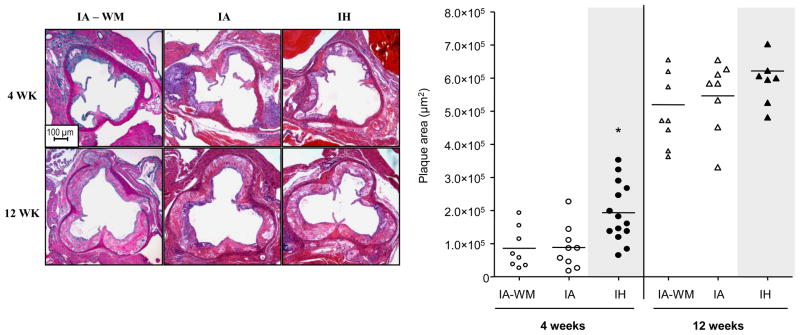
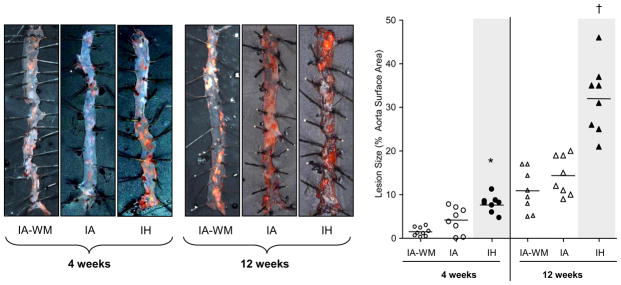
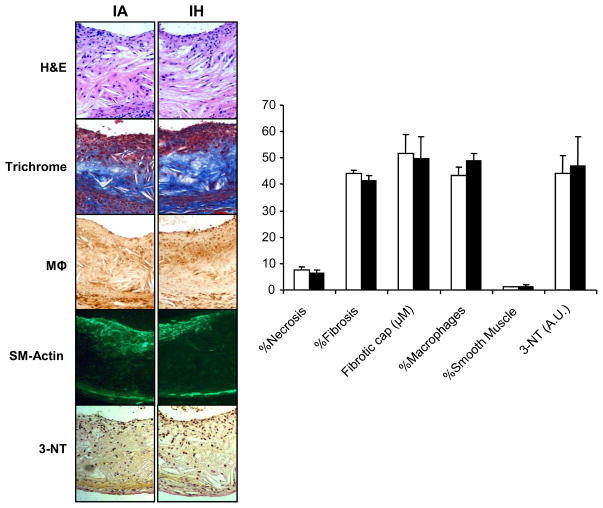
As expected, the size of atherosclerotic plaques increased with time in all groups (Figs 2, 3; p<0.001). As shown in figure 2, after 4 weeks, IH increased plaque area in the proximal aorta by twofold. However, after 12 weeks, there was no difference in lesion size between IA and IH groups. In the descending aorta (Figure 3), IH increased lesion area at both time points, compared to IA and IA-WM controls. Lesion area was slightly smaller in the IA-WM mice compared to IA mice (p<0.05). Lesion composition was compared at 12 weeks, when they were large enough for accurate analysis. At this point, plaques exhibited characteristics of mature atheroma with fibrotic caps and collagen deposition. Lesions in IA, IA-WM, and IH groups were similar in terms of necrosis, fibrosis, fibromuscular cap thickness, foam cell area, smooth muscle content, and nitrotyrosine staining (Figure 4). No intra-plaque neo-vessels were evident in any group using both anti-vWF and anti CD34 antibodies (data not shown)
Figure 2.
Images and quantification of lesions in the aortic root of ApoE−/− mice exposed to intermittent air control (IA), IA with weight matching (IA-WM), or intermittent hypoxia (IH). *P<0.01 for 4 weeks: IH vs. (IA or IA-WM). Each replicate and the mean for the group are shown.
Figure 3.
En face lesions of the descending aorta in ApoE−/− mice exposed to intermittent air control (IA), IA with weight matching (IA-WM) or intermittent hypoxia (IH). Lesions are stained with Sudan IV and shown at 10X magnification. *P<0.05 for 4 weeks: IH vs.(IA or IA-WM); †P<0.001 for 12 weeks: IH vs (IA or IA-WM). Weight-matching reduced plaque area (p<0.05). All replicates and the mean for each group are shown.
Figure 4.
Composition of plaques in ApoE−/− mice exposed to 12 weeks of intermittent air control (IA) or intermittent hypoxia (IH). Representative images were obtained at the level of the aortic sinus above the valve cusps. Plaques in the IA, IA-WM (not shown) and IH group exhibited similar necrotic area (H&E), fibrosis (trichrome), macrophage area (mΦ), and smooth muscle actin content (SM-Actin). Levels of nitrotyrosine (3-NT) staining were also not different between IH and IA groups (A.U.= arbitrary units).
DISCUSSION
We showed previously that IH mimicking the oxygen profile of subjects with OSA in a non-atherogenic host induced early atherosclerotic lesions. In this study, we show that IH accelerates the growth of advanced atherosclerotic plaques in ApoE−/− mice, without altering their composition, suggesting that IH had no effect on stability of the plaques. In the discussion below, we will explore the connections between IH and atherosclerosis and the clinical implications of our work.
Intermittent Hypoxia and Atherosclerosis
The atherogenic effect of IH was most notable in the descending aorta, whereas in the ascending aorta, a difference was only apparent at 4 weeks. One interpretation of this finding is that atherogenesis in this region slowed after 4 weeks during IH. Alternatively, a maximal rate of atherogenesis in the aortic sinus may have been reached with no apparent effect of IH. We found no obvious differences in plaque composition between IH and controls. IH thus promoted the development of larger but structurally similar lesions. The effects occurred independently of the weight loss incurred by IH; IA-WM mice consumed, on average, 83% the amount food as ad libitum control mice and exhibited less atherosclerosis. Similarly, Guo et al 17 induced 60% calorie restriction in apoE−/− mice and found even more dramatic attenuation of lesions. Thus, weight loss induced by our model of IH is accompanied by detrimental cardiovascular consequences, as opposed to the beneficial effects of weight loss induced by food restriction.
Intermittent Hypoxia and Dyslipidemia
Serum cholesterol was clearly affected by IH, with LDL-C increasing by ~44% at 4 weeks, and ~23% at 12 weeks. In prior studies of C57BL/6J mice exposed to IH, HDL-C and TG increased in 5 days 18, and shifted towards LDL-C predominance after 4 weeks19. The fact that IH-induced lipid increases were stable rather than additive over time is an important finding, suggesting either early attainment of higher steady-state levels, or transient hyperlipidemia during IH that normalizes during normoxia. The mechanism of these increases is not entirely clear, but lipid mobilization, as evidenced by increased FFA, is likely to play a role. A rise in hepatic FFA substrate would be expected to result in TG synthesis and enhanced secretion of VLDL lipoproteins 20. In addition, we have previously shown that chronic IH up-regulates transcriptional pathways of hepatic lipid biosynthesis21.
Intermittent Hypoxia, Hypertension, and Endothelial Function
IH led to increases in blood pressure and a stable increase in vascular stiffness as measured by PWV. Blood pressure increases have been reported in similar IH experiments arising from sympathetic activation22 and oxidative stress23. An increase in PWV could also be a consequence of hypertension, but blood pressure under isoflurane at the time of PWV assessment was similar in all groups. PWV increases might also be a consequence of advanced atherosclerosis, but an increase in PWV occurred relatively early in this study. In addition, other groups observed similar increases in PWV during IH in the absence of atherosclerosis24. Our experimental findings illustrate the potentially interacting yet independent effects of hypertension, arterial stiffness, and OSA. OSA in the absence of hypertension is associated with increased arterial stiffness7, while OSA and concurrent hypertension is associated with additive increases in carotid-femoral PWV25 and carotid atherosclerosis26.
Intermittent Hypoxia and Oxidative Stress
Oxidative stress, another factor in the development of atherosclerosis, has been reported in OSA and IH27. The increase in oxLDL with a preserved oxLDL/LDL ratio does not support an effect of IH on lipid peroxidation apart from an overall increase in LDL cholesterol. Plaque nitrotyrosine levels were not different between IH and controls, suggesting that IH exposure did not induce significant levels of peroxynitrite in these regions. Thus, although we do not discount the potential role of ROS in IH-induced increases in blood pressure and PWV, we did not find evidence for an intra-lesion role of ROS on IH-induced atherogenesis. Similarly, 4 weeks of IH did not alter several aortic oxidative stress parameters in C57BL/6J mice28.
Limitations
A few important caveats apply to this investigation. First, IH has limitations as a model of OSA, lacking features of airway closure, thoracic pressure variation, complete synchrony with sleep, and hypercapnea29. Second, aortic lesions of ApoE−/− mice are relatively resistant to plaque rupture - the litmus test of their stability. Nevertheless, the parameters we assessed are established features of vulnerable lesions2. Third, we identified not one but several possible mechanisms, and this list may be incomplete. On the other hand, we have identified which effects of IH are cumulative (hypertension), persistent (PWV, TG, FFA) or attenuated (LDL-C) by exposure time. Finally, mice exposed to IH lost weight and ate less food than controls, a known consequence of chronic hypoxia30. Food restriction was athero-protective, supporting a role of IH on atherogenesis independent from its growth-suppressing effects.
Conclusion and Significance
In a murine model of advanced atherosclerosis, we have shown that exposure to chronic IH accelerates the growth of aortic lesions. Atherosclerotic lesions generally progressed more rapidly, but were not rendered more structurally vulnerable. Dyslipidemia, hypertension, and arterial stiffness are potential therapeutic targets for intervention during IH to attenuate lesion growth.
Supplementary Material
Acknowledgments
A. Funding Sources
Jonathan Jun: NIH (T32 HL07534) and American Lung Association-National Sleep Foundation Pickwick Fellowship (SF-78568-N); Jianguo Li: American Heart Association Mid-Atlantic Affiliate Postdoctoral Fellowship (0625514U); Christian Reinke: Research Fellowship grant RE 2842/1-1, German Research Foundation (DFG); Vsevolod Y Polotsky: NIH (R01 HL80105 and SCCOR 5P50HL084945) and American Heart Association Mid-Atlantic Affiliate 0765293U.
We are indebted to Allen Myers, Ph.D. for his assistance with immunohistochemistry.
Footnotes
C. Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 December 17;106(25):3143–421. [PubMed] [Google Scholar]
- 2.Halvorsen B, Otterdal K, Dahl TB, Skjelland M, Gullestad L, Oie E, Aukrust P. Atherosclerotic plaque stability--what determines the fate of a plaque? Prog Cardiovasc Dis. 2008 November;51(3):183–94. doi: 10.1016/j.pcad.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Geng YJ, Sukhova GK, Simon DI, Lee RT. Molecular determinants of atherosclerotic plaque vulnerability. Ann N Y Acad Sci. 1997 April 15;811:134–42. doi: 10.1111/j.1749-6632.1997.tb51996.x. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 April 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008 August 1;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009 August;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of CPAP on Early Signs of Atherosclerosis in Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2007 June 7;176(7):706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000 May 11;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.McArdle N, Hillman D, Beilin L, Watts G. Metabolic Risk Factors for Vascular Disease in Obstructive Sleep Apnea: A Matched Controlled Study. Am J Respir Crit Care Med. 2007 January 15;175(2):190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 10.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004 September;59(9):777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004 February 1;27(1):123–8. [PubMed] [Google Scholar]
- 12.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007 June 15;175(12):1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004 June;24(6):1006–14. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 14.Hartley CJ, Taffet GE, Michael LH, Pham TT, Entman ML. Noninvasive determination of pulse-wave velocity in mice. Am J Physiol. 1997 July;273(1 Pt 2):H494–H500. doi: 10.1152/ajpheart.1997.273.1.H494. [DOI] [PubMed] [Google Scholar]
- 15.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995 November;36(11):2320–8. [PubMed] [Google Scholar]
- 16.Gidez LI, Miller GJ, Burstein M, Slagle S, EDER HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982 November;23(8):1206–23. [PubMed] [Google Scholar]
- 17.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002 April 30;123(8):1121–31. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O’Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005 September 30;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007 February;102(2):557–63. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 20.Sniderman AD, Cianflone K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler Thromb. 1993 May;13(5):629–36. doi: 10.1161/01.atv.13.5.629. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics. 2007 October 22;31(2):273–80. doi: 10.1152/physiolgenomics.00082.2007. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992 November;20(5):612–9. doi: 10.1161/01.hyp.20.5.612. [DOI] [PubMed] [Google Scholar]
- 23.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006 August 15;575(Pt 1):229–39. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008 January 15;177(2):227–35. doi: 10.1164/rccm.200702-238OC. [DOI] [PubMed] [Google Scholar]
- 25.Tsioufis C, Thomopoulos K, Dimitriadis K, Amfilochiou A, Tousoulis D, Alchanatis M, Stefanadis C, Kallikazaros I. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007 Jan;25(1):141–6. doi: 10.1097/HJH.0b013e32801092c1. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension. 2009 Jan;53(1):64–9. doi: 10.1161/HYPERTENSIONAHA.108.119420. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009 June;33(6):1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 28.Jun JC, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent Hypoxia has Organ-Specific Effects on Oxidative Stress. Am J Physiol Regul Integr Comp Physiol. 2008 Oct;295(4):R1274–81. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun J, Polotsky VY. SLEEP DISORDERED BREATHING AND METABOLIC EFFECTS: EVIDENCE FROM ANIMAL MODELS. Sleep Med Clin. 2007 June;2(2):263–77. doi: 10.1016/j.jsmc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raguso CA, Guinot SL, Janssens JP, Kayser B, Pichard C. Chronic hypoxia: common traits between chronic obstructive pulmonary disease and altitude. Curr Opin Clin Nutr Metab Care. 2004 July;7(4):411–7. doi: 10.1097/01.mco.0000134372.78438.09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.