Abstract
Purpose
To assess results of a multi-institutional study of Intensity-Modulated Radiation Therapy (IMRT) for early oropharyngeal cancer.
Patients and Methods
Patients with oropharyngeal carcinoma stage T1-2, N0-1, M0 requiring treatment of the bilateral neck were eligible. Chemotherapy was not permitted. Prescribed planning target volumes (PTVs) doses to primary tumor and involved nodes was 66 Gy at 2.2 Gy/fraction over 6 weeks. Sub-clinical PTVs received simultaneously 54-60 Gy at 1.8-2.0 Gy/fraction. Participating institutions were pre-approved for IMRT, and quality assurance review performed by the Image–Guided Therapy Center.
Results
69 patients accrued from 14 institutions. At median follow-up for surviving patients 2.8 years, 2-year estimated local-regional failure (LRF) rate was 9%. 2/4 patients (50%) with major under-dose deviations had LRF, compared with 3/49 (6%) without such deviations (p=0.04). All cases of LRF, metastasis, or second primary cancer, occurred among patients who were current/former smokers, and none among patients who never smoked. Maximal late toxicities grade ≥ 2 were skin 12%, mucosa 24%, salivary 67%, esophagus 19%, osteoradionecrosis 6%. Longer follow-up revealed reduced late toxicity in all categories. Xerostomia grade ≥ 2 was observed in 55% of patients at 6 months but reduced to 25% and 16% at 12 and 24 months, respectively. In contrast, salivary output did not recover over time.
Conclusions
Moderately accelerated hypofractionatd IMRT without chemotherapy for early oropharyngeal cancer is feasible, achieving high tumor control rates and reduced salivary toxicity compared with similar patients in previous RTOG studies. Major target under-dose deviations were associated with higher LRF rate.
Keywords: Oropharynx cancer, IMRT, head and neck cancer
Introduction
Recent improvements in tumor control rates for head and neck (HN) cancer have been achieved through altered fractionation radiotherapy (RT) and the addition of concurrent chemotherapy. In tandem with these advances, technological innovations including conformal 3D RT and subsequently intensity modulated RT (IMRT) have become more broadly available. Reports from several institutions suggested that IMRT for HN cancer, and specifically oropharyngeal cancer, achieved important goals in reducing treatment toxicity, notably xerostomia, and in yielding high local-regional (LR) tumor control rates. Few marginal failures were observed following judicious utilization of the principles of target definition and delineation (1-6).
In an effort to investigate whether the early successes of IMRT reported by few institutions could be reproduced in a multi-institutional setting, the Radiation Therapy Oncology Group (RTOG) embarked on a prospective study of IMRT for early oropharyngeal cancer, RTOG 00-22. Altered fractionated RT was initially desired for this study, however, it was recognized that multiple daily fractions would not be feasible for many institutions due to the lengthy IMRT treatment time. Therefore, a regimen of high fraction doses to the gross tumor volumes (GTVs), simultaneously with standard fraction doses to the clinical target volumes (CTVs), was devised such that the biologically effective GTV doses would be nearly equivalent to 70 Gy delivered by conventional fractionated RT. This regimen was delivered over 6 weeks, representing a moderate acceleration over a standard course. It was hypothesized that the dose conformality provided by IMRT would allow the delivery of moderate GTV dose hypofractionation, without increasing acute or late sequelae, while maintaining the tumor control benefits associated with shortened overall treatment time.
This study was the first multi-institutional trial incorporating IMRT. It included target dose prescription and tissue dose constraints which were formalized by many clinicians and physicists after considerable debate. The trial also utilized central quality assurance processes assessing the ability of the participating institutions to plan and execute IMRT, and the quality of the individual IMRT plans. This paper reports the mature clinical results of this study.
Patients and methods
Eligible patients had clinical early stage (T1-2, N0-1, M0) oropharyngeal squamous cell carcinoma in whom primary RT was indicated and in whom both sides of the neck were judged to be at risk of metastatic disease. Patients with small N2 disease by imaging were also eligible. Previous surgery of the primary tumor or lymph nodes was limited to excisional or incisional biopsies. Patients had Zubrod performance status 0-1 and no previous therapy for HN cancer. Chemotherapy, salivary stimulants, or radiation protectors were not allowed.
Whole mouth unstimulated salivary flow rate measurements were made over a collection time of 5 minutes, followed with a stimulation by swabbing 2% citrate solution to the lateral tongue bilaterally over a 2-minute period. The mouth was then emptied, followed with saliva collection over 5 minutes.
Radiation Therapy
The immobilization device included both neck and shoulder immobilization. The definition of the target volumes was in accordance with the 1993 ICRU Report #50: The gross target volume (GTV) was defined as all known disease determined by clinical examination and from CT, PET, or MRI. The clinical target volumes (CTVs) were defined as GTVs plus areas considered to contain potential microscopic disease with typical margins of 1-2 cm, or lymph node levels at risk of sub-clinical disease (levels II-IV bilaterally, Ib ipsilaterally, and level V and retropharyngeal nodes if the jugular nodes were involved). An on-line atlas detailing the CTVs for the N0 neck was posted at the RTOG web site to aid and standardize nodal CTV delineation (http://www.rtog.org/hnatlas/main.html). The planning target volume (PTV), aimed to accommodate set-up uncertainties, was at least 5 mm, unless individual institution set-up uncertainty data supported different margins. Uninvolved organs to be contoured included skin surfaces, brainstem, spinal cord, mandible, glottic larynx, parotid and submandibular salivary glands. Spinal cord contours were defined as 5 mm larger in the radial dimension than the anatomical spinal cord boundary. Tissues outside the targets and critical organs were defined as unspecified tissues.
Planning
The PTVs included PTV66, encompassing the primary targets, and PTV54, encompassing the secondary targets. The prescribed doses were 66 Gy and 54 Gy, respectively, delivered simultaneously over 30 fractions, at daily fraction sizes of 2.2 Gy and 1.8 Gy, respectively, 5 days a week, over 6 weeks. An optional intermediate-risk CTV encompassing the volume in the immediate vicinity of the GTV, or first-echelon nodal areas, was prescribed 60 Gy (PTV60), delivered simultaneously at 2.0 Gy/fraction.
The prescription dose was defined as the isodose encompassing at least 95% of the PTV. Target dose restrictions included the following: no more than 20% of any PTV could receive >110% of its prescribed dose, no more than 1% of any PTV would receive < 93% of the prescribed dose, and no more than 1% or 1 cc of the tissue outside the PTV would receive >110% of the dose prescribed to the primary target (66 Gy). The protocol did not specify trimming the PTV away from skin surfaces to reduce skin toxicity when it was not a target.
The reported doses for each PTV included the prescribed doses as well as the maximal dose points, % target volume receiving >110% and 115% of the prescribed dose and the % target volume receiving < 93% of the prescribed dose, as well as mean PTV dose. The doses were to be based on dose distributions corrected for heterogeneities.
The following critical normal structure dose constraints were used: The glottic larynx was expected to receive < 50 Gy to at least 2/3 of its volume. The brainstem, spinal cord and mandible maximal doses were 54 Gy, 45 Gy, and 70 Gy, respectively. Unspecified tissue maximal dose was restricted to ≤ 110% prescribed PTV66 dose. Parotid salivary glands planning objectives were mean dose < 26 Gy or 50% receiving < 30 Gy, to at least one gland. In addition, reducing as much as possible to doses to the oral cavity were planning objectives.
Either whole-neck IMRT or split-field technique, in which the low neck was treated with an anterior beam matched with upper-neck IMRT, were allowed. No specific image guidance was included in the protocol. All types of IMRT delivery were allowed and re-planning during therapy was not specified.
Quality assurance (QA)
QA of target and organ delineation was performed by the study chair on initial cases submitted by each institution, followed by spot checks of subsequent cases. The Image-Guided Therapy Center (IGTC) at Washington University reviewed initial portal films and DRRs from each institution followed with spot-checking. Calculated isodose distributions were verified by the QA center and scored according to the following criteria:
No Variation: all criteria for maximal and minimal doses fulfilled.
Minor Variation for PTV66: the prescription criteria are not met, but all the following are fulfilled: the 60 Gy isodose covers ≥ 99% of PTV66, and the 66 Gy isodose surface covers ≥ 90% of PTV66. Also, the 72.6 Gy isodose surface (110% prescribed dose) covers ≤ 25% of PTV66.
Minor Variation for PTV60 or PTV54: The prescription criteria are not met, but the following are fulfilled: the 47 Gy isodose surface covers ≥ 99% of PTV54 and the 54 Gy isodose covers ≥ 90% of PTV54. The 52 Gy isodose covers > 90% of PTV60 and the 72.6 Gy isodose covers ≤ 20% PTV54 and PTV60 (except when it coincides with PTV66).
Major Variations were defined as having met dose limits for neither No Variation nor Minor Variation.
Parotid gland scoring was defined as No Variation, Minor Variation if dose goals were not met but < 60% of one gland received > 30 Gy, and Major Variation if > 60% of each parotid gland received > 30 Gy.
QA physics/dosimetry
The participating institution had to be pre-approved for IMRT treatment by the IGTC. In brief, pre-approval consisted of submitting an IMRT treatment plan and the results of treatment of an anthropomorphic phantom provided by the IGTC, detailed elsewhere (7). The IGTC compared the submitted DVHs for the targets and critical tissues with the DVHs calculated by the center. Once the IMRT treatment plan and phantom irradiation results were approved, the institution was deemed eligible to participate in the study and was required to send all treatment plans to the IGTC, including all GTV/CTV/PTV contours, DVHs, simulation and portal films, and a copy of the daily treatment record.
Follow-up and Statistical Considerations
Patients underwent weekly examinations during treatment and follow-up including H&P and toxicity evaluation every 3 months in years 1-2, every 6 months in years 3-5, and then annually. Whole mouth saliva measurements were made 3, 6, and 12 months after therapy. Acute toxicity (within 90 days of start therapy) was scored according to National Cancer Institute Common Toxicity Criteria version 2.0, and late toxicity according to RTOG/European Organisation for Research and Treatment of Cancer criteria.
The primary objective of the study was assessment of the feasibility of adequate target coverage and parotid gland sparing. Secondary objectives were to determine the rate and pattern of locoregional (LR) tumor recurrence and the nature and prevalence of acute and late side effects, and their relationships to the doses to the relevant organs.
The treatment regimen was to be considered for further study if the requirements were met for both xerostomia and LR failure. Based on the RTOG database, a LR failure rate of 20% was targeted with 35% considered unacceptable. Using the method of Fleming (8) with types I and II errors 0.10, the required sample size was 57 patients. LR failure was to be considered acceptable if ≤ 15 patients (out of 57) failed within the first 2 years. In the amifostine study, acute xerostomia (≥ grade 2) was reduced from 78% to 51% in patients receiving conventional RT (9), for a relative reduction of 35%. From the RTOG database of patients with early stage oropharyngeal cancer, an acute grade ≥ 2 xerostomia was observed in 84%. A relative reduction of 35%, in similar patients receiving IMRT, would reduce this rate to 55%. Xerostomia was to be considered acceptable if ≤ 31 patients (out of 57) experienced acute xerostomia grade ≥ 2. The sample size was adjusted by 10% to 64 patients to allow for ineligible patients and loss to follow-up.
Toxicity rates ≥ grade 2 were estimated along with 95% confidence intervals. LR failure was persistent or recurrent local or regional disease; patients with persistent disease were considered a failure at day 1. Rates were estimated using the cumulative incidence method to account for the competing risk of death without LR failure (10). Disease-free and overall survival rates were estimated using the Kaplan-Meier method. Failure for disease-free survival was defined as LR failure, distant metastasis, second primary tumor, or death. The proportions of patients with LR failure with and without major under-dose variations and by smoking status were tested using Fisher's Exact Test with significance level 0.05.
Results
Between February 2001 and January 2005, 69 patients from 14 institutions were accrued to the protocol. Two patients were retrospectively deemed ineligible (due to pre-RT chemotherapy in one and tumor stage T3 in one). Median follow-up for surviving patients is 2.8 years (range 1.4 - 4.8); only 2 surviving patients have been followed for less than 2 years. Patient and tumor characteristics for the 67 eligible patients are detailed in Table 1. Seven patients (10%) were upstaged to N2 by imaging.
Table 1. Patient and Tumor Characteristics for 67 Patients.
| Age | ||
| Median | 56 | |
| Range | 33-84 | |
| No. pts | % | |
| Gender | ||
| Male | 52 | 78 |
| Female | 15 | 22 |
| Zubrod Performance Status | ||
| 0 | 44 | 66 |
| 1 | 23 | 34 |
| Primary Site | ||
| Tonsil | 33 | 49 |
| Base of tongue | 26 | 39 |
| Soft palate | 8 | 12 |
| T stage | ||
| T1 | 17 | 25 |
| T2 | 50 | 75 |
| N stage, clinical | ||
| N0 | 38 | 57 |
| N1 | 29 | 43 |
| N stage, radiographic | ||
| N0 | 31 | 46 |
| N1 | 26 | 39 |
| N2a | 2 | 3 |
| N2b | 5 | 7 |
| Unknown | 3 | 4 |
A review of the treatment plans is summarized in Table 2. Of the 67 analyzable cases, 14 (21%) could not be fully evaluated for target dose coverage because they were treated with one manufacturer unit whose digital data submission for the PTVs was not initially possible. Overall, there was no case judged to be according to protocol without any variation. Of the evaluable cases, 47 (89%) were scored with minor and 6 (11%) with major variations, of whom 5 had major variations in PTV66 coverage. Of the patients with major PTV66 variations, one had an over-dose variation, and 4 had major under-dose variations (all had <90% of PTV66 encompassed within the 66 Gy isodose volume). The compliance with protocol directives regarding parotid gland doses was much higher: 95% had no variation and 5% had minor variations.
Table 2. IMRT plans review for 67 Patients.
| No. pts | % | |
|---|---|---|
| Overall | ||
| Minor variation | 47 | 70 |
| Major variation | 6 | 9 |
| Inevaluable | 14 | 21 |
| PTV66 | ||
| Minor variation | 48 | 72 |
| Major variation | 5 | 7 |
| Inevaluable | 14 | 21 |
| PTV60 | ||
| Not applicable | 34 | 51 |
| No variation | 1 | 1 |
| Minor variation | 26 | 39 |
| Major variation | 1 | 1 |
| Inevaluable | 5 | 7 |
| PTV54 | ||
| No variation | 10 | 15 |
| Minor variation | 40 | 60 |
| Major variation | 3 | 4 |
| Inevaluable | 14 | 21 |
| Parotid gland | ||
| No variation | 61 | 91 |
| Minor variation | 3 | 4 |
| Inevaluable | 3 | 4 |
Acute toxicity grade ≥ 2 is summarized in Table 3. The most prevalent toxicities were skin and oropharyngeal (OP). Break-down of the OP toxicities revealed that most were related to dysphagia [52% grade 2-3, 95% confidence interval (40, 65)], mucositis [58% grade 2-4 (46, 70)], and salivary gland disorders, both dryness [49% grade 2 (37, 61)] and thick sticky saliva (42% grade 2 (30, 54)]. Twenty-six of the first 57 patients and 33 of all 67 patients experienced acute grade 2 dryness (xerostomia).
Table 3. Acute toxicity grade ≥ 2 for 67 Patients.
| Grade | |||
|---|---|---|---|
| 2 | 3 | 4 | |
| Gastrointestinal | 46% | 31% | 4% |
| Dysphagia | 37% | 15% | 0 |
| Mucositis | 31% | 25% | 1% |
| Esophagitis | 3% | 0 | 0 |
| Dry mouth | 49% | 0 | 0 |
| Salivary gland changes | 42% | 0 | 0 |
| Taste disturbance | 16% | 0 | 0 |
| Nausea | 6% | 3% | 0 |
| Vomiting | 3% | 3% | 0 |
| Dehydration | 12% | 1% | 0 |
| Anorexia | 0 | 3% | 3% |
| Other | 0% | 1% | 0 |
| Skin | 21% | 10% | 0 |
| Pain | 16% | 4% | 0 |
| Pulmonary | 1% | 1% | 0 |
| Blood | 0 | 4% | 0 |
| Constitutional symptoms | 28% | 0 | 0 |
| Auditory | 1% | 0 | 0 |
| Infection febrile neutropenia | 1% | 0 | 0 |
| Neurology | 1% | 0 | 0 |
Maximal late toxicities and late toxicities observed at 6, 12, 18, and 24 months from start of RT are summarized in Table 4. Most of the maximal late toxicities grade ≥ 2 were observed within the first year from start of RT, and late toxicity rates generally declined over time. Notably, salivary toxicity grade ≥ 2 was observed in 55% of patients at 6 months from start of RT but was reduced to 25% at 12 months and 16% at 24 months. Six patients had new or continuing grade 3 or 4 toxicity 15 or more months from the start of RT, including 3 cases of osteoradionecrosis (ORN). Dosimetry of the ORN sites was available for 2 patients. In both patients the ORN sites received the highest doses delivered to the mandible in the same patient: 69 and 70 Gy (mandibular mean dose was 45 Gy in both patients).
Table 4. Late toxicity grade ≥ 2.
| Maximal Toxicity (n=67) Grade | 6 Months from Start of RT (n=64) Grade | 12 Months from Start of RT (n=59) Grade | 18 Months from Start of RT (n=52) Grade | 24 Months from Start of RT (n=51) Grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Skin | 12% | 0 | 0 | 5% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucosa | 16% | 0 | 8% | 9% | 0 | 2% | 3% | 0 | 2% | 4% | 0 | 2% | 2% | 0 | 4% |
| Subcutaneous | 12% | 0 | 0 | 3% | 0 | 0 | 5% | 0 | 0 | 2% | 0 | 0 | 0 | 0 | 0 |
| Salivary | 63% | 4% | 0 | 52% | 3% | 0 | 25% | 0 | 0 | 15% | 0 | 0 | 16% | 0 | 0 |
| Esophagus | 15% | 4% | 0 | 5% | 3% | 0 | 7% | 2% | 0 | 4% | 2% | 0 | 2% | 0 | 0 |
| Larynx | 6% | 0 | 0 | 3% | 0 | 0 | 2% | 0 | 0 | 2% | 0 | 0 | 0 | 0 | 0 |
| Spinal cord | 1% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bone | |||||||||||||||
| (incl. osteoradionecrosis) | 1% | 1% | 3% | 0 | 0 | 2% | 0 | 0 | 2% | 0 | 0 | 2% | 0 | 0 | 4% |
| Joint | 3% | 0 | 0 | 2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal | 1% | 0 | 0 | 0 | 0 | 0 | 2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 19% | 3% | 1% | 14% | 2% | 0 | 2% | 0 | 0 | 2% | 2% | 0 | 2% | 0 | 0 |
The reduction in whole mouth salivary flow rates 3 months after therapy was substantial: median 80% and 86% in stimulated and unstimulated saliva, respectively. Small improvements in the relative loss of both stimulated and unstimulated salivary flow rates from 3 months through 12 months after RT were noted, but were not statistically significant (p=0.38 and 0.57, respectively).
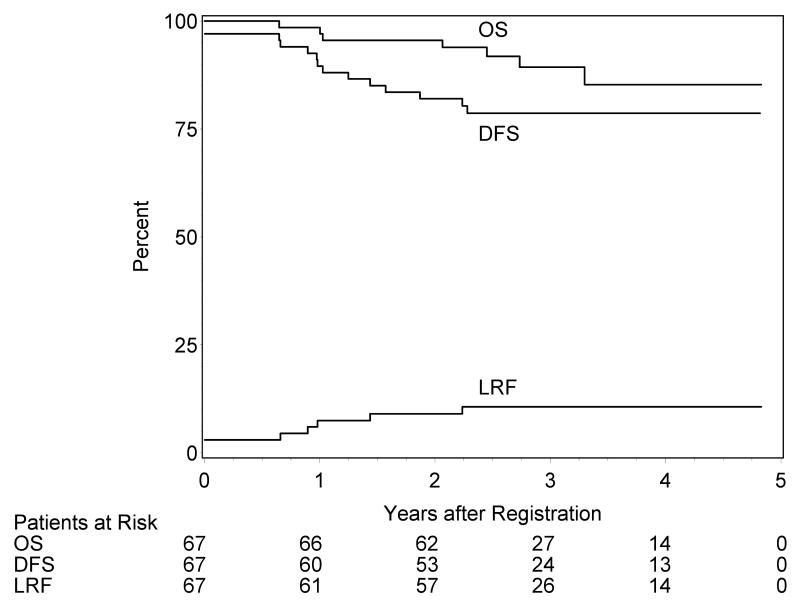
LR failures were noted in 7 patients; 2 had persistent disease, 4 had local recurrence and one had regional recurrence. Two of these patients were salvaged. The estimated 2-year LR failure rate is 9.0% (2.1, 15.9) [Figure 1]. Distant metastasis was reported in only one patient. Five patients had second primary tumors (2 lung cancer and one each in skin, larynx, and oral cavity). Seven patients have died; the estimated 2-year overall survival rate is 95.5% (90.6, 100.0) and the disease-free survival rate is 82.0% (72.7, 91.2) [Figure 1].
Figure 1.
Kaplan-Meier estimates of overall survival (OS) and disease-free survival (DFS) and cumulative incidence of local-regional failure (LRF).
Of the 7 patients with LR failures, 2 had major dosimetric variations in PTV66 coverage (both failed locally), and 2 patients had inevaluable treatment plans. The major variations in the 2 who failed locally were 66 Gy covering 88% or 80% of PTV66 (rather than 95% per protocol). Overall, 2 of the 4 patients with major under-dose variations and evaluable plans failed locally, compared with 3 LR failures in 49 patients with evaluable plans and no major variations (p=0.04).
Smoking status was known in 88% of the patients. Of the 36 (54%) current/former smokers, 7 had LR failures, one had metastatic disease, and 5 had second primary cancers. Of the 23 (34%) never-smokers (<5 packs lifetime), there were no LR failures, no distant metastasis, and no second primary cancers. The differences between smokers and never-smokers in tumor recurrences was statistically significant (p=0.02), while the differences in second primary cancers did not reach statistical significance (p=0.15).
Discussion
This was the first multi-institutional study of IMRT which laid out strict criteria for target delineation by physicians, target and organ dose prescription, and implementing quality control assessment of the dosimetric/physical aspects of IMRT delivery. All the aspects of quality assurance were enacted through a centralized quality control process, paving the way for subsequent multi-institutional studies for HN cancer which incorporated IMRT. The quality control process has been described in part elsewhere (11). This process sought to ensure that the definition of the targets and normal tissues, their dose prescriptions and constraints, and their use in the dosimetric and clinical implementation of IMRT, followed published consensus guidelines (12-14). The value of the guidelines stated in the protocol is highlighted by the results of the study: patients with major underdosage of the primary tumor PTV, as defined by the protocol, had a significantly higher rate of local-regional recurrence compared with patients without major variations. Similar findings have recently been reported from another multi-institutional study using mostly conventional RT (15). Thus, this protocol's guidelines can serve as a template for institutional studies and for routine clinical practice of IMRT for HN cancer. Compared with the importance of the major variations in target doses, the impact of the minor variations, which were very common, could not be elicited in this study. It is likely that these criteria were too stringent, and they have been slightly relaxed in current RTOG trials involving IMRT.
The results of therapy of oropharyngeal cancer with IMRT have mostly been reported by academic centers treating a large number of patients (1-6). It is likely that a learning curve exists in the use of IMRT for HN cancer, and that experience treating many patients reduces the risk of failures. Recent data from community centers using IMRT shows a high rate of tumor control and complication-free survival, attesting to the success achieved in emulating IMRT principles (16). The high rate of tumor control achieved in recent years in the series of IMRT cited above relates in part to the increasing frequency of non-smoking, Human Papillomavirus (HPV)-associated oropharyngeal (OP) cancer in the developed world, whose prognosis is better than that of smoking-related OP cancer (17). The results of the current study mirror these recent series: All tumor-related failures as well as all second primary cancers were observed in current or previous smokers, and none in the never-smokers, who likely had HPV-related tumors. To the best of our knowledge, this is the first series of early oropharyngeal cancer in which significant differences in tumor outcome in favor of never-smokers have been reported.
Acceleration of the treatment course was achieved in this protocol by increasing daily GTV fraction doses. This strategy is conceptually different from accelerated courses which achieve their goal by hyperfractionation. Few previous studies of IMRT for HN cancer have relied on the high dose conformality to deliver high daily fraction doses to the GTVs, as well as increasing the total BED beyond standard levels, in an effort to increase local tumor control rates (18). However, within the targets in the HN there are embedded mucosa, submucosa, blood vessels, and nerves, whose recovery and integrity after therapy determine the risk of long-term local sequelae. These risks may depend not only on the daily and total doses, but also on the extent of dose inhomogeneity and “hot spots” within the targets, as well as the volume of the target receiving a high prescribed dose (19). Notably, the addition of concurrent chemotherapy to a regimen of IMRT delivering total 60 Gy at 2.4 Gy/fraction was reported to be non-tolerable due to excessive acute mucositis (20). The implications of this study are therefore limited to early tumors treated without concurrent chemotherapy. Using the study's IMRT regimen for more advanced cancer and concurrent with chemotherapy should be done within the context of controlled studies.
ORN was observed in 6% of the patients, a rate which was higher than expected using IMRT (21). Dosimetry of the ORN sites was available in 2 patients and indicated a total dose close to 70 Gy, which over 30 fractions indicates fraction doses of 2.3 Gy, increasing the total BED to the ORN sites to well above 70 Gy. While these “hot spots” to the mandible could be the main cause of ORN, another potential factor was the lack of detailed prophylactic dental care recommendations in the protocol. In recent years, all RTOG protocols for HN cancer contain an appendix specifying prophylactic dental care guidelines, and they are available on the RTOG web site (www.rtog.org/members/active.html#headneck). Adherence to these guidelines is expected to reduce ORN risks.
Almost all treatment plans adhered to protocol rules regarding parotid gland sparing. As a consequence, observer-rated xerostomia improved steadily after therapy, representing a significant improvement compared with similar patients treated on previous RTOG protocols. An improvement over time in observer-rated and/or patient-reported xerostomia after parotid-sparing IMRT has been described previously (22, 23), in contrast with conventional RT, after which xerostomia does not usually improve (23). The studies reporting improvement in xerostomia have also noted a parallel improvement in the salivary output, likely contributed by the spared parts of the salivary glands (22, 23). In contrast, the current study has not demonstrated an improvement of whole mouth saliva during the first year after IMRT. We do not know the reason for this discrepancy. Notably, quality control for whole mouth saliva collection was not included in the study.
In conclusion, RTOG 00-22 is a pioneer multi-institutional study whose primary importance was setting clear target dose prescriptions and tissue dose constraints for IMRT of HN cancer. The results show that adherence to protocol guidelines is a significant factor associated with LR tumor control. The moderate treatment acceleration was associated with a low rate of sequelae. This regimen is therefore acceptable for the therapy of early oropharyngeal cancer. Modifying this regimen for advanced cancer, especially concurrent with chemotherapy, should be done within a clinical study setting.
Acknowledgments
Supported by NCI grants ATC U24 CA81647, RTOG U10 CA21661, CCOP U10 CA37422,
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chao KS, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 2.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: The MSKCC experience. Int J Radiat Oncol Biol Phys. 2006;64:363–373. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Rad Onc Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Garden AS, Morrison WH, Wong PF, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:438–444. doi: 10.1016/j.ijrobp.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao M, Nguyen T, Buatti JM, et al. Changing failure patterns in oropharyngeal squamous cell carcinoma treated with IMRT. Am J Clin Oncol. 2006;29:606–612. doi: 10.1097/01.coc.0000242294.89536.d6. [DOI] [PubMed] [Google Scholar]
- 6.Sanguineti G, Gunn GB, Endres EJ, et al. Pattern of locoregional failure after exclusive IMRT for oropharyngeal carcinoma. Int J Rad Onc Biol Phys. 2008;72:737–46. doi: 10.1016/j.ijrobp.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Molineu A, Followil DS, Balter PA, et al. Design and implementation of an anthropomorphic quality assurance phantom for IMRT. Int J Radiat Oncol Biol Phys. 2005:577–88. doi: 10.1016/j.ijrobp.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Fleming T. One-Sample Multiple Testing Procedure for Phase II Clinical Trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 9.Brizel DM, Wasserman TH, hence M, et al. Phase III randomized trial of amifostine in head and neck cancer. J Clin Oncol. 2000;18:3339–45. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 10.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 11.Palta JR, Deye JA, Ibbott GS, et al. Credentialing of institutions for IMRT. Int J Rad Onc Biol Phys. 2004;59:1257–1262. doi: 10.1016/j.ijrobp.2004.03.007. letter. [DOI] [PubMed] [Google Scholar]
- 12.International Commission on Radiation Units and Measurements. Report 62. Bethesda:MD: ICRU; 1999. Prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 13.IMRT Collaborative Working Group. Intensity modulated radiation therapy: Current status. Int J Rad Onc Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 14.Ezzell GA, Galvin JM, Low D, et al. Guidance document on delivery, treatment planning, and clinical implementation of IMRT. Med Phys. 2003;30:2089–2115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 15.Rischin D, Peters L, O'Sullivan B, et al. Phase III study of tirapazamine, cisplatin and radiation versus cisplatin and radiation for advanced head and neck cancer. J Clin Oncol. 2008;26:18S–1010s. abstract #LBA6008. [Google Scholar]
- 16.Seung S, Bae J, Solhjem M, et al. IMRT for head-and-neck cancer in the community. Int J Rad Onc Biol Phys. 2008;72:1075–81. doi: 10.1016/j.ijrobp.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauve A, Morris M, Schmidt-Ullrich R, et al. Simultaneous integrated boost IMRT for head-and-neck carcinomas: II--clinical results. Int J Radiat Oncol Biol Phys. 2004;60:374–87. doi: 10.1016/j.ijrobp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Eisbruch A, Gregoire V. Balancing Risk and Reward in Target Delineation for Highly Conformal Radiotherapy in Head and Neck Cancer. Sem Rad Onc. 2009;19:43–52. doi: 10.1016/j.semradonc.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amosson CM, The BS, Garg AK, et al. Accelerated fractionation for head and neck cancer using SMART boost. Int J Rad Onc Biol Phys. 2003;57:S306. abstract #1076. [Google Scholar]
- 21.Ben David MA, Diamante M, Radawski JD, et al. Lack of osteoradionecrosis of the mandible after IMRT for head and neck cancer. Int J Rad Onc Biol Phys. 2007;68:396–402. doi: 10.1016/j.ijrobp.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 23.Kam MMK, Leung SF, Zee B, et al. Impact of IMRT on salivary function in nasopharyngeal carcinoma. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]