Abstract
Clamp protein or clamp, initially identified as the processivity factor of the replicative DNA polymerase, is indispensable for the timely and faithful replication of DNA genome. Clamp encircles duplex DNA and physically interacts with DNA polymerase. Clamps from different organisms share remarkable similarities in both structure and function. Loading of clamp onto DNA requires the activity of clamp loader. Although all clamp loaders act by converting the chemical energy derived from ATP hydrolysis to mechanical force, intriguing differences exist in the mechanistic details of clamp loading. The structure and function of clamp in normal and translesion DNA synthesis has been subjected to extensive investigations. This review summarizes the current understanding of clamps from three kingdoms of life and the mechanism of loading by their cognate clamp loaders. We also discuss the recent findings on the interactions between clamp and DNA, as well as between clamp and DNA polymerase (both the replicative and specialized DNA polymerases). Lastly the role of clamp in modulating polymerase exchange is discussed in the context of translesion DNA synthesis.
Keywords: DNA polymerase, Clamp loader, PCNA, Polymerase exchange, Translesion DNA synthesis
1. Introduction
Accurate and efficient synthesis of genomic DNA in all three kingdoms of life is carried out by the multiple-protein complex called DNA replisome [1–4]. DNA polymerase constitutes the core of the replisome and has the ability of synthesizing complementary DNA in a 5′-to-3′ direction on both leading and lagging strands [5,6]. The replicative DNA polymerases possess remarkable fidelity that guarantees the faithful replication of genomic DNA [7,8]. Most DNA polymerases are intrinsically low-processivity enzymes [9,10] despite some noticeable exceptions, such as the phage Φ29 DNA polymerase [11]. Processivity is defined as the ability of DNA polymerase to carry out continuous DNA synthesis on a template DNA without frequent dissociation. It can be measured by the average number of nucleotides incorporated by a DNA polymerase on a single association/disassociation event. DNA polymerase alone produces short DNA product strand per binding event [12]. The low processivity of DNA polymerase alone is insufficient for the timely replication of a large DNA genome.
Nature has solved this dilemma by utilizing a processivity factor that vastly improves the processivity of DNA polymerase [12–16]. A common processivity factor, clamp protein or clamp, can be found in prokaryotes, eukaryotes and archaea, attesting its essential role in replication of the DNA genome. Thioredoxin, unrelated to clamp protein, functions as the processivity factor for bacterial phage T7 DNA polymerase, gp5 [17,18]. In this review we focus on clamp protein as the processivity factor for DNA polymerases.
Clamp proteins are usually multimeric protein with a toroidal shape, and are capable of encircling duplex DNA. Once loaded onto duplex DNA clamp can move in either 5′-to-3′ or 3′-to-5′ direction without dissociation. The DNA polymerase is physically linked to clamp through a clamp-interacting sequence motif. This interaction serves to increase the processivity of DNA polymerase.
Most clamp proteins exist as a closed ring in solution, although unusual clamp proteins with an incomplete ring structure have also been discovered in several human viruses. In order to be loaded onto the genomic DNA in a site distant from the ends, the ring structure of clamp needs to be transiently opened, allowing the threading of DNA strand into the central cavity of clamp. This process requires a protein complex called clamp loader. Clamp loader is likewise found in organisms from different domains of life. All clamp loaders belong to the AAA+ (ATPases associated with a variety of cellular activities) protein family. In an ATP-dependent process clamp loader acts as a catalytic ‘matchmaker’ to properly position, orient, and load the clamp onto DNA. This sequence of reactions is fueled by ATP hydrolysis in the clamp loader active site.
Although initially identified as the processivity factor for the replicative DNA polymerase, clamp proteins also play other essential functions in a variety of DNA-processing events. This is exemplified by the eukaryotic clamp, proliferating cell nuclear antigen (PCNA), which physically interacts with a large group of proteins and coordinates their functions in various DNA transactions. The proper regulation of DNA polymerases and other DNA-processing proteins through their interactions with clamp, a common ‘platform’ on DNA, is essential for the highly accurate replication of genomic DNA. Recent progresses pointed to post-translational modification of PCNA by ubiquitin and SUMO (small ubiquitin-like modifier) as a means of modulating the function of PCNA-interacting proteins in eukaryotes.
In this review we will compare the structural and functional features of clamp from different domains of life. The interactions of clamp with polymerase and DNA will be discussed. The mechanism of clamp loading in bacteriophage T4, E. coli and S. cerevisiae will be compared to point out both the commonalities and interesting distinctions in the mechanistic details. Furthermore, the role of clamp in mediating the polymerase exchange process will be reviewed in the context of translesion DNA synthesis.
2. Structure of clamp proteins
The first high-resolution X-ray crystal structure of a clamp protein, the E. coli β clamp, was reported by the Kuriyan and O′Donnell labs in the early 1990s [19]. Heretofore the high-resolution structures of many other clamps have been reported, including gp45 in bacteriophage T4 [20] and RB69 [21], the β subunit in Gram-positive bacterium (Streptococcus pyogenes) [22], the PCNA from yeast S. cerevisiae [23] and human [24], as well as the archaeal PCNA from Pyrococcus furiosus and Sulfolobus solfataricus [25,26]. Remarkably despite the large sequence diversity observed, most clamps exist as a circular protein with a central cavity large enough to accommodate a B-form DNA duplex. Most clamps are homomultimers (PCNA is a homotrimer and β clamp a homodimer). More recent biochemical and structural characterization of the archaeal clamps revealed unique heterotrimeric structures [26]. Not all clamp proteins exist as closed ring. Several human viral processivity factors were recently found to exist as a C-shaped dimer or monomer [27,28]. In this section the structural features and the related functions of the representative clamps from several different organisms are discussed.
2.1. The bacterial β clamp
The E. coli β clamp is required for the processivity of DNA polymerase III (PolIII) [29]. In the crystal structure β clamp exists as a head-to-tail dimer (Fig. 1A). The overall structure can be described as a toroid with a central hole of ca. 35 Å. The structure is highly symmetric and the two subunits are related to each other through an 180° rotation about the axis perpendicular to the plane. Each monomer of β clamp consists of three independently folded domains with similar chain topology and domain structure. The interface between two monomers is held up by a total of thirteen hydrogen bonds located on both the neighboring β strands and α helices that delineate the subunit interface. The inner surface of the ring is lined with 12 α helices and can cover one full turn of the B-form duplex DNA. One unique feature of the clamp structure is its central cavity. The size of the central cavity is large enough to accommodate a B-form duplex DNA with a diameter of 25 Å. Once loaded onto DNA, the clamp can freely slide on DNA in both directions. Another striking feature of the β clamp is that despite an overall negative electrostatic potential determined for the clamp, the central cavity is positively charged. This charge distribution suggests the existence of electrostatic interactions between the inner rim of β clamp and the negatively charged backbone of duplex DNA.
Fig. 1.
The three-dimensional structure of the representative clamps from different domains of life. (A) Escherichia coli β clamp. The β homodimer is formed via head-to-tail interaction between the two crescent-shaped subunits; (B) Gp45 in bacteriophage RB69. Three identical subunits are arranged head-to-tail to form a homotrimer; (C) Human PCNA. The PCNA ring is composed of three identical monomers joined in a head-to-tail arrangement; (D) Human heterotrimeric checkpoint clamp Rad9–Rad1–Hus1. The ring is colored as follows: Rad9 (red), Rad1 (blue) and Hus1 (yellow); (E) Sulfolobus solfataricus PCNA. The PCNA ring is formed by PCNA1 (red), PCNA2 (blue), and PCNA3 (yellow).
2.2. The bacteriophage clamp gp45
The DNA polymerase holoenzyme in the T4 and RB69 bacteriophages consists of the DNA polymerase gp43 and clamp gp45. The high-resolution X-ray crystal structures of gp45 in bacteriophage RB69 and T4 have been obtained [20,21]. The two clamps are highly similar in primary sequence (~80% identical), as well as the three-dimensional structure. Both proteins are homotrimeric toroids assembled from a 25 kDa subunit (Fig. 1B). The overall dimension of the phage clamp is ca. 85 Å in the outer diameter, 35 Å in the inner diameter and 25–26 Å in the thickness [20]. The trimeric ring is highly symmetric with a three-fold symmetry. Further symmetry is observed within each monomer. Structural studies revealed that each monomer consists of two domains that share structural similarity, despite that no apparent sequence similarity is found between the two domains. Another notable feature of the two-domain subunit is that a long extended loop connects the two domains. This arrangement suggests a flexible nature of the subunit, an important requirement for the dynamic opening and closing of the clamp interface for loading onto DNA.
Three identical subunit interfaces exist in each gp45 clamp. The subunit interface is held together by four pairs of hydrogen bonds between two β strands donated by the neighboring subunits. Three identical subunit interfaces provide the site that can be topologically breached for the loading of the clamp onto DNA duplex. The hydrogen-bonding interaction can be restored to re-establish the closed-ring structure once the clamp is delivered to the proper site on DNA by clamp loader.
2.3. The eukaryotic clamp PCNA
Structures of the S. cerevisiae and human PCNA have been reported [23,24]. Both PCNAs exist as a homotrimer with a three-fold symmetry (Fig. 1C). Remarkably the overall structure of the yeast and human PCNAs resemble that of the β clamp despite the significant difference in the subunit size. Unlike the β clamp each subunit of PCNA contains two domains. Nonetheless the homotrimeric structure of PCNA contains six structurally conserved domains, which equals the total domains in the homodimeric β clamp. Therefore both PCNA and β clamp possess the pseudo-six-fold symmetry. In contrast the difference in the structures of PCNA and phage gp45 are clear. The PCNA ring is hexagonal in shape rather than the triangular shape seen in gp45 (Fig. 1).
PCNA is also different from its phage counterpart gp45 in stability. PCNA apparently exists as a more stable homotrimer with a dissociation constant of ca. 21 nM (compared to ca. 250 nM for gp45 and <67 pM for E. coli β clamp) [30]. The interaction at the subunit interfaces in PCNA is more extensive than gp45 with a total eight pairs of hydrogen bonds as compared to four hydrogen bonds in gp45. Almost twice more total surface area is buried in PCNA than in gp45. Therefore PCNA is more stable once loaded onto DNA, similar to β clamp. In contrast gp45 readily falls off DNA in the absence of DNA polymerase.
Although initially identified as the processivity factor of the eukaryotic DNA polymerases, PCNA has been shown to interact with a large group of proteins functioning in diverse processes including DNA replication, DNA repair, translesion DNA synthesis, cell-cycle control and chromatin remodeling [31,32]. Besides the replicative DNA polymerases δ and ε, other prominent PCNA-interacting proteins include flap endonuclease 1 (FEN1), DNA ligase I, Topo I, WRN helicase, Uracil-DNA glycosylase, cyclin, P21, as well as a group of specialized DNA polymerases, e.g. Polη and Polκ. This observation has led to the model that PCNA serves to recruit the different DNA-processing proteins through direct physical interactions. Analysis of the primary sequences of the PCNA binding proteins revealed a conserved PIP or “PCNA-interacting protein” motif that can be defined as Qxx(M/L/I)xxF(Y/F) (x stands for any residue). The remarkable ability of PCNA to interact with its protein partners was satisfactorily explained by the co-crystal structure of PCNA and the p21 peptide [24]. p21 is a small cell-cycle protein that inhibits cell-cycle progression and DNA replication in response to DNA damage. In the co-crystal structure, the p21 peptide binds to PCNA in an extended conformation interacting with both domains in the PCNA subunit and the interdomain connector loop (ICL). The C-terminal nine residues of p21 peptide form a β strand that interacts extensively with the parallel ICL in PCNA. Moreover the hydrophobic residues in the central part of p21 peptide also contribute to the binding by interacting with a hydrophobic pocket on the PCNA surface formed partially by the ICL loop. Further structural determinations between PCNA and the PIP peptide from the p66 subunit of Polδ and FEN1 revealed that both peptides bind to the same site on PCNA as the p21 peptide [33]. However the binding affinity for the p66 and FEN1 peptides are 189- and 725-fold lower compared to the p21 peptide. The varied affinity between the PIP peptides and PCNA has been attributed to the sequence variation in the PIP motif as well as the flanking sequences. Given the large number of proteins interacting with PCNA, the fine-tuned affinity may provide a possible way of coordinating the sequential binding events on a common platform encircling duplex DNA.
2.4. The heterotrimeric clamp in eukaryotes
Besides the homotrimeric PCNA eukaryotes also contain heterotrimeric clamps. In humans the checkpoint (or 9-1-1) clamp, formed by Rad9, Hus1 and Rad1, is involved in the checkpoint response in S phase. Although there are little sequence similarities between the subunits of the checkpoint clamp and the PCNA subunit, initial bioinformatic analysis predicted a closed trimeric structure for the checkpoint 9-1-1 clamp [34]. Further biochemical and electron microscopic analyses provided convincing evidence for a PCNA-like structure in the 9-1-1 clamp [35–37]. Similar checkpoint clamps were also identified in S. pombe [38] and S. cerevisiae [39]. The S. pombe checkpoint clamp is similar to the human 9-1-1 clamp formed by Rad9, Hus1 and Rad1. In S. cerevisiae the checkpoint clamp is composed of Ddc1, Mec3 and Rad17. Remarkably the checkpoint clamp requires a dedicated clamp loader for loading. For human 9-1-1 clamp, the clamp loader is hRad17–RFC complex that shares four small subunits with the RFC complex [35,36].
Very recently the crystal structure of the human Rad9–Rad1–Hus1 clamp was reported. As expected the heterotrimeric 9-1-1 clamp forms a toroid similar to the homotrimeric PCNA in both shape and size (Fig. 1D) [40]. Unlike PCNA the interfaces between the 9-1-1 subunits are unequivalent with more extensive interactions at the Rad9–Hus1 and Rad1–Hus1 interfaces. The Rad1–Rad9 interface, significantly weaker compared to the other two interfaces, was proposed to be the interface for opening during clamp loading. The three subunits in 9-1-1 clamp are significantly different in structural details despite having a similar fold, particularly in the interdomain connector loop (ICL) region. Given that the Rad9 subunit possesses an ICL similar to that in PCNA, Rad9 is predicted to bind PIP box motif. In comparison the Rad1 subunit lacks the hydrophobic pocket observed in PCNA and the Rad9 subunit. Therefore Rad1 is unlikely to bind protein through the PIP box motif. The PIP binding of the Hus1 subunit cannot be unequivocally assessed based on the structure alone. Unlike PCNA, 9-1-1 clamp binds FEN1 and p15PAF in a PIP box-independent mechanism [40]. A competition binding experiment revealed that 9-1-1 clamp cannot bind FEN1 and p21Cip1/Waf1 simultaneously.
2.5. The archaeal PCNA
Two forms of PCNA have been identified in archaea, exemplified by the homotrimeric PCNA from Pyrococcus furiosus and the heterotrimeric PCNA from Sulfolobus solfataricus. The P. furiosus PCNA structure resembles that of the eukaryotic PCNA, adopting a pseudo-six-fold toroidal structure. More ion pairs are found in the P. furiosus PCNA trimer, presumably contributing to its higher thermostability. Less hydrogen-bond pairs are found at the subunit interfaces of P. furiosus PCNA as compared to the eight hydrogen bonds found in human PCNA. Thus P. furiosus PCNA is intrinsically less stable compared to human PCNA, in line with the biochemical observation that the P. furiosus PCNA alone can stimulate DNA synthesis by the archaeal DNA polymerase in the absence of clamp loader activity. In Sulfolobus solfataricus three distinct PCNA subunits (PCNA1, 2, and 3) were found to form a unique heterotrimer [41] (Fig. 1E). Recent structural determinations have provided further structural details [26,42,43]. The overall structure of the S. solfataricus PCNA heterotrimer is highly similar to that of the eukaryotic PCNA and β clamp. The subunits PCNA1, 2 and 3 superimpose well with a small root-mean-square deviation (r.m.s.d) of 1.5 Å. Distinct from the homotrimeric PCNA, the S. solfataricus PCNA1 and PCNA2 subunits can form a stable opened dimer, while PCNA3 subunit can exist as a stable monomer. In the V-shaped dimer an angle of about 130° was observed between the PCNA1 and PCNA2 subunits. Moreover the dimer adopts a more opened conformation as compared to that in the closed trimer structure [43]. As in the eukaryotic PCNA, each subunit of the S. solfataricus PCNA contains the conserved PIP-binding site. However the three sites are not identical and presumably bind distinct peptide sequences derived from different DNA-processing proteins in S. solfataricus. A co-crystal structure of the S. solfataricus PCNA trimer and DNA ligase revealed that the ligase binds specifically to the PCNA3 subunit [42]. This unique structural feature suggests that multiple proteins can be retained to the DNA-processing site by interacting with one of the three subunits, thus facilitating multiple steps of DNA transaction in a coordinated fashion. These observations lend support for a so-called ‘tool-belt’ model proposed for eukaryotic DNA replication and repair processes [44].
2.6. The C-shaped clamp
The genomes of certain human viruses encode unconventional processivity factors for the viral DNA polymerase. For example the herpes simplex virus (HSV) DNA polymerase complex comprises a 1235-amino acid catalytic subunit and a processivity factor UL42. The processivity factor UL42 binds to DNA with high affinity and is essential for the long-chain DNA synthesis [45]. Intriguingly the N-terminal two-thirds of the UL42 sequence adopts a similar two-domain fold as human PCNA [28]. However the relative rotation between the two domains in UL42 is approximately 40°, compared to the sixty-degree rotation in human PCNA that generates a PCNA molecule with pseudo-six-fold symmetry. Thus a flatter UL42 structure is not likely to form a homotrimer as in human PCNA. The DNA binding mode of UL42 likely relies on the positively-charged C-terminal domain. The interaction between the HSV polymerase and UL42 is mediated by the C-terminal peptide of the polymerase as in other polymerase–clamp interactions. Different from the PIP–PCNA interaction the polymerase C-terminal peptide adopts αβα structure, rather than an extended β strand. Nonetheless the peptide binding site on UL42 is located at the conserved interdomain-connecting loop. Several models have been proposed for the processivity of HSV Pol–UL42 complex, including one model proposing that UL42 can slide on DNA despite its high affinity for DNA [46]. Similar to the HSV UL42 another processivity factor UL44 was described for human cytomegalovirus as the processivity factor for the viral DNA polymerase UL54 [47]. Like UL42, human cytomegalovirus UL44 displays high affinity for DNA. Crystal structure of the UL44 again revealed a PCNA-like two-domain fold [48]. However a comparison of UL44, UL42, and PCNA structure revealed the marked difference in the curvature of their structure, with UL44 and PCNA being more concaved compared to UL42. In crystal structure UL44 exists as a dimer, which was corroborated by gel-filtration and ultracentrifugation studies. Remarkably the C-shaped dimer of UL44 is formed in a head-to-head arrangement as compared to the head-to-tail arrangement observed in PCNA. A cavity of approximately 25 Å in UL44 dimer was proposed to accommodate double-stranded DNA, thus rendering the viral DNA polymerase processive.
3. Clamp–polymerase interaction
3.1. Replicative DNA polymerase–clamp interaction
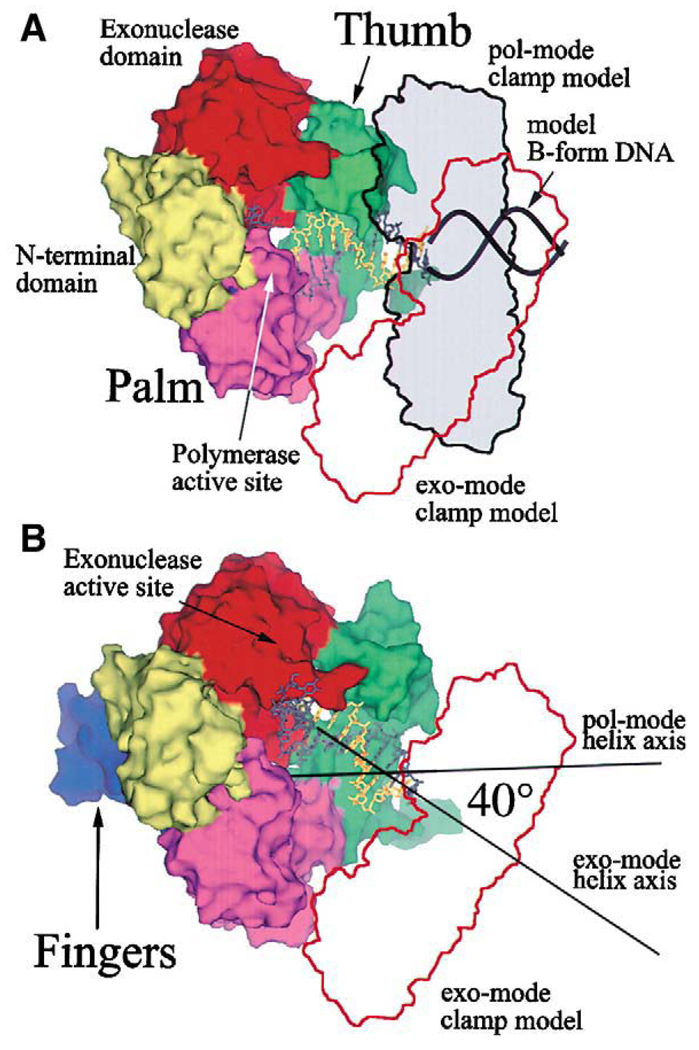
The essential role of the interaction between DNA polymerase and its processivity factor in processive DNA synthesis has been well documented for the DNA polymerases in both prokaryotic and eukaryotic organisms. However a high-resolution view of the polymerase–clamp complex has not been reported. Shamoo and Steitz have built a model of the RB69 DNA polymerase complexed with its processivity factor gp45 based on two separate structures [21]. In one structure the RB69 DNA polymerase is in the editing mode with a primer-template DNA bound in the polymerase active site. In a separate structure the C-terminal peptide (11 amino acids) of the RB69 polymerase is bound to a pocket on the gp45 surface mainly through hydrophobic interactions. By combining the two available structures, a DNA polymerase–clamp holoenzyme model was built as guided by the duplex DNA in the polymerase active site. In this model the clamp is attached to the RB69 DNA polymerase through a flexible linkage between the polymerase C-terminal peptide and a hydrophobic binding pocket on gp45. There is no extensive interaction between the surfaces of the polymerase and clamp. This loose and flexible interaction has been instrumental in explaining the conformational changes of RB69 DNA polymerase between the polymerizing and editing modes [49] (Fig. 2). When the DNA polymerase switches from polymerizing to editing mode, the gp45 clamp needs to move a large distance to accommodate a 40-degree rotation of the double-stranded DNA axis.
Fig. 2.
A model of the RB69 DNA polymerase in complex with its cognate clamp in the polymerizing and editing modes. (A) The RB69 polymerase in polymerizing mode shown as a molecular surface. The primer-template DNA is shown in stick form and the model-built B-form extension of this DNA is shown as a backbone worm only. (B) The RB69 polymerase (editing mode) complexed with clamp in a different orientation from that in the polymerizing mode. This figure is adapted from [49].
It should be noted that the RB69 DNA polymerase is a single-subunit polymerase belonging to the Pol α family. The interaction between other multi-subunit DNA polymerases and their processivity factors is likely more complex. The eukaryotic replicative DNA polymerases are usually a complex of three or four subunits and more than one interaction site exists between the polymerase subunits and the processivity factor PCNA. To date a high-resolution structure of the multi-subunit DNA polymerase alone or in complex with PCNA has not been reported. Biochemical studies of the S. cerevisiae and human polymerase δ-PCNA holoenzyme have provided useful information on the nature of the polymerase–clamp interaction. The S. cerevisiae Polδ, consisting of three subunits Pol3, Pol31, and Pol32, interacts with PCNA through at least two subunits (Pol3 and Pol32). Although the PIP motif in the Pol32 subunit interacts with PCNA either on or off DNA, the Pol3 subunit only interacts with PCNA on DNA. These interactions are modulated differently by free PCNA or PCNA loaded onto duplex DNA [50]. Despite the multiple interaction sites, the resulting DNA polymerase holoenzyme likely retains a flexible conformation. This notion is supported by a recent biochemical investigation showing the S. cerevisiae DNA polymerase δ can undergo an exchange with an error-prone DNA polymerase η when the polymerase holoenzyme is stalled [14]. A similar polymerase exchange has also been observed for the T4 phage DNA polymerase gp43 and E. coli Pol III [13,51,52]. Thus the dynamic nature of the polymerase–clamp interaction is likely shared among replicative polymerases from prokaryotes to eukaryotes despite that the complexity of the replicative polymerases varies largely.
3.2. Translesion DNA synthesis polymerase–clamp interaction
Translesion DNA synthesis (TLS), a mechanism utilized by cells to synthesize past a DNA lesion, is evolutionarily conversed in organisms from prokaryotes to eukaryotes. Like the replicative DNA polymerases, a group of specialized DNA polymerases (or TLS polymerases) also interacts with clamp proteins (PCNA and β clamp). Most of these TLS polymerases belong to the Y-family polymerase (see review by Washington et al. in this issue and [53]). Analyses of the primary sequences of the eukaryotic TLS polymerases reveal that many of them contain a PIP motif outside the catalytic domain and often close to the C-terminus. Polη has been shown to interact with PCNA through the PIP motif; this interaction stimulates the DNA synthesis on large DNA substrates, but does not increase the processivity of Polη [54]. Moreover the same interaction was recently found to be essential for the ability of Polη to access the stalled Polδ-PCNA holoenzyme complex [14,55]. Recent structures of PCNA bound with the PIP peptides from three TLS polymerases, Polη, Polι and Polκ, revealed that the PIP peptides bind to the same region on the PCNA surface as the p21 peptide [56]. Despite notable differences in their conformation from that of the p21 peptide, the PIP peptides from Polη and Polι bind to PCNA with a moderately high affinity (Kd of 0.4 µM and 0.39 µM respectively). In general these observations are in accord with both the in vivo and in vitro studies suggesting an essential role of TLS Pol's interaction with PCNA through the PIP motif. Notably a second PIP motif was recently identified in human Polη. The newly identified PIP was named PIP1, to be distinguished from the PIP located at the C-terminus of the sequence (renamed as PIP2) [55]. Interestingly the two PIP motifs are both functional in interacting with PCNA and can substitute for one another in an in vivo UV-resistance assay. The abolishment of both PIP motifs results in a complete loss of Polη′s function in vivo. This finding raises the possibility that one Polη can bind to two PCNA subunits simultaneously. It is not yet clear whether regions on Polη other than the PIP motifs interact with PCNA. A recent structural and functional characterization of the PCNA mutant G178S, which was known to cause deficiency in translesion DNA synthesis by both Polη and Polζ in yeast cells [57], raised an interesting possibility that Polη may interact simultaneously with both faces of PCNA [58]. The secondary site of interaction is defined by a loop (J-loop) located opposite to the PIP motif binding side.
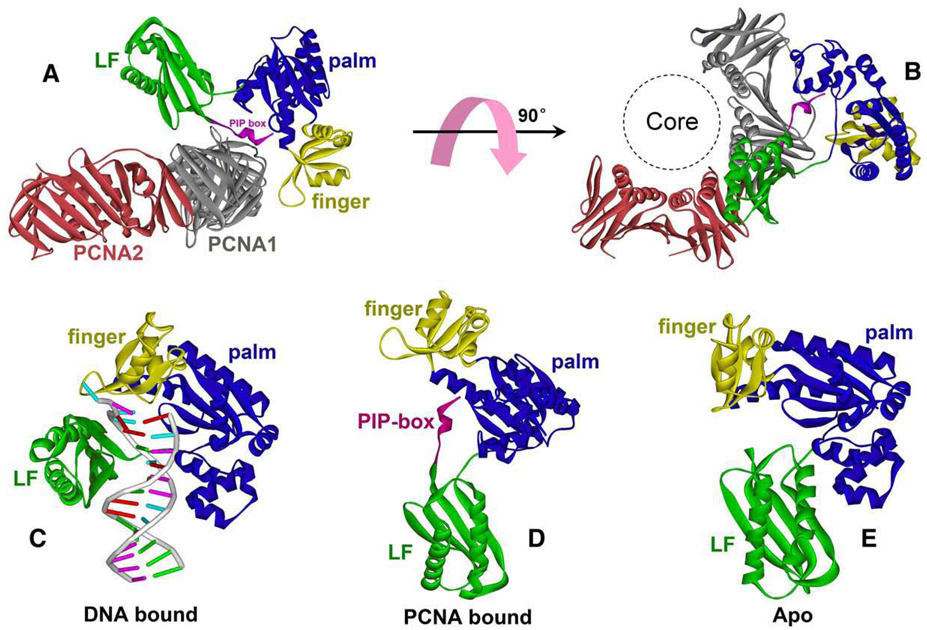
Useful information on the TLS polymerase–clamp interaction can be inferred from several costructures of the prokaryotic and archaeal TLS Pols with their cognate clamps. A recent co-crystal structure of the archaeal Dpo4 complex with a PCNA1–PCNA2 dimer showed that Dpo4 makes multiple contacts with the PCNA subunits [59] (Fig. 3). Besides the common interaction between the Dpo4 C-terminal tail and the conserved PIP-binding site on PCNA1, extensive contacts are observed between the little-finger (LF), finger, and thumb domains of Dpo4 and the PCNA1 surface away from the PIP-binding site. The structure of the full-length Dpo4 revealed a potentially flexible conformation due to the modular structure of Dpo4 connected by two peptide hinges. The flexible conformation of Dpo4 on PCNA may be instrumental in regulating the accessibility of Dpo4 to DNA during translesion DNA synthesis. A similar modular interaction between TLS Pol and clamp was observed in a structure of E. coli Pol IV little-finger domain and the β clamp [60]. Besides the conserved interaction between the Pol IV C-terminal peptide with the β clamp (equivalent to the PIP–PCNA interaction), a substantial interaction was observed between the Pol IV little-finger and the interface region between two β subunits. This secondary interaction was proposed to bring the Pol IV into a nonproductive conformation in which the polymerase active site would not have a productive access to the DNA running through the central cavity of β clamp. It is still not clear whether the modular interactions seen in the Dpo4–PCNA complex and the Pol IV little-finger–β clamp complex can be generalized to other TLS polymerases. Further functional and structural analyses will be needed for a better understanding of TLS Pol–clamp interactions that occur during translesion DNA synthesis.
Fig. 3.
Two orthogonal views of Dpo4–PCNA1, 2 complex (A and B) and a comparison of the multiple conformation of Dpo4 polymerase in the DNA-bound (C), PCNA-bound (D) and apo form (E).
4. The clamp–DNA interaction
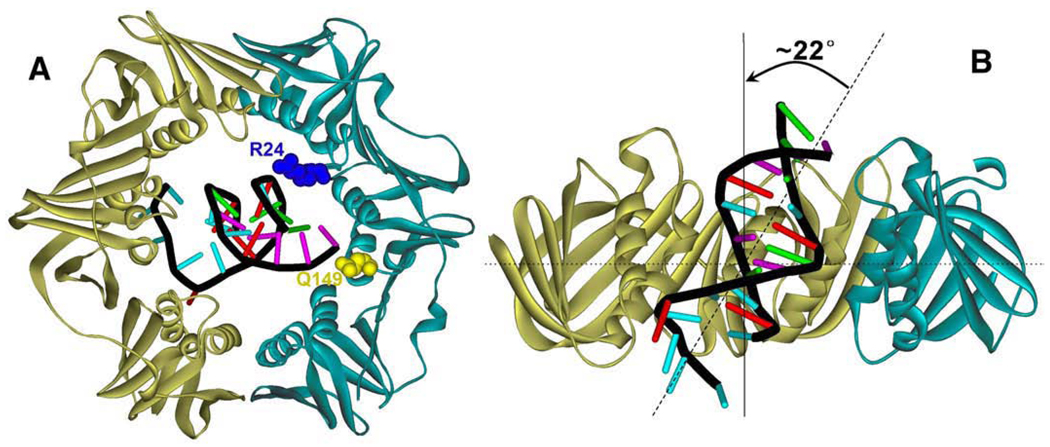
Given the symmetric structure of most clamp proteins, the duplex DNA has been often depicted to run through the central cavity of clamp perpendicularly. A recent high-resolution structure of the E. coli β clamp bound with a primer/template (P/T) DNA revealed unexpected features of the clamp–DNA interaction [61]. In the co-crystal structure the P/T DNA transverses the central cavity of the β clamp. Intriguingly the duplex DNA adopts a tilted conformation with an angle of 22° between the axis of the B-form duplex DNA and the C2 rotation axis of the β clamp (Fig. 4). This tilted conformation of DNA allows direct contact between the two residues on the clamp surface (Arg24 and Gln149) and the duplex DNA. The single-stranded portion of the P/T DNA makes crystal contact with an adjacent β clamp surface in the crystal lattice. It should be noted that the DNA is bound in a direction opposite to the normal in which the polymerase-interacting surface of the clamp faces the single-stranded portion of the P/T DNA. Although the significance of this unusual directionality is not apparent, one possible explanation may be the crystal contact between the single-stranded portion in the P/T DNA and the neighboring β clamp. Biochemical evidence suggests that the interactions between DNA and β clamp are required for clamp loading [61]. Of particular interest is that the single-stranded portion of the P/T DNA binds to β clamp in the same site that has been shown to bind other β clamp-binding proteins, exemplified by the binding of a C-terminal peptide of the Pol III α subunit. One attractive proposal for the functional significance of this interaction is that the interaction may serve as a “placeholder” to keep β clamp from sliding away from the P/T junction following loading by clamp loader. This likely facilitates the subsequent interaction between Pol III and β clamp for assembling the functional polymerase holoenzyme. The interaction between the single-stranded DNA and β clamp may be subsequently disrupted by the polymerase–clamp interaction. A recent single-molecule investigation of the movement of β clamp on duplex DNA suggested a rather slow diffusion of β clamp alone along the duplex DNA (the measured diffusion constant is at least three orders of magnitude lower than free diffusion in aqueous solution) [62]. Moreover, β clamp remains at the 3′ end of the P/T DNA when the E. coli single-stranded DNA binding protein (SSB) is present at the primer end. These observations are in accord with the notion that direct interactions exist between clamp and DNA.
Fig. 4.
E. coli β clamp–DNA interaction. (A) top view of the homodimeric clamp with a duplex DNA threading through the interior cavity in a tilted conformation. The residues (Arg24 and Gln149) that interact with DNA are shown. (B) Side view of the clamp–DNA complex showing the duplex DNA in the central channel of clamp tilted by 22° from the C2 rotation axis of β clamp.
Since β clamp is a symmetric dimer, it is likely that the tilted DNA partitions equally between two tilted orientations. An interesting implication is that such an alternation of the DNA orientation may facilitate the switch of the 3′ end of the primer DNA strand between the active sites of two polymerases bound to β clamp simultaneously.
For a long time the PCNA–DNA interaction has also been thought to be merely topological. However existing evidence suggests that the interaction between PCNA and DNA can influence PCNA structure and thus affect its interaction with other proteins. One example is the catalytic subunit, Pol3, of Polδ, which has been shown to only interact with PCNA loaded onto DNA. Although a co-crystal structure is not yet available, the eukaryotic PCNA was also shown to make specific contacts with DNA [61]. Interestingly a computational simulation suggested that DNA can thread through the interior cavity of PCNA in a tilted conformation with a angle close to 20° [63]. The molecular dynamic simulation showed that the charged residues lining the inner rim of the central cavity of PCNA interacts with the phosphate backbone that tracks along the minor groove of the DNA duplex. A recent single-particle electron microscopic reconstruction of the DNA ligase•PCNA•DNA complex revealed that DNA passes through the PCNA interior cavity with a tilted angle of 16° relative to the three-fold axis of PCNA [64]. Moreover the contacts between the rod-shaped DNA density and the inner wall of the PCNA ring was consistently observed. Because this is a costructure of a ternary complex consisting of DNA ligase, PCNA and DNA, the tilted DNA configuration may be influenced by its interaction with DNA ligase. The recent progress in characterizing PCNA–DNA interaction points to additional mechanisms for modulating the activities of various proteins that interact with PCNA.
5. Loading of processivity factor by clamp loader
In order to load clamp onto DNA, the clamp loader complex is required to induce the opening of clamp at its subunit interface, deliver the open clamp to the proper site on DNA, and subsequently close the clamp onto DNA. Clamp loaders in all domains of life exist as a pentameric protein complex, albeit with varied subunit composition. The clamp loader from bacteriophage T4 (gp44/62) comprises four gp44 subunits and one gp62 subunit. The core of the E. coli clamp loader, γ complex, comprises one δ, one δ′ and three γ subunits. Higher complexity is seen in the eukaryotic clamp loader, replication factor C (RFC) complex, which is composed of five different subunits. The archaeal clamp loader closely resembles the eukaryotic RFC complex. Most clamp loader subunits belong to the AAA+ protein family [65]. The AAA+ family proteins are ATPases functioning in assembly, remodeling and disassembly of macromolecular complexes. Clamp loader is one of the founding members of this diverse protein family.
In the process of DNA replication clamp loaders from different organisms act by coupling the ATP binding and hydrolysis with topological change in the structure of clamp for site-specific loading onto the primed template DNA strand. However, each clamp loader possesses mechanistic characteristics that are likely dictated by its primary sequence and quaternary structure. The phage T4 gp44/62 [66–77], E. coli γ complex [78–86], eukaryotic RFC (S. cerevisiae [87–93] and human [94–97]) and archaeal RFC [98–101] have been targeted for extensive mechanistic studies. A common clamp-loading process involves an initial binding of clamp by the ATP-loaded clamp loader and the formation of an open clamp–clamp loader complex. Subsequent binding of DNA to the complex results in the closing of clamp onto DNA and the ejection of clamp loader. Nonetheless, subtle differences in the timing and stoichiometry of ATP binding and hydrolysis in the specific steps of clamp loading have been observed for the clamp loaders from different organisms. In E. coli binding of three molecules of ATP to γ complex powers the opening of β clamp [102]. Following the loading of clamp onto DNA hydrolysis of three ATPs is required for the closing of β clamp onto DNA and the subsequent departure of clamp loader [79]. In comparison gp44/62 in bacteriophage T4 binds four molecules of ATP. Upon binding of gp45 two equivalents of ATP are hydrolyzed by gp44/62 to form an open clamp–clamp loader complex [70,103]. Hydrolysis of two additional ATPs by gp44/62 is required for closing of clamp onto DNA and the departure of gp44/62 [69]. The difference between the mechanisms of the two prokaryotic clamp loaders may be dictated by the numbers of competent ATPase subunits in the clamp loader complexes (four in gp44/62 and three in γ complex) and the different subunit composition in clamp (homotrimer in gp45 and homodimer in β clamp). However, it should be noted that other independent studies indicate that gp44/62 can form an open clamp–clamp loader complex without ATP hydrolysis [72,77].
The eukaryotic clamp loader consists of five non-identical subunits, usually referred to as subunits A through E. Based on their position in the RFC complex, subunit A and E are designated as equivalent to the δ and δ′ subunits respectively in the E. coli γ complex, whereas subunits B through D are equivalent to the three γ subunits in the E. coli γ complex. The eukaryotic RFC complex contains four functional ATPase sites, resembling the T4 gp44/62 complex. It is notable that ATP binding by RFC complex is modulated by its interaction with PCNA and DNA. A study using a RFC complex containing an N-terminally truncated RFC-A subunit revealed that the initial binding of two molecules of ATPγS, a slowly hydrolyzable ATP analogue, to RFC enables the binding of RFC to PCNA [88]. The PCNA•RFC(2ATP) complex is capable of binding a third ATP as the result of a conformational change in the RFC complex. The resulting PCNA RFC(3ATP) complex is able to bind a primer/template DNA and form a ternary complex, DNA•PCNA•RFC(3ATP). The binding of a fourth ATP triggers the loading of PCNA and ejection of RFC [88]. A recent study using a RFC complex containing a full-length RFC-A subunit reported that three ATPγS binds to RFC in the absence of PCNA and DNA, and two more ATPγS binds to the RFC complex when RFC binds to PCNA, DNA or both [104]. The differences in the stoichiometry of ATPγS binding to the two different forms of RFC suggests that the N-terminal domain of RFC-A subunit may affect the binding of ATP to RFC.
The loading of PCNA by RFC shares some common mechanistic features with the E. coli γ complex. In the current model the ATP-loaded RFC binds to PCNA and triggers the opening of PCNA without ATP hydrolysis. The RFC(ATP)•PCNA complex then binds to the 3′ end of the recessed primer DNA, followed by the release of PCNA onto DNA and the ejection of RFC, which are accompanied by ATP hydrolysis. It should be noted that the ATP-loaded RFC alone binds DNA transiently, and the subsequent hydrolysis of ATP causes the dissociation of RFC from DNA [88,104]. This provides a safety mechanism that prevents the formation of an incompetent clamp-loading complex. Despite the similarity to its E. coli counterpart γ complex, RFC is different in the requirement of specific subunits in clamp loading. Particularly the ATPase domain of the RFC-A subunit, which is equivalent to the δ subunit in the γ complex, is dispensable for opening PCNA [89,91]. Instead, the RFC-E subunit, equivalent to the δ′ subunit in the γ complex, is required for the normal function of RFC [91].
The details of ATP hydrolysis in the yeast RFC have been investigated with both steady-state and pre-steady-state kinetic analyses. Johnson et al. proposed that hydrolysis of one ATP is associated with PCNA closure and the hydrolysis of the rest of the ATP molecules is required for PCNA closure and ejection of RFC [92]. In a recent pre-steady-state kinetic analysis Chen et al. reported that three equivalents of ATP were rapidly hydrolyzed by the S. cerevisiae RFC in one turnover [104]. Interestingly the archaeal RFC, which is closely related to the eukaryotic RFC, demonstrated very different ATP utilization in PCNA loading (three ATPs are hydrolyzed by A. fulgidus RFC for the release of PCNA•DNA from RFC and a fourth ATP is hydrolyzed for recycling RFC) [99].
Given the extensive interactions between clamp and clamp loader it is not surprising that the actual mechanism of clamp loading has been tailored to fit the unique characteristics of each clamp. This is exemplified by the phage T4 clamp-loading system. As detailed in the previous section, although the T4 gp45 clamp exists as a homotrimeric toroid in crystal structure, the interaction that holds the three subunits together is much weaker compared to the E. coli β clamp and the eukaryotic PCNA. Structural studies of the engineered monomeric β clamp suggest that a ‘spring tension’ is built in the closed clamp [105]. Therefore, despite the fact that in a crystal structure gp45 exists as a closed trimer, in solution gp45 exists in an asymmetric open state [68,71]. This unique solution structure of gp45 makes possible an alternative loading pathway in which gp44/62 binds to DNA first and then recruits an open clamp to the site for loading. This pathway has been demonstrated in both the ensemble and single-molecule experiments [66,67].
An elegant structural analysis of the S. cerevisiae RFC(ATPγS)•PCNA complex provided the first high-resolution image of the clamp loader–clamp complex (Fig. 5A) [106]. Remarkably the RFC pentamer forms a central cavity to which a spiral B-form duplex DNA can fit snugly. This unique feature explains with satisfaction the recognition of the primer/template (P/T) DNA by RFC for clamp loading. Although the duplex region of the DNA fits to the interior of the RFC complex, the 3′ end of the primer strand runs into an interior wall of the “collar” formed by the third subunit of each of the five RFC subunits. The single-stranded portion of the P/T DNA snakes out through a crevice between the subunit A and E. This structural feature explains the specificity of RFC complex for a 3′-recessed DNA in PCNA loading. This model of DNA recognition by clamp loader was corroborated in E. coli γ complex by measuring the DNA-dependent clamp release and DNA binding affinity of a series of γ complex mutants, in which the positively-charged and polar residues proposed to interact with DNA in the γBCD and δ′E subunits were mutated [107]. More recently a high-resolution structure of the E. coli γ complex bound to a P/T DNA provided further molecular details of clamp loader–DNA interaction [108] (Fig. 5B). The structural features of the γ complex•DNA complex are in remarkable agreement with the model proposed based on the previous RFC•PCNA costructure [106]. Bound with ADP•BeF3 (an ATP analog) the four subunits of the γ complex, namely B(γ), C(γ), D(γ), E(δ′) form a right-handed spiral that tracks the phosphate backbone of the DNA template strand. Notably the γ complex subunit A(δ) is disengaged from DNA. It is proposed that clamp binding can bring domain 1 of the A(δ) subunit into contact with DNA and form a complete five-subunit spiral. The fact that the primer strand does not make close contact with the inner chamber of clamp loader (except the 3′ terminal nucleotide in the primer strand) provides a satisfactory explanation for the observation that clamp loader can recognize both DNA and RNA primers. The γ complex•DNA structure also defines the exit channel of the single-stranded DNA portion of the P/T DNA. The single-stranded DNA exits from the central chamber of the clamp loader and makes contacts with the outer surface on the collar domain of subunit A(δ). A comparison with the structure of γ complex in the absence of DNA [109,110] suggests that DNA binding brings γ complex into an active spiral conformation, in which all three ATPase sites are likely catalytically competent.
Fig. 5.
(A) The S. cerevisiae RFC–PCNA complex. The subunits of RFC complex (A through E) are colored as blue, red, yellow, magenta and green respectively. The PCNA trimer is shown as ribbon and colored grey. (B) The structure of the E. coli clamp loader, γ complex, bound to a primer/template DNA (black). The γ complex contains three γ subunits (red, yellow and magenta), one δ subunit (blue) and one δ′ subunit (green).
In the RFC(ATPγS)•PCNA complex PCNA was shown to interact with RFC complex through three of the five RFC subunits. Notably a closed PCNA was observed in the complex structure despite the binding of ATPγS to the RFC ATPase sites. This observation is likely due to the Arg to Gln mutation introduced to the critical arginine finger in order to preserve the nucleotide for prolonged incubation with the ATPase [92]. The closed PCNA•RFC complex may represent an intermediate state of PCNA loading in which PCNA is just closed onto DNA and RFC is poised for hydrolyzing the bound ATP and for subsequent departure. Alternatively this complex may represent an intermediate formed early in the pathway when RFC encounters PCNA (prior to ring opening).
An open PCNA•RFC structure, although suggested by prior biochemical analysis [111,112], has remained elusive until the report of two electron microscopy images of the archaeal Pyrococcus furiosus RFC•PCNA•DNA complex. The initial EM structure at 23 Å revealed two discernable components: a larger horseshoe and a smaller closed ring, which were interpreted as the corresponding RFC and PCNA respectively [113]. Subsequent refinement of the EM structure of the same complex to a 12 Å resolution, revealed a remarkable spring–washer-shaped open PCNA in complex with RFC [114]. The opened PCNA ring structure has also been observed in solution using a fluorescence approach, in which a FRET (fluorescence resonance energy transfer) pair was introduced across the subunit interface to report the opening of PCNA interface [93]. These studies confirmed that binding of ATP or ATPγS to RFC is sufficient in opening PCNA. The spiral conformation of the opened PCNA ring as shown in the EM structure enables more extensive interactions between RFC and PCNA as compared to a planar PCNA ring. The interactions likely serve to stabilize the opened conformation of PCNA. In accord with the static structure derived from EM analysis a molecular dynamic simulation demonstrated significant out-of-plane movement of the PCNA subunits, as well as lateral relaxation of the PCNA ring [115]. Together, these observations support a RFC clamp-loading mechanism with the formation of an open PCNA•RFC(ATP) intermediate that is competent for subsequent loading of PCNA onto DNA. Further biochemical studies pointed to the essential roles of the RFC arginine finger in sensing ATP binding and promoting DNA association with open PCNA•RFC(ATP) complex [92]. The same conclusion has also been reached for Archaeoglobus fulgidus RFC [98]. In this archaeal RFC the mutation of arginine finger in the small subunit resulted in a non-functional complex despite its normal ability to bind ATP.
Although PCNA is a symmetric homotrimer by itself, the three subunits become non-equivalent in the RFC•PCNA complex due to the lack of three-fold symmetry in RFC complex. This leads to the question whether a specific interface of PCNA is subjected to opening during loading. The Sulfolobus solfataricus clamp-loading system possesses unique advantages in addressing this question since the S. solfataricus clamp is a heterotrimer. A series of circularly permuted PCNA was constructed by linking the C- and N-termini of the neighboring PCNA subunits [116]. Various combinations were tested to probe the specific interface that is subjected to opening. It was found that the opening of a single subunit interface between PCNA1 and PCNA3 is required for productive PCNA loading.
6. The role of clamp in polymerase exchange
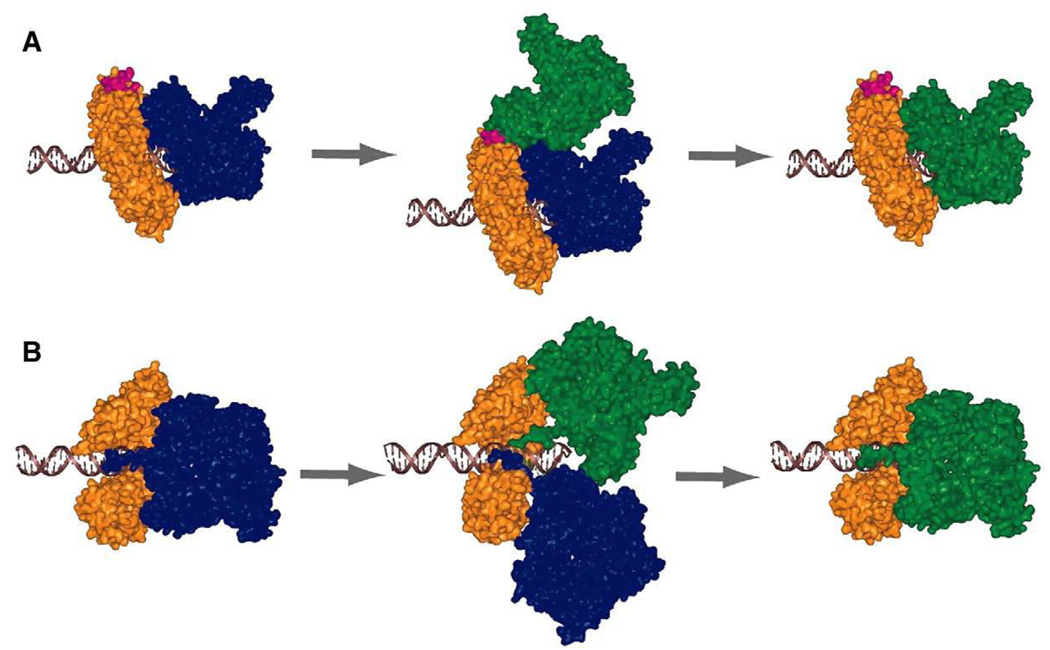
In accord with its high processivity, DNA polymerase holoenzymes formed by DNA polymerase and clamp usually demonstrate high stability once assembled on DNA strand. This is exemplified by a dissociation half-life of ca. 9 min for the bacteriophage T4 gp43•gp45 complex [13]. However the T4 DNA polymerase holoenzyme is likely a highly dynamic entity given that during processive DNA synthesis an active exchange process frequently takes place between the DNA polymerase in solution and the polymerase in the holoenzyme. This phenomenon was initially discovered for the T4 bacteriophage polymerase (gp43) holoenzyme [13]. By using a gp43 mutant that is compromised in its polymerase activity, but retains normal interaction with its cognate clamp (gp45), it was found that gp43 undergoes rapid exchange during the highly processive DNA synthesis. A model was proposed for the polymerase exchange process (Fig. 6). In this model the incoming polymerase binds to the same gp45 trimer, but at a different site, subsequently replacing the resident polymerase through an active process. The rapid kinetics of polymerase exchange in T4 phage suggests that ~90 polymerase exchange events per replication fork would occur during the 15 min time span for replicating the entire T4 genome. Remarkably the polymerase exchange processes have also been demonstrated for the replicative DNA polymerases from other prokaryotes. In bacteriophage T7 polymerase exchange was demonstrated during the coordinated leading and lagging strand DNA synthesis [117]. Unlike the T4 DNA polymerase holoenzyme, in T7 phage polymerase exchange was only observed in the presence of the T7 helicase (gp4). This difference can be understood in view that the T7 DNA polymerase (gp5) uses E. coli thioredoxin as a processivity factor, rather than a clamp that can be used as a ‘landing pad’ for the incoming polymerase [13]. It was suggested that instead the T7 helicase (gp4) is required to recruit the DNA polymerase from solution. In E. coli an exchange between the low-fidelity polymerase Pol IV and the replicative polymerase Pol III have also been reported [51,52]. The exchange processwas found to be strictly regulated by the movement of the Pol III holoenzyme [52]. The stalling of Pol III is required for Pol IV to take control of the β clamp and DNA. However once the DNA synthesis is restarted Pol III can rapidly regain control of DNA.
Fig. 6.
Structural models proposed for polymerase exchange in T4 bacteriophage. The clamp, gp45, is colored in orange. The resident DNA polymerase gp43 and the incoming polymerase are colored in blue and green respectively. (A) Interdomain binding intermediate model is based on binding of the incoming gp43 at the interdomain binding site (pink) on gp45 as identified by Shamoo and Steitz [21]. A conformational change is required to displace the bound polymerase and insert the C-terminal tail of gp43 into the subunit interface of gp45 to form the new polymerase holoenzyme. (B) Direct displacement model through a short-lived complex, in which the incoming C-terminal tail of gp43 displaces the bound gp43 at the open subunit interface of gp45. This model is not favored due to excessive clashes between two polymerases. The figure is adapted from [13]. Copyright (2004) National Academy of Sciences, U.S.A.
7. Post-translational modification of PCNA and polymerase exchange
Polymerase exchange is a central step in translesion DNA synthesis. Because replicative DNA polymerases strongly discriminate against damaged DNA bases, DNA lesions that escape the DNA repair machineries will stall the replicative DNA polymerase during active DNA synthesis in S phase. An unrepaired DNA lesion could thus cause the collapse of the replication fork and lead to DNA breaks. To cope with these potentially deleterious cellular events cells have evolved specialized polymerases capable of translesion DNA synthesis. One such polymerase is Polη that is capable of synthesizing through the cyclobutane pyrimidine dimer (CPD) caused by UV irradiation. In general the remarkable activity of translesion DNA synthesis comes with a sacrifice of the fidelity of TLS polymerases. On a normal DNA template the fidelity of most TLS polymerases is several orders of magnitude lower than that of the high-fidelity DNA polymerases. Thus the recruitment of TLS polymerases to stalled replication forks requires strict spatial and temporal control.
Genetic studies in S. cerevisiae showed that following DNA damage and/or stalling of the DNA replication fork, PCNA undergoes either monoubiquitination or polyubiquitination [118,119]. Both modifications occur at residue Lys164 on PCNA. The polyubiquitin chain, linked through K63 on ubiquitin, is distinct from the K48 linked ubiquitin chain in proteasome-mediated protein degradation. PCNA also undergoes SUMOylation at Lys164 and, to a less extent, at Lys127 [118].
Although polyubiquitination of PCNA has been proposed to trigger the error-free branch of postreplication repair (PRR), the mechanistic details are largely unknown. SUMOylation of PCNA has been suggested to channel lesions into the Rad6-dependent error-prone branch of PRR [119] by recruiting the Srs2 helicase to the replication forks [120,121]. Srs2 exerts an anti-recombinogenic effect by disrupting the Rad51 nucleoprotein filaments [122,123]. Monoubiquitination of PCNA has been linked to translesion DNA synthesis, the error-prone branch of PRR. One important step of TLS is the recruitment of TLS Pol to the damage site through their interaction with PCNA. One common feature of TLS Pols is that many possess a PIP motif that is responsible for interaction with PCNA [53]. Deletion of this motif greatly compromises the ability of TLS Pols in carrying out successful translesion DNA synthesis. Although indispensible for TLS the PIP–PCNA interaction alone is not sufficient for regulating TLS. A successful translesion DNA synthesis requires the recruitment of a specialized TLS Pol prior to the nucleotide incorporation opposite the lesion, and the subsequent removal of the specialized Pol from DNA once the synthesis is completed. The relative affinity of TLS Pol for PCNA needs to be modulated at the two distinct steps of TLS. Existing evidences suggest that monoubiquitination of PCNA is instrumental for such modulation. It has been demonstrated for several TLS Pols (including human Polη [124–126], yeast Polη [127], Rev1 [128,129], Polι [126] and Polκ [130]) that monoubiquitination of PCNA enhances the affinity of TLS Pols for PCNA. This monoubiquitin-mediated interaction between TLS Pols and PCNA presumably recruits the specialized Pols to the DNA damage site. Once recruited to the site on DNA an exchange step between the lesion-specific polymerase and the replicative DNA polymerase presumably follow. Although the molecular details of this exchange step remain to be worked out, studies using a reconstituted system demonstrated that monoubiquitination of PCNA facilitates the ‘switching-in’ of S. cerevisiae Polη in the presence of Polδ [14]. Moreover it was found that the movement of the polymerase holoenzyme is also critical for the regulation of the polymerase exchange step. Effective exchange only occurs when the Polδ holoenzyme is stalled, while Polδ holoenzyme undergoing processive DNA synthesis is refractory to exchange with Polη [14]. The exact mechanism of how monoubiquitination of PCNA promotes polymerase exchange remains to be determined. Monoubiquitination of PCNA may allow Polη to compete more effectively with the replicative DNA polymerase for the 3′ end of the nascent DNA strand through a conformational effect on Polη. Given that three available polymerase binding pockets exist on a single PCNA trimer a ‘tool-belt’ model provides possible means for regulating polymerase exchange. The essence of this model is that more than one polymerase can bind to the same PCNA, e.g. Polη and Polδ can bind to two different PCNA subunits simultaneously. Although the direct evidence for such a model in the eukaryotic replication system is yet to be found, in E. coli Pol III and Pol IV have been shown to bind β clamp simultaneously [52].
The actual lesion bypass synthesis comprises two steps: the insertion of nucleotide opposite the lesion and the extension from the lesion. Depending on the nature of the lesion, the two steps can be either completed by a single specialized polymerase (in the case of CPD lesion) or two different specialized polymerases (for most other lesions). Polζ possesses the unique ability of extending from the lesion [53,131]. Following the lesion bypass synthesis a reverse polymerase exchange is presumably required to restore the high-fidelity replicative DNA polymerase to the growing DNA end. The signal for such a reverse exchange is not yet clear. Deubiquitination of PCNA may be required for reinstating the replicative DNA polymerase [14,132]. In S. cerevisiae the enzyme that deubiquitinates PCNA has yet to be identified. In human the ubiquitin specific protease 1 (USP1) is able to deubiquitinate PCNA [133]. USP1 was found to undergo auto-cleavage following DNA damage [133]. Moreover the cellular expression of USP1 is down-regulated in response to DNA damaging events [134]. Based on these findings it was proposed that monoubiquitination of PCNA is upregulated by the degradation or inactivation of USP1. In this model the cellular level of USP1 will be depressed following DNA damage, which suggests that PCNA will be persistently monoubiquitinated during DNA damage response. Thus the timing of deubiquitination of PCNA by USP1 is still not clear. A recent finding showed that the monoubiquitinated PCNA persists for several hours following the removal of UV damage by exogenous photolyase overexpressed in human cells [135]. Therefore the exact role of deubiquitination of PCNA in TLS warrants further investigation.
8. Concluding remarks
In recent years both structural and mechanistic insights have been obtained into the molecular function of clamp proteins. The availability of high-resolution structures of clamps from each domain of life greatly advanced our understanding of clamp as a processivity factor for DNA replication. A wealth of information on the mechanism of clamp loading was obtained as the results of the in-depth structural determination of clamp loaders in isolation and in complex with clamp, as well as biochemical studies that largely complement the structural data. A relatively less well-understood aspect of clamp function is the physical and functional interactions between clamp and DNA polymerases, including both the replicative DNA polymerase and the TLS polymerase. The structural determinations of such complexes, although challenging, will provide valuable information on our understanding of the many DNA transaction events involving processivity factors.
An exciting development is the elucidation of the clamp's role in DNA transactions other than DNA replication, including DNA repair, translesion DNA synthesis and cell-cycle control. It has become clear that clamp plays diverse roles other than as a processivity factor for DNA polymerase. The mechanism of how clamp coordinates multiple-protein interactions in the context of replication fork stalling is of particular interest, which is central to the precise control of many DNA transactions following the disruption in normal DNA synthesis. Among many outstanding questions is how the different forms of post-translational modification of PCNA by ubiquitin and SUMO in eukaryotes regulate the various pathways of DNA damage response. Given the essential role of clamp in cell proliferation, clamp represents an untapped target that can be exploited for novel antiviral, antibacterial and anticancer therapy.
Acknowledgements
We thank the Zhuang laboratory for critical reading of the manuscript. We are grateful for colleagues in the field for stimulating discussion. We also thank the reviewers of the original manuscript for their helpful comments and suggestions. We wish to apologize to colleagues whose work has not been cited due to space limitation. This work was supported in part by grants from the National Institute of Health and the University of Delaware Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yao NY, O'Donnell M. Replisome dynamics and use of DNA trombone loops to bypass replication blocks. Mol. Biosyst. 2008;4:1075–1084. doi: 10.1039/b811097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benkovic SJ, Valentine AM, Salinas F. Replisome-mediated DNA replication. Annu. Rev. Biochem. 2001;70:181–208. doi: 10.1146/annurev.biochem.70.1.181. [DOI] [PubMed] [Google Scholar]
- 3.Garg P, Burgers PM. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit. Rev. Biochem. Mol. Biol. 2005;40:115–128. doi: 10.1080/10409230590935433. [DOI] [PubMed] [Google Scholar]
- 4.Frouin I, Montecucco A, Spadari S, Maga G. DNA replication: a complex matter. EMBO Rep. 2003;4:666–670. doi: 10.1038/sj.embor.embor886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–527. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson A, O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu. Rev. Biochem. 2005;74:283–315. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- 7.McCulloch SD, Kunkel TA. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008;18:148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyce CM, Benkovic SJ. DNA polymerase fidelity: kinetics, structure, and checkpoints. Biochemistry. 2004;43:14317–14324. doi: 10.1021/bi048422z. [DOI] [PubMed] [Google Scholar]
- 9.Stukenberg PT, Studwell-Vaughan PS, O'Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. J. Biol. Chem. 1991;266(17):11328–11334. [PubMed] [Google Scholar]
- 10.Capson TL, Peliska JA, Kaboord BF, Frey MW, Lively C, Dahlberg M, Benkovic SJ. Kinetic characterization of the polymerase and exonuclease activities of the gene 43 protein of bacteriophage T4. Biochemistry. 1992;31:10984–10994. doi: 10.1021/bi00160a007. [DOI] [PubMed] [Google Scholar]
- 11.Kamtekar S, Berman AJ, Wang J, Lazaro JM, de Vega M, Blanco L, Salas M, Steitz TA. Insights into strand displacement and processivity from the crystal structure of the protein-primed DNA polymerase of bacteriophage phi29. Mol. Cell. 2004;16:609–618. doi: 10.1016/j.molcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Mace DC, Alberts BM. T4 DNA polymerase: rates and processivity on single-stranded DNA templates. J. Mol. Biol. 1984;177:295–311. doi: 10.1016/0022-2836(84)90458-3. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhuang Z, Roccasecca RM, Trakselis MA, Benkovic SJ. The dynamic processivity of the T4 DNA polymerase during replication. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8289–8294. doi: 10.1073/pnas.0402625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang Z, Johnson RE, Haracska L, Prakash L, Prakash S, Benkovic SJ. Regulation of polymerase exchange between Poleta and Poldelta by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5361–5366. doi: 10.1073/pnas.0801310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langston LD, O'Donnell M. DNA polymerase delta is highly processive with proliferating cell nuclear antigen and undergoes collision release upon completing DNA. J. Biol. Chem. 2008;283:29522–29531. doi: 10.1074/jbc.M804488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgers PM. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J. Biol. Chem. 1991;266(33):22698–22706. [PubMed] [Google Scholar]
- 17.Himawan JS, Richardson CC. Amino acid residues critical for the interaction between bacteriophage T7 DNA polymerase and Escherichia coli thioredoxin. J. Biol. Chem. 1996;271:19999–20008. doi: 10.1074/jbc.271.33.19999. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Hamdan SM, Cook TE, Richardson CC. Interactions of Escherichia coli thioredoxin, the processivity factor, with bacteriophage T7 DNA polymerase and helicase. J. Biol. Chem. 2008;283:32077–32084. doi: 10.1074/jbc.M805062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong XP, Onrust R, O'Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992;69:425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- 20.Moarefi I, Jeruzalmi D, Turner J, O'Donnell M, Kuriyan J. Crystal structure of the DNA polymerase processivity factor of T4 bacteriophage. J. Mol. Biol. 2000;296:1215–1223. doi: 10.1006/jmbi.1999.3511. [DOI] [PubMed] [Google Scholar]
- 21.Shamoo Y, Steitz TA. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell. 1999;99:155–166. doi: 10.1016/s0092-8674(00)81647-5. [DOI] [PubMed] [Google Scholar]
- 22.Argiriadi MA, Goedken ER, Bruck I, O'Donnell M, Kuriyan J. Crystal structure of a DNA polymerase sliding clamp from a Gram-positive bacterium. BMC Struct. Biol. 2006;6:2. doi: 10.1186/1472-6807-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 24.Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 25.Matsumiya S, Ishino Y, Morikawa K. Crystal structure of an archaeal DNA sliding clamp: proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Sci. 2001;10:17–23. doi: 10.1110/ps.36401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams GJ, Johnson K, Rudolf J, McMahon SA, Carter L, Oke M, Liu H, Taylor GL, White MF, Naismith JH. Structure of the heterotrimeric PCNA from Sulfolobus solfataricus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;62:944–948. doi: 10.1107/S1744309106034075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appleton BA, Brooks J, Loregian A, Filman DJ, Coen DM, Hogle JM. Crystal structure of the cytomegalovirus DNA polymerase subunit UL44 in complex with the C terminus from the catalytic subunit. Differences in structure and function relative to unliganded UL44. J. Biol. Chem. 2006;281:5224–5232. doi: 10.1074/jbc.M506900200. [DOI] [PubMed] [Google Scholar]
- 28.Zuccola HJ, Filman DJ, Coen DM, Hogle JM. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol. Cell. 2000;5:267–278. doi: 10.1016/s1097-2765(00)80422-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuriyan J, O'Donnell M. Sliding clamps of DNA polymerases. J. Mol. Biol. 1993;234:915–925. doi: 10.1006/jmbi.1993.1644. [DOI] [PubMed] [Google Scholar]
- 30.Yao N, Turner J, Kelman Z, Stukenberg PT, Dean F, Shechter D, Pan ZQ, Hurwitz J, O'Donnell M. Clamp loading, unloading and intrinsic stability of the PCNA, beta and gp45 sliding clamps of human, E. coli and T4 replicases. Genes Cells. 1996;1:101–113. doi: 10.1046/j.1365-2443.1996.07007.x. [DOI] [PubMed] [Google Scholar]
- 31.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell. Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 33.Bruning JB, Shamoo Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure. 2004;12:2209–2219. doi: 10.1016/j.str.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Venclovas C, Thelen MP. Structure-based predictions of Rad1, Rad9, Hus1 and Rad17 participation in sliding clamp and clamp-loading complexes. Nucleic Acids Res. 2000;28:2481–2493. doi: 10.1093/nar/28.13.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsey-Boltz LA, Bermudez VP, Hurwitz J, Sancar A. Purification and characterization of human DNA damage checkpoint Rad complexes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11236–11241. doi: 10.1073/pnas.201373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiomi Y, Shinozaki A, Nakada D, Sugimoto K, Usukura J, Obuse C, Tsurimoto T. Clamp and clamp loader structures of the human checkpoint protein complexes, Rad9-1-1 and Rad17-RFC. Genes Cells. 2002;7:861–868. doi: 10.1046/j.1365-2443.2002.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Griffith JD, Lindsey-Boltz LA, Sancar A. Structures of the human Rad17-replication factor C and checkpoint Rad 9-1-1 complexes visualized by glycerol spray/low voltage microscopy. J. Biol. Chem. 2002;277:15233–15236. doi: 10.1074/jbc.C200129200. [DOI] [PubMed] [Google Scholar]
- 38.Kaur R, Kostrub CF, Enoch T. Structure–function analysis of fission yeast Hus1–Rad1–Rad9 checkpoint complex. Mol. Biol. Cell. 2001;12:3744–3758. doi: 10.1091/mbc.12.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majka J, Burgers PM. Yeast Rad17/Mec3/Ddc1: a sliding clamp for the DNA damage checkpoint. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2249–2254. doi: 10.1073/pnas.0437148100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dore AS, Kilkenny ML, Rzechorzek NJ, Pearl LH. Crystal structure of the Rad9–Rad1–Hus1 DNA damage checkpoint complex—implications for clamp loading and regulation. Mol. Cell. 2009;34:735–745. doi: 10.1016/j.molcel.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 41.Dionne I, Nookala RK, Jackson SP, Doherty AJ, Bell SD. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol. Cell. 2003;11:275–282. doi: 10.1016/s1097-2765(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 42.Pascal JM, Tsodikov OV, Hura GL, Song W, Cotner EA, Classen S, Tomkinson AE, Tainer JA, Ellenberger T. A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Mol. Cell. 2006;24:279–291. doi: 10.1016/j.molcel.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Hlinkova V, Xing G, Bauer J, Shin YJ, Dionne I, Rajashankar KR, Bell SD, Ling H. Structures of monomeric, dimeric and trimeric PCNA: PCNA-ring assembly and opening. Acta Crystallogr. D Biol. Crystallogr. 2008;64:941–949. doi: 10.1107/S0907444908021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pages V, Fuchs RP. How DNA lesions are turned into mutations within cells? Oncogene. 2002;21:8957–8966. doi: 10.1038/sj.onc.1206006. [DOI] [PubMed] [Google Scholar]
- 45.Chow CS, Coen DM. Mutations that specifically impair the DNA binding activity of the herpes simplex virus protein UL42. J. Virol. 1995;69:6965–6971. doi: 10.1128/jvi.69.11.6965-6971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randell JC, Coen DM. Linear diffusion on DNA despite high-affinity binding by a DNA polymerase processivity factor. Mol. Cell. 2001;8:911–920. doi: 10.1016/s1097-2765(01)00355-0. [DOI] [PubMed] [Google Scholar]
- 47.Loregian A, Appleton BA, Hogle JM, Coen DM. Specific residues in the connector loop of the human cytomegalovirus DNA polymerase accessory protein UL44 are crucial for interaction with the UL54 catalytic subunit. J. Virol. 2004;78:9084–9092. doi: 10.1128/JVI.78.17.9084-9092.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appleton BA, Loregian A, Filman DJ, Coen DM, Hogle JM. The cytomegalo-virus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol. Cell. 2004;15:233–244. doi: 10.1016/j.molcel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Franklin MC, Wang J, Steitz TA. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 50.Johansson E, Garg P, Burgers PM. The Pol32 subunit of DNA polymerase delta contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 2004;279:1907–1915. doi: 10.1074/jbc.M310362200. [DOI] [PubMed] [Google Scholar]
- 51.Furukohri A, Goodman MF, Maki H. A dynamic polymerase exchange with Escherichia coli DNA polymerase IV replacing DNA polymerase III on the sliding clamp. J. Biol. Chem. 2008;283:11260–11269. doi: 10.1074/jbc.M709689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Indiani C, McInerney P, Georgescu R, Goodman MF, O'Donnell M. A sliding-clamp toolbelt binds high- and low-fidelity DNA polymerases simultaneously. Mol. Cell. 2005;19:805–815. doi: 10.1016/j.molcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 54.Haracska L, Kondratick CM, Unk I, Prakash S, Prakash L. Interaction with PCNA is essential for yeast DNA polymerase eta function. Mol. Cell. 2001;8:407–415. doi: 10.1016/s1097-2765(01)00319-7. [DOI] [PubMed] [Google Scholar]
- 55.Acharya N, Yoon JH, Gali H, Unk I, Haracska L, Johnson RE, Hurwitz J, Prakash L, Prakash S. Roles of PCNA-binding and ubiquitin-binding domains in human DNA polymerase eta in translesion DNA synthesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17724–17729. doi: 10.1073/pnas.0809844105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hishiki A, Hashimoto H, Hanafusa T, Kamei K, Ohashi E, Shimizu T, Ohmori H, Sato M. Structural basis for novel interactions between human translesion synthesis polymerases and PCNA. J. Biol. Chem. 2009 doi: 10.1074/jbc.M809745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Gibbs PE, Lawrence CW. The Saccharomyces cerevisiae rev6-1 mutation, which inhibits both the lesion bypass and the recombination mode of DNA damage tolerance, is an allele of POL30, encoding proliferating cell nuclear antigen. Genetics. 2006;173:1983–1989. doi: 10.1534/genetics.106.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freudenthal BD, Ramaswamy S, Hingorani MM, Washington MT. Structure of a mutant form of proliferating cell nuclear antigen that blocks translesion DNA synthesis. Biochemistry. 2008;47:13354–13361. doi: 10.1021/bi8017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing G, Kirouac K, Shin YJ, Bell SD, Ling H. Structural insight into recruitment of translesion DNA polymerase Dpo4 to sliding clamp PCNA. Mol. Microbiol. 2009;71:678–691. doi: 10.1111/j.1365-2958.2008.06553.x. [DOI] [PubMed] [Google Scholar]
- 60.Bunting KA, Roe SM, Pearl LH. Structural basis for recruitment of translesion DNA polymerase Pol IV/DinB to the beta-clamp. EMBO J. 2003;22:5883–5892. doi: 10.1093/emboj/cdg568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgescu RE, Kim SS, Yurieva O, Kuriyan J, Kong XP, O'Donnell M. Structure of a sliding clamp on DNA. Cell. 2008;132:43–54. doi: 10.1016/j.cell.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laurence TA, Kwon Y, Johnson A, Hollars CW, O'Donnell M, Camarero JA, Barsky D. Motion of a DNA sliding clamp observed by single molecule fluorescence spectroscopy. J. Biol. Chem. 2008;283:22895–22906. doi: 10.1074/jbc.M800174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanov I, Chapados BR, McCammon JA, Tainer JA. Proliferating cell nuclear antigen loaded onto double-stranded DNA: dynamics, minor groove interactions and functional implications. Nucleic Acids Res. 2006;34:6023–6033. doi: 10.1093/nar/gkl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayanagi K, Kiyonari S, Saito M, Shirai T, Ishino Y, Morikawa K. Mechanism of replication machinery assembly as revealed by the DNA ligase–PCNA–DNA complex architecture. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0811196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 66.Smiley RD, Zhuang Z, Benkovic SJ, Hammes GG. Single-molecule investigation of the T4 bacteriophage DNA polymerase holoenzyme: multiple pathways of holoenzyme formation. Biochemistry. 2006;45:7990–7997. doi: 10.1021/bi0603322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang Z, Berdis AJ, Benkovic SJ. An alternative clamp loading pathway via the T4 clamp loader gp44/62–DNA complex. Biochemistry. 2006;45:7976–7989. doi: 10.1021/bi0601205. [DOI] [PubMed] [Google Scholar]
- 68.Millar D, Trakselis MA, Benkovic SJ. On the solution structure of the T4 sliding clamp (gp45) Biochemistry. 2004;43:12723–12727. doi: 10.1021/bi048349c. [DOI] [PubMed] [Google Scholar]
- 69.Trakselis MA, Berdis AJ, Benkovic SJ. Examination of the role of the clamp-loader and ATP hydrolysis in the formation of the bacteriophage T4 polymerase holoenzyme. J. Mol. Biol. 2003;326:435–451. doi: 10.1016/s0022-2836(02)01330-x. [DOI] [PubMed] [Google Scholar]
- 70.Alley SC, Abel-Santos E, Benkovic SJ. Tracking sliding clamp opening and closing during bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry. 2000;39:3076–3090. doi: 10.1021/bi992377r. [DOI] [PubMed] [Google Scholar]
- 71.Alley SC, Shier VK, Abel-Santos E, Sexton DJ, Soumillion P, Benkovic SJ. Sliding clamp of the bacteriophage T4 polymerase has open and closed subunit interfaces in solution. Biochemistry. 1999;38:7696–7709. doi: 10.1021/bi9827971. [DOI] [PubMed] [Google Scholar]
- 72.Pietroni P, von Hippel PH. Multiple ATP binding is required to stabilize the “activated” (clamp open) clamp loader of the T4 DNA replication complex. J. Biol. Chem. 2008;283:28338–28353. doi: 10.1074/jbc.M804371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latham GJ, Dong F, Pietroni P, Dozono JM, Bacheller DJ, von Hippel PH. Opening of a monomer–monomer interface of the trimeric bacteriophage T4-coded GP45 sliding clamp is required for clamp loading onto DNA. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12448–12453. doi: 10.1073/pnas.96.22.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Latham GJ, Bacheller DJ, Pietroni P, von Hippel PH. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. III. The Gp43 DNA polymerase binds to the same face of the sliding clamp as the clamp loader. J. Biol. Chem. 1997;272:31685–31692. doi: 10.1074/jbc.272.50.31685. [DOI] [PubMed] [Google Scholar]
- 75.Latham GJ, Bacheller DJ, Pietroni P, von Hippel PH. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. II. The Gp44/62 clamp loader interacts with a single defined face of the sliding clamp ring. J. Biol. Chem. 1997;272:31677–31684. doi: 10.1074/jbc.272.50.31677. [DOI] [PubMed] [Google Scholar]
- 76.Pietroni P, Young MC, Latham GJ, von Hippel PH. Structural analyses of gp45 sliding clamp interactions during assembly of the bacteriophage T4 DNA polymerase holoenzyme. I. Conformational changes within the gp44/62–gp45–ATP complex during clamp loading. J. Biol. Chem. 1997;272:31666–31676. doi: 10.1074/jbc.272.50.31666. [DOI] [PubMed] [Google Scholar]
- 77.Pietroni P, Young MC, Latham GJ, von Hippel PH. Dissection of the ATP-driven reaction cycle of the bacteriophage T4 DNA replication processivity clamp loading system. J. Mol. Biol. 2001;309:869–891. doi: 10.1006/jmbi.2001.4687. [DOI] [PubMed] [Google Scholar]
- 78.Ason B, Handayani R, Williams CR, Bertram JG, Hingorani MM, O'Donnell M, Goodman MF, Bloom LB. Mechanism of loading the Escherichia coli DNA polymerase III beta sliding clamp on DNA. Bona fide primer/templates preferentially trigger the gamma complex to hydrolyze ATP and load the clamp. J. Biol. Chem. 2003;278:10033–10040. doi: 10.1074/jbc.M211741200. [DOI] [PubMed] [Google Scholar]
- 79.Anderson SG, Williams CR, O'Donnell M, Bloom LB. A function for the psi subunit in loading the Escherichia coli DNA polymerase sliding clamp. J. Biol. Chem. 2007;282:7035–7045. doi: 10.1074/jbc.M610136200. [DOI] [PubMed] [Google Scholar]
- 80.Bertram JG, Bloom LB, O'Donnell M, Goodman MF. Increased dNTP binding affinity reveals a nonprocessive role for Escherichia coli beta clamp with DNA polymerase IV. J. Biol. Chem. 2004;279:33047–33050. doi: 10.1074/jbc.C400265200. [DOI] [PubMed] [Google Scholar]
- 81.Snyder AK, Williams CR, Johnson A, O'Donnell M, Bloom LB. Mechanism of loading the Escherichia coli DNA polymerase III sliding clamp: II. Uncoupling the beta and DNA binding activities of the gamma complex. J. Biol. Chem. 2004;279:4386–4393. doi: 10.1074/jbc.M310430200. [DOI] [PubMed] [Google Scholar]
- 82.Williams CR, Snyder AK, Kuzmic P, O'Donnell M, Bloom LB. Mechanism of loading the Escherichia coli DNA polymerase III sliding clamp: I. Two distinct activities for individual ATP sites in the gamma complex. J. Biol. Chem. 2004;279:4376–4385. doi: 10.1074/jbc.M310429200. [DOI] [PubMed] [Google Scholar]
- 83.Bertram JG, Bloom LB, Hingorani MM, Beechem JM, O'Donnell M, Goodman MF. Molecular mechanism and energetics of clamp assembly in Escherichia coli. The role of ATP hydrolysis when gamma complex loads beta on DNA. J. Biol. Chem. 2000;275:28413–28420. doi: 10.1074/jbc.M910441199. [DOI] [PubMed] [Google Scholar]
- 84.Ason B, Bertram JG, Hingorani MM, Beechem JM, O'Donnell M, Goodman MF, Bloom LB. A model for Escherichia coli DNA polymerase III holoenzyme assembly at primer/template ends. DNA triggers a change in binding specificity of the gamma complex clamp loader. J. Biol. Chem. 2000;275:3006–3015. doi: 10.1074/jbc.275.4.3006. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Coman MM, Sakato M, O'Donnell M, Hingorani MM. Conserved residues in the delta subunit help the E. coli clamp loader, gamma complex, target primer-template DNA for clamp assembly. Nucleic Acids Res. 2008;36:3274–3286. doi: 10.1093/nar/gkn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magdalena Coman M, Jin M, Ceapa R, Finkelstein J, O'Donnell M, Chait BT, Hingorani MM. Dual functions, clamp opening and primer-template recognition, define a key clamp loader subunit. J. Mol. Biol. 2004;342:1457–1469. doi: 10.1016/j.jmb.2004.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomes XV, Burgers PM. ATP utilization by yeast replication factor C. I. ATP-mediated interaction with DNA and with proliferating cell nuclear antigen. J. Biol. Chem. 2001;276:34768–34775. doi: 10.1074/jbc.M011631200. [DOI] [PubMed] [Google Scholar]
- 88.Gomes XV, Schmidt SL, Burgers PM. ATP utilization by yeast replication factor C. II. Multiple stepwise ATP binding events are required to load proliferating cell nuclear antigen onto primed DNA. J. Biol. Chem. 2001;276:34776–34783. doi: 10.1074/jbc.M011743200. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt SL, Gomes XV, Burgers PM. ATP utilization by yeast replication factor C. III. The ATP-binding domains of Rfc2, Rfc3, and Rfc4 are essential for DNA recognition and clamp loading. J. Biol. Chem. 2001;276:34784–34791. doi: 10.1074/jbc.M011633200. [DOI] [PubMed] [Google Scholar]
- 90.Yao N, Coryell L, Zhang D, Georgescu RE, Finkelstein J, Coman MM, Hingorani MM, O'Donnell M. Replication factor C clamp loader subunit arrangement within the circular pentamer and its attachment points to proliferating cell nuclear antigen. J. Biol. Chem. 2003;278:50744–50753. doi: 10.1074/jbc.M309206200. [DOI] [PubMed] [Google Scholar]
- 91.Yao NY, Johnson A, Bowman GD, Kuriyan J, O'Donnell M. Mechanism of proliferating cell nuclear antigen clamp opening by replication factor C. J. Biol. Chem. 2006;281:17528–17539. doi: 10.1074/jbc.M601273200. [DOI] [PubMed] [Google Scholar]
- 92.Johnson A, Yao NY, Bowman GD, Kuriyan J, O'Donnell M. The replication factor C clamp loader requires arginine finger sensors to drive DNA binding and proliferating cell nuclear antigen loading. J. Biol. Chem. 2006;281:35531–35543. doi: 10.1074/jbc.M606090200. [DOI] [PubMed] [Google Scholar]
- 93.Zhuang Z, Yoder BL, Burgers PM, Benkovic SJ. The structure of a ring-opened proliferating cell nuclear antigen-replication factor C complex revealed by fluorescence energy transfer. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2546–2551. doi: 10.1073/pnas.0511263103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uhlmann F, Cai J, Gibbs E, O'Donnell M, Hurwitz J. Deletion analysis of the large subunit p140 in human replication factor C reveals regions required for complex formation and replication activities. J. Biol. Chem. 1997;272:10058–10064. doi: 10.1074/jbc.272.15.10058. [DOI] [PubMed] [Google Scholar]
- 95.Uhlmann F, Gibbs E, Cai J, O'Donnell M, Hurwitz J. Identification of regions within the four small subunits of human replication factor C required for complex formation and DNA replication. J. Biol. Chem. 1997;272:10065–10071. doi: 10.1074/jbc.272.15.10065. [DOI] [PubMed] [Google Scholar]
- 96.Cai J, Gibbs E, Uhlmann F, Phillips B, Yao N, O'Donnell M, Hurwitz J. A complex consisting of human replication factor C p40, p37, and p36 subunits is a DNA-dependent ATPase and an intermediate in the assembly of the holoenzyme. J. Biol. Chem. 1997;272:18974–18981. doi: 10.1074/jbc.272.30.18974. [DOI] [PubMed] [Google Scholar]
- 97.Zhang G, Gibbs E, Kelman Z, O'Donnell M, Hurwitz J. Studies on the interactions between human replication factor C and human proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1869–1874. doi: 10.1073/pnas.96.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seybert A, Singleton MR, Cook N, Hall DR, Wigley DB. Communication between subunits within an archaeal clamp–loader complex. EMBO J. 2006;25:2209–2218. doi: 10.1038/sj.emboj.7601093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seybert A, Wigley DB. Distinct roles for ATP binding and hydrolysis at individual subunits of an archaeal clamp loader. EMBO J. 2004;23:1360–1371. doi: 10.1038/sj.emboj.7600130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seybert A, Scott DJ, Scaife S, Singleton MR, Wigley DB. Biochemical characterisation of the clamp/clamp loader proteins from the euryarchaeon Archaeoglobus fulgidus. Nucleic Acids Res. 2002;30:4329–4338. doi: 10.1093/nar/gkf584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pisani FM, De Felice M, Carpentieri F, Rossi M. Biochemical characterization of a clamp–loader complex homologous to eukaryotic replication factor C from the hyperthermophilic archaeon Sulfolobus solfataricus. J. Mol. Biol. 2000;301:61–73. doi: 10.1006/jmbi.2000.3964. [DOI] [PubMed] [Google Scholar]
- 102.Hingorani MM, O'Donnell M. ATP binding to the Escherichia coli clamp loader powers opening of the ring-shaped clamp of DNA polymerase III holoenzyme. J. Biol. Chem. 1998;273:24550–24563. doi: 10.1074/jbc.273.38.24550. [DOI] [PubMed] [Google Scholar]
- 103.Trakselis MA, Alley SC, Abel-Santos E, Benkovic SJ. Creating a dynamic picture of the sliding clamp during T4 DNA polymerase holoenzyme assembly by using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8368–8375. doi: 10.1073/pnas.111006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen S, Levin MK, Sakato M, Zhou Y, Hingorani MM. Mechanism of ATP-driven PCNA clamp loading by S. cerevisiae RFC. J. Mol. Biol. 2009;388:431–442. doi: 10.1016/j.jmb.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davey MJ, Jeruzalmi D, Kuriyan J, O'Donnell M. Motors and switches: AAA+ machines within the replisome. Nat. Rev. Mol. Cell Biol. 2002;3:826–835. doi: 10.1038/nrm949. [DOI] [PubMed] [Google Scholar]
- 106.Bowman GD, O'Donnell M, Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp–clamp loader complex. Nature. 2004;429:724–730. doi: 10.1038/nature02585. [DOI] [PubMed] [Google Scholar]
- 107.Goedken ER, Kazmirski SL, Bowman GD, O'Donnell M, Kuriyan J. Mapping the interaction of DNA with the Escherichia coli DNA polymerase clamp loader complex. Nat. Struct. Mol. Biol. 2005;12:183–190. doi: 10.1038/nsmb889. [DOI] [PubMed] [Google Scholar]
- 108.Simonetta KR, Kazmirski SL, Goedken ER, Cantor AJ, Kelch BA, McNally R, Seyedin SN, Makino DL, O'Donnell M, Kuriyan J. The mechanism of ATP-dependent primer-template recognition by a clamp loader complex. Cell. 2009;137:659–671. doi: 10.1016/j.cell.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeruzalmi D, O'Donnell M, Kuriyan J. Crystal structure of the processivity clamp loader gamma (gamma) complex of E. coli DNA polymerase III. Cell. 2001;106:429–441. doi: 10.1016/s0092-8674(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 110.Kazmirski SL, Podobnik M, Weitze TF, O'Donnell M, Kuriyan J. Structural analysis of the inactive state of the Escherichia coli DNA polymerase clamp–loader complex. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16750–16755. doi: 10.1073/pnas.0407904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burgers PM. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J. Biol. Chem. 1991;266:22698–22706. [PubMed] [Google Scholar]
- 112.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J. Biol. Chem. 1991;266:1950–1960. [PubMed] [Google Scholar]
- 113.Miyata T, Oyama T, Mayanagi K, Ishino S, Ishino Y, Morikawa K. The clamp-loading complex for processive DNA replication. Nat. Struct. Mol. Biol. 2004;11:632–636. doi: 10.1038/nsmb788. [DOI] [PubMed] [Google Scholar]
- 114.Miyata T, Suzuki H, Oyama T, Mayanagi K, Ishino Y, Morikawa K. Open clamp structure in the clamp-loading complex visualized by electron microscopic image analysis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13795–13800. doi: 10.1073/pnas.0506447102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kazmirski SL, Zhao Y, Bowman GD, O'Donnell M, Kuriyan J. Out-of-plane motions in open sliding clamps: molecular dynamics simulations of eukaryotic and archaeal proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13801–13806. doi: 10.1073/pnas.0506430102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dionne I, Brown NJ, Woodgate R, Bell SD. On the mechanism of loading the PCNA sliding clamp by RFC. Mol. Microbiol. 2008;68:216–222. doi: 10.1111/j.1365-2958.2008.06150.x. [DOI] [PubMed] [Google Scholar]
- 117.Johnson DE, Takahashi M, Hamdan SM, Lee SJ, Richardson CC. Exchange of DNA polymerases at the replication fork of bacteriophage T7. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5312–5317. doi: 10.1073/pnas.0701062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 119.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 120.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- 121.Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Krejci L, Damborsky J, Thomsen B, Duno M, Bendixen C. Molecular dissection of interactions between Rad51 and members of the recombination–repair group. Mol. Cell. Biol. 2001;21:966–976. doi: 10.1128/MCB.21.3.966-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004;23:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 126.Plosky BS, Vidal AE, Fernandez de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, Woodgate R. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 2006;25:2847–2855. doi: 10.1038/sj.emboj.7601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parker JL, Bielen AB, Dikic I, Ulrich HD. Contributions of ubiquitin- and PCNA-binding domains to the activity of Polymerase eta in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35:881–889. doi: 10.1093/nar/gkl1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, Ulrich HD, Friedberg EC. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell. 2006;23:265–271. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 129.Wood A, Garg P, Burgers PM. A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage. J. Biol. Chem. 2007;282:20256–20263. doi: 10.1074/jbc.M702366200. [DOI] [PubMed] [Google Scholar]
- 130.Guo C, Tang TS, Bienko M, Dikic I, Friedberg EC. Requirements for the interaction of mouse Polkappa with ubiquitin and its biological significance. J. Biol. Chem. 2008;283:4658–4664. doi: 10.1074/jbc.M709275200. [DOI] [PubMed] [Google Scholar]
- 131.Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- 132.Waters LS, Minesinger BK, Wiltrout ME, D'Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D'Andrea AD. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 134.Cohn MA, Kowal P, Yang K, Haas W, Huang TT, Gygi SP, D'Andrea AD. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell. 2007;28:786–797. doi: 10.1016/j.molcel.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 135.Niimi A, Brown S, Sabbioneda S, Kannouche PL, Scott A, Yasui A, Green CM, Lehmann AR. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16125–16130. doi: 10.1073/pnas.0802727105. [DOI] [PMC free article] [PubMed] [Google Scholar]