Abstract
This review presents an overview of the emerging field of prostaglandin signaling in neurological diseases, focusing on PGE2 signaling through its four E-prostanoid (EP) receptors. A large number of studies have demonstrated a neurotoxic function of the inducible cyclooxygenase COX-2 in a broad spectrum of neurological disease models in the central nervous system (CNS), from models of cerebral ischemia to models of neurodegeneration and inflammation. Since COX-1 and COX-2 catalyze the first committed step in prostaglandin synthesis, an effort is underway to identify the downstream prostaglandin signaling pathways that mediate the toxic effect of COX-2. Recent epidemiologic studies demonstrate that chronic COX-2 inhibition can produce adverse cerebrovascular and cardiovascular effects, indicating that some prostaglandin signaling pathways are beneficial. Consistent with this concept, recent studies demonstrate that in the CNS, specific prostaglandin receptor signaling pathways mediate toxic effects in brain but a larger number appear to mediate paradoxically protective effects. Further complexity is emerging, as exemplified by the PGE2 EP2 receptor, where cerebroprotective or toxic effects of a particular prostaglandin signaling pathway can differ depending on the context of cerebral injury, for example in excitotoxicity/hypoxia paradigms versus inflammatory-mediated secondary neurotoxicity. The divergent effects of prostaglandin receptor signaling will likely depend on distinct patterns and dynamics of receptor expression in neurons, endothelial cells, and glia and the specific ways in which these cell types participate in particular models of neurological injury.
Keywords: COX-2, PGE2, EP1 receptor, EP2 receptor, EP3 receptor, EP4 receptor, excitotoxicity, cerebral ischemia, inflammation, Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS)
COX-1 and COX-2
The inducible isoform of cyclooxygenase, COX-2, is rapidly upregulated in neurons following N-methyl-D-aspartate (NMDA) receptor-dependent synaptic activity 1, consistent with a physiologic role in modulating synaptic plasticity 2, 3. COX-2 activity is also induced in neurons in vivo in acute paradigms of excitotoxicity such as cerebral ischemia and seizures 1, 4-6, where it can promote injury to neurons 7-10. COX-2 is also induced in brain in inflammatory paradigms in non-neuronal cells, including microglia, astrocytes and endothelial cells, where it contributes to inflammatory injury in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis 11-20. Thus, COX activity and its downstream prostaglandin production function pathologically in promoting neuronal injury both in acute excitotoxic insults but also in chronic neurodegenerative diseases where inflammation is a major pathological component. To better understand mechanisms of COX neurotoxicity, it is essential therefore to study the downstream prostaglandin signaling pathways that are the effectors of COX-mediated neurotoxicity. This review centers on the function of the prostaglandin receptors in models of neurological disease, and specifically on the function of the PGE2 EP receptors. For a review of the cyclooxygenases, the reader is referred to several excellent reviews on the cyclooxygenases COX-1 and inducible COX-2 in brain 21-25.
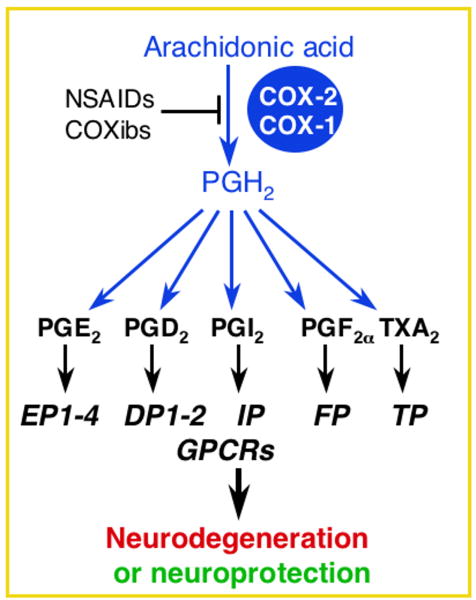
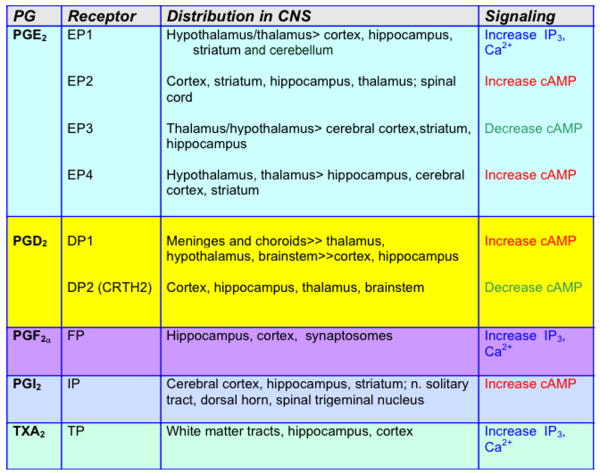
Prostaglandins are derived from the metabolism of arachidonic acid (AA) by COX-1 and COX-2 to PGH2 (Figure 1). PGH2 then serves as the substrate for the generation of prostaglandins and thromboxane A2: PGE2, PGF2α, PGD2, PGI2 (prostacyclin), and thromboxane A2 (TXA2). These prostanoids bind to specific G protein-coupled receptors designated EP (for E-prostanoid receptor), FP, DP, IP, and TP, respectively (reviewed in 26). PG receptor subtypes are distinguished by the signal transduction pathway that is activated upon ligand binding. Activation leads to changes in the production of cAMP and/or phosphoinositol turnover and intracellular Ca2+ mobilization. Further complexity occurs in the case of PGE2, which binds four receptor subtypes (EP1, EP2, EP3, and EP4) and PGD2 which binds two receptor subtypes with distinct and potentially antagonistic signaling cascades. All nine PG receptors have been identified in CNS (Figure 2).
Figure 1.
Prostaglandin receptors mediate both toxic and protective effects in models of neurological disease.
Figure 2.
CNS distribution and primary signaling characteristics of the nine PG receptors.
Recently however, deleterious cardiovascular side-effects arising from chronic use of COX-2 inhibitors have been demonstrated 27-29, suggesting that some prostaglandin (PG) signaling pathways downstream of COX-2 are beneficial 30-32. The concept of toxic and beneficial PG signaling pathways is now applicable to the CNS as well, as is described below for the PGE2 EP1-4 receptors.
A. The EP1 receptor
In the CNS, the EP1 receptor is expressed in brain under basal conditions in cerebral cortex and hippocampus and in cerebellar Purkinje cells 33, 34 The EP1 receptor is unique among the PGE2 EP receptors in that it is coupled to Gαq, and activation of EP1 receptor results in increased phosphatidyl inositol hydrolysis and elevation of the intracellular Ca2+ concentration. In brain, EP1 is involved in specific behavioral paradigms. Pharmacologic inhibition or genetic deletion of EP1 receptor in mice subjected to environmental or social stressors resulted in behavioral disinhibition and was associated with increased dopamine turnover in striatum 35. A subsequent study demonstrated that activation of EP1 receptors in striatum amplified dopamine receptor signaling via modulation of DARPP-32 phosphorylation 36.
With respect to a pathological role for EP1 signaling in the CNS, it was noted that administration of PGE2 to cortical and hippocampal primary neuronal cultures at physiological concentrations (1nM to 1μM) protected neurons from N-methyl-d-asparate (NMDA) or glutamate toxicity 37-39. However, in the presence of a COX-2 inhibitor, excitotoxicity-induced neuronal death could be elicited with an EP1/EP3 receptor agonist (17-phenyl trinor PGE2), suggesting that among the four EP receptors, there were protective as well as toxic EP receptors 40. In findings that expanded on these observations, Gendron et al. 41 demonstrated that in a model of oxygen-glucose deprivation (OGD), the neuroprotection elicited by COX-2 inhibition could be reversed by administration of PGE2, and this was mediated by the EP1 receptor. In vivo administration of the EP1/EP3 agonist 17-phenyl trinor PGE2 into neocortex also reversed the protection against NMDA excitotoxicity elicited by COX-2 inhibition 42. The identity of the EP1 receptor as a major EP receptor that transduces COX-2 neurotoxicity was subsequently demonstrated in vivo in models of NMDA excitotoxicity and focal cerebral ischemia, where inhibition of the receptor with a selective antagonist or global genetic deletion of EP1 reduced cerebral injury 34, 43. In the former study, the reduction in NMDA excitotoxic injury in COX-2-/- mice was reversed with local administration of a PGE2 analogue, and this effect was blocked by pharmacological inhibition of the EP1 receptor; moreover, PGE2 administration to EP1-/- mice did not increase cerebral infarction. Finally, pharmacological antagonism of the EP1 receptor did not further reduce the protection induced with genetic deletion of COX-2. Translational relevance of EP1 antagonism in a model of cerebral ischemia was demonstrated in a later study where significant cerebroprotection and rescue of behavioral deficits occurred even when EP1 antagonist was administered 12 hours after ischemia 44.
A primary mechanism of NMDA-induced neurotoxicity is increased Ca2+ flux through the NMDA receptor and disrupted intracellular Ca2+ homeostasis. In vitro studies of cultured neurons demonstrated that pharmacologic antagonism of EP1 or genetic deletion of EP1 resulted in a normalization of intracellular Ca2+ concentrations as measured by the Ca2+ indicator Fura-2; the normalization of Ca2+ levels was not caused by EP1-mediated effects on Ca2+ influx through the NMDA receptor or voltage-gated Ca2+ channels. The normalization of intracellular Ca2+ with EP1 blockade or genetic deletion was associated with improved function of the Na+/Ca2+ exchangers 34. A subsequent in vitro study examined whether inhibition of the toxic EP1 receptor caused neuronal protection by enhancing the protective phospho-AKT pathway. It was determined that inhibition of EP1 resulted in increased AKT phosphorylation following OGD as well as under basal conditions 45, suggesting that EP1 functions constitutively in negatively regulating the phosphorylation state of AKT. The phosphatase and tensin homologue on chromosome ten (PTEN) is a phosphatase that opposes the action of phophatidylinositol-3-kinase (PI3K) in phosphorylating a broad range of substrates including AKT; previously the EP1 receptor had been shown to enhance PTEN phosphatase in a model of lung fibroblast migration 46. The EP1-mediated regulation of phospho-AKT levels was associated with activation of the phosphatase PTEN that via its depletion of PIP3 could inactivate AKT. At this point, it is not known if there is a link between the two in vitro EP1 effects on Na+/Ca2+ exchangers and PTEN/AKT, and whether these mechanisms are active in vivo. A recent study by Carlson et al. 47 highlights an important issue relating to the in vivo mechanism of EP toxicity, namely that of neuron-glial interaction. In this study, EP1 antagonists were protective in pure neuronal cultures stimulated with NMDA, however addition of microglia to the cultures reduced the protective effects of EP1 antagonists on neurons.
EP1 is known to induce vasoconstriction in the peripheral vasculature, opening up the possibility that EP1 may exert its toxic effects, at least in models of cerebral ischemia, via deleterious effects on cerebral blood flow. Studies by Saleem et al indicate that deletion of the EP1 receptor may have beneficial effects on cerebral blood flow, thus reducing the severity of cerebral ischemia 48. In a model of focal transient cerebral ischemia, EP1-/- mice demonstrated increased intraischemic absolute cerebral blood flow as well as increased blood flow in early reperfusion after termination of ischemia. These findings are consistent with previous studies in non-CNS vascular models, where EP1 induced vasoconstriction 49, 50. Thus, in models of cerebral ischemia, inhibition of EP1 signaling may lead to cerebroprotection via neuronal-specific mechanisms, beneficial effects on cerebral blood flow, or both.
Direct effects of EP1-mediated neurotoxicity have been demonstrated in additional models of neurodegeneration, in particular in models of Parkinson's disease (PD) examining survival of cultured dopaminergic neurons. Stimulation of cultured mesencephalic neurons with PGE2 resulted in neurotoxicity; this effect was mediated by the EP1 receptor but not by the EP2 receptor, which is also expressed on dopaminergic neurons 51. In the setting of 6-hydroxy dopamine (6-OHDA) oxidative stress, a stimulus that induces COX-2 expression and PGE2 signaling in dopaminergic mesencephalic neurons 52, inhibition of the EP1 receptor successfully rescued dopaminergic neurons. These in vitro studies point to a potential role of EP1 in dopaminergic neuronal viability, both basally and in the setting of oxidative stress.
B. The EP2 receptor
The EP2 receptor is positively coupled to cAMP production and is widely expressed in neurons in forebrain structures as well as in thalamus, hypothalamus, brainstem and spinal cord 37, 53, 54. In terms of its physiologic function in brain, a role of EP2 signaling in activity-dependent synaptic plasticity is supported by an emerging literature in widely differing models. In developing hypothalamus, PGE2, acting via the EP2 and EP3 receptors in the developing preoptic area increases dendritic spines by an alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor dependent mechanism 55 and regulates levels of spinophilin 56. In a model of inflammatory hyperalgesia in spinal cord dorsal horn, PGE2 facilitates pain transmission via blockade of inhibitory glycine receptors 57, 58 and this response is blocked with deletion of the EP2 receptor 59. In hippocampus, long-term potentiation (LTP) in perforant path-dentate granule cell synapses can be blocked by COX-2 inhibitors, but rescued with administration of PGE23. PGE2 increases the probability of glutamatergic synaptic transmission in hippocampus via pre-synaptic EP2 signaling in a PKA-dependent fashion 60. In cerebral cortex, in a model of visual cortical theta-burst stimulation (TBS)-evoked LTP, blocking post-synaptic EP2 expression with RNA interference induced LTP, and blocking post-synaptic EP3 expression reduced LTP; moreover, in this model TBS was associated with differential trafficking of surface EP2 and EP3 receptors between the cell membrane and the cytosol 61. This last observation is particularly intriguing because differential trafficking of AMPA receptors to and from the post-synaptic membrane is believed to play an important role in the changes in synaptic strength in LTP and long-term depression (LTD), respectively. In recent studies, deletion of the EP2 receptor led to significant cognitive deficits in standard tests of fear, anxiety, and social memory, recapitulating some aspects of human psychopathology related to schizophrenia. This complex behavioral phenotype of EP2-/- mice was associated with a deficit in hippocampal LTD 62.
In terms of a function of the EP2 receptor in COX-2 mediated neurotoxicity, it is important to note an expanding literature that documents pro-survival and anti-apoptotic functions of GPCRαs. In vitro studies of dispersed hippocampal neurons and organotypic hippocampal slices demonstrate that activation of the EP2 receptor is neuroprotective in paradigms of NMDA toxicity and OGD 37, 63, however stimulation with PGE2 has no effect, consistent with the idea of toxic (EP1) and protective EP (EP2) receptor activities. In acute glutamate toxicity models, inhibition of protein kinase A (PKA) activation reversed the protective effect of EP2 signaling, indicating that neuronal EP2-mediated protection is dependent on cAMP signaling 37. In vivo, in the middle cerebral artery occlusion/reperfusion (MCAO-RP) model of focal transient 37 and permanent 63 forebrain ischemia, genetic deletion of the EP2 receptor significantly increased cerebral infarction in cerebral cortex and subcortical structures, consistent with the in vitro protective effect of pharmacologic stimulation with EP2 agonist. EP2 receptor stimulation was also protective in a model of striatal excitotoxicity 64. Stimulation of the EP2 receptor rescued neurons in additional in vitro models of neurodegenerative disease, including the threo-hydroxyaspartate (THA) model of glutamate-induced motor neuron toxicity 53, a model of human amyotrophic lateral sclerosis (ALS) where chronic glutamate toxicity is induced by blocking astrocyte glutamate transporters. In the 6-OHDA model of dopaminergic neuronal degeneration, a model of Parkinson's disease, EP2 signaling was also neuroprotective 65. In both cases, as in hippocampal neuroprotection, the EP2-dependent protective effects were dependent on cAMP/PKA activation.
Although the mechanism of EP2 protection in vitro involves neuronal cAMP dependent EP2 signaling, EP2 is also expressed basally in the cerebral vasculature and appears dynamically upregulated in the setting of cerebral ischemia and reperfusion 66. EP2 signaling induces vasodilation in non-cerebral vascular systems 67 and this additional function suggests a hypothetical role for EP2 in increasing blood flow in settings of cerebral ischemia. Although measurement of absolute cerebral blood flow in vivo in EP2-/- and wild type control mice has not demonstrated significant differences between genotypes, studies so far have been limited to measurement of cerebral blood flow during intra-ischemic periods, and have not examined what happens during reperfusion 37.
Interestingly, the EP2 receptor elicits a very different response in the context of neuroinflammatory conditions. Activation of the EP2 receptor in organotypic hippocampal slices can exacerbate lipopolysaccharide (LPS)-mediated neurotoxicity 68, in stark contrast to the neuroprotection elicited by EP2 activation in the setting of NMDA toxicity or OGD described above. Accumulating evidence now indicates a pro-inflammatory neurotoxic effect of EP2 receptor signaling in activated microglia in vitro 69-71 and in vivo in models of inflammatory neurodegeneration including models of Familial Alzheimer's disease, Familial ALS, and Parkinson's disease (PD) 72-74.
In brain, expression of the PGE2 EP2 receptor is highly inducible in cerebral cortex and hippocampus in the lipopolysaccharide (LPS) model of innate immunity 75. The bacterial endotoxin LPS has been used extensively to model the innate immune response and secondary neurotoxicity that occur with inflammation. LPS is a potent immunogen and binds microglial CD14 receptor/TLR4; this in turn causes activation of mitogen-activated protein kinases and NFkappaB that induce transcription of pro-inflammatory genes such as COX-2 and iNOS important in microglial activation. LPS stimulation also leads to activation of microglial NADPH oxidase, a major source of superoxide in inflammation.
The EP2 receptor plays a critical role in the generation of reactive oxygen species (ROS) and increased NOS activity in response to intraventricular administration of LPS 71. Following administration of LPS, EP2-/- mice fail to mount the inflammatory oxidative response seen in wild type mice, as quantified by levels of lipid peroxidation. Moreover, conditioned medium from EP2-/-microglia stimulated with LPS fails to induce secondary neurotoxicity as compared to wild type microglia 69. This suggests that PGE2 signaling through the microglial EP2 receptor plays a central role in the inflammatory oxidative response and secondary neurotoxicity. The EP2 receptor is similarly induced outside the CNS in peripheral macrophages and antigen presenting cells 75-79 where it regulates the expression of downstream inflammatory mediators, including TNF-α 76, 80, 81, IL-6 76, 82, MCP-1 83, ICAM 84, and iNOS 85, 86.
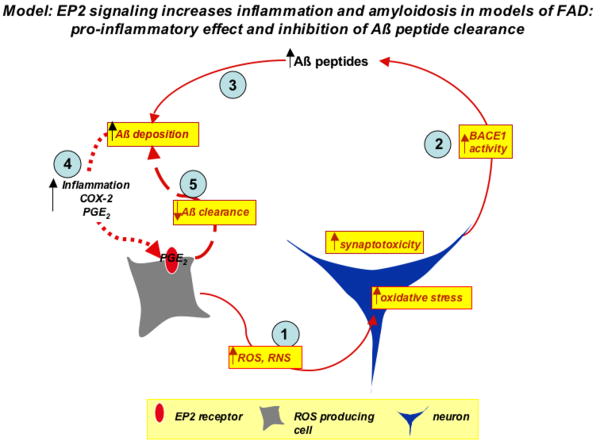
Recent studies indicate a significant overlap in molecular mechanisms between models of innate immune responses and models of neurodegeneration such as AD, ALS, and PD. The CD14-dependent innate immune response to LPS is relevant to the immune response to amyloid Aβ peptides for example, which are abundantly produced in murine transgenic models of Familial Alzheimer's disease (FAD). In this genetic model of FAD, microglial activation and elaboration of inflammatory mediators are in part CD14-dependent 70, 87. In the APPSwe-PS1ΔE9 (APPS) transgenic model, deletion of the EP2 receptor leads to significantly lower levels of lipid peroxidation 72, similar to what was found in the LPS model. In addition, deletion of the EP2 receptor in this model led to dramatic decreases in Aβ1-40 and Aβ1-42 peptide levels, a finding subsequently confirmed in an second APPSwe model 88. The reduction in Aβ peptide levels could be the result of EP2 mediated effects on Aβ peptide production and/or clearance. In terms of production of Aβ peptide, deletion of EP2 in the APPS model resulted in significant decreases in levels of β-CTF 72, the product of BACE1 cleavage of amyloid precursor protein (APP), suggesting that EP2 directly or indirectly regulated BACE-1 activity and Aβ peptide production. Direct stimulation of neurons expressing APPSwe-PS1ΔE9 transgenes with EP2 agonist did not increase Aβ peptide generation, lending support for an indirect effect. In addition, the EP2 receptor may also play a role in Aβ peptide phagocytosis and clearance in ex vivo preparations 89; in these studies, postnatal EP2-/-microglia demonstrated an enhanced ability to phagocytose physiologically aggregated Aβ peptide from post-mortem tissue sections of patients with AD. This enhanced phagocytic potential by microglial cells lacking EP2 is supported by studies in models of pulmonary infection where deletion of the EP2 receptor potentiates phagocytosis of bacteria by lung macrophages 90. Thus, the lower Aβ peptide levels in response to deletion of EP2 in APPS mice may be due to one or more factors, including a reduction of inflammatory oxidative stress, which secondarily can decrease BACE activity and Aβ peptide generation, or improved clearance from microglial phagocytosis and clearance of Aβ peptide (Figure 3). Experiments using ex vivo preparations of mesolimbic cortex from patients with Lewy body disease suggest that deletion of EP2 may enhance clearance of α-synuclein aggregates as well 73. In the same study, EP2-/- mice were also found to be more resistant to MPTP toxicity as measured by levels of striatal dopamine.
Figure 3.
The EP2 receptor functions both in promoting inflammation and inhibiting clearance of Aβ peptides in a transgenic model of Familial AD. A model is proposed in which EP2 signaling in glia (gray cell) results in the production of oxidant species from NADPH oxidase complex, iNOS, and COX-2. This inflammatory oxidative stress will injure neurons (blue cell; step 1). The increased oxidative stress in the neuron increases BACE-1 activity and generation of Aβ peptides (step 2), and will induce synaptic injury. Increased levels of Aβ peptides lead to Aβ peptide accumulation and deposition (step 3), which amplifies the inflammatory response (step 4) and further stimulates EP2 signaling on pro-inflammatory glial cells. Functional EP2 receptor inhibits Aβ peptide phagocytosis (step 5), further amplifying the cycle of amyloid accumulation and reactive inflammation.
Recent studies have confirmed a significant role of glial-mediated secondary neurotoxicity in motor neuron degeneration in the G93A SOD transgenic mouse model of Familial ALS 91-93. Inhibition of COX-2 in this model improves motor strength and survival, suggesting that downstream prostaglandin signaling pathways participate in disease progression 94. Genetic deletion of the PGE2 EP2 receptor in the G93A SOD model significantly improves motor strength and extends survival 74. Consistent with its role in promoting inflammatory injury, EP2 immunoreactivity in spinal cord is highly induced in microglia and astrocytes in aging G93A SOD but not wild type mice, and this is associated with increased expression of COX-2, iNOS, and NADPH oxidase subunits and increased neuronal lipid peroxidation and motor neuron loss. Deletion of the EP2 receptor in G93A SOD mice significantly reduced expression of these oxidative enzymes at the mRNA and protein levels 74 indicating that the EP2 receptor regulates a class of enzymes responsible for oxidative inflammatory neurotoxicity. EP2 signaling also appears to regulate a similar cassette of pro-inflammatory genes in the LPS and in the APPS models as well 71, 74, suggesting a conserved pro-inflammatory mechanism of action across multiple neuroinflammatory degenerative disease models.
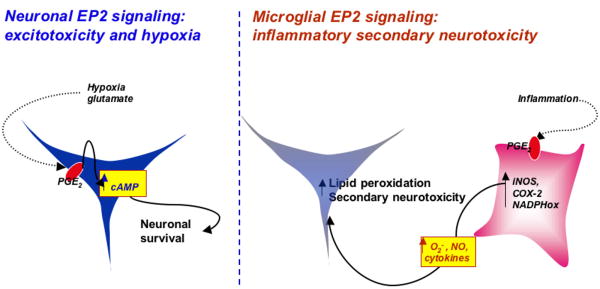
The data so far in the CNS therefore suggest a dichotomy of action of the EP2 receptor, depending on the type of injury (acute excitotoxicity vs chronic inflammation; Figure 4). EP2 signaling mediates significant neuroprotection selectively in acute models of cerebral ischemia and excitotoxicity, where neuronal EP2 receptor mediates protection by a PKA-dependent mechanism. In contrast, in models of chronic inflammation and neurodegeneration, microglial EP2 may lead to secondary neurotoxicity from increases of ROS producing enzymes and pro-inflammatory cytokines. Thus, the net effect of the EP2 signaling on neuronal viability in neurodegenerative disorders will depend on (1) the context of the stimulus and the degree of inflammatory response versus excitotoxicity in the specific injury model, (2) the specific cell type in which EP2 signaling is activated (neuronal versus glial), and (3) the cell-specific and model-specific downstream targets of EP2 signaling in these cells. This dichotomy of action is reminiscent of the dual function of the transcriptional regulator NFKappa-b, which regulates pro-survival and pro-plasticity genes in neurons, but regulates pro-inflammatory neurotoxic genes in microglia 95, 96.
Figure 4.
Model of opposing effects of EP2 signaling depending on the injury context. Basally, EP2 is expressed in neurons, and in the setting of glutamate toxicity or OGD, PGE2 signaling through the EP2 receptor is sufficient to rescue neurons, and rescues in a PKA dependent manner. In models of secondary neurotoxicity, where glial cells are activated by immunogens such as LPS or Aβ peptides to produce toxic cytokines and reactive oxygen and nitrogen species, glial EP2 promotes the expression of pro-inflammatory genes such as iNOS, COX-2, and components of the NADPH oxidase complex. These pro-inflammatory and pro-oxidant molecules injure neurons and lead to synaptic and neuronal degeneration.
C. The EP3 receptor
The EP3 receptor is coupled primarily to Gαi, however is certain conditions and because of differential splicing at the carboxy-terminus can also be coupled to Gq 97. EP3 is expressed mainly in subcortical structures, in particular the hypothalamus 98, consistent with one of its principal physiologic functions of regulating the febrile response 99, 100. In pathologic paradigms of neurological diseases, including neuroinflammatory disease models and models cerebral ischemia, the function of the EP3 receptor so far is not firmly defined. In vitro evidence in models of glutamate toxicity points to a protective function of EP3 neuronal signaling in both dispersed hippocampal neurons and organotypic slices, where EP3 receptor stimulation is associated with increased levels of the pro-survival phospho-AKT 53, 68. In vivo however, genetic deletion of the EP3 receptor does not alter infarct volume or behavioral outcome in a model of transient focal ischemia 66. A subsequent study noted a decrease in infarct volume in EP3-/- mice at 48 hours after ischemia, however this was not sustained and no difference was observed 96h after ischemia 101. These genetic findings are in contrast to a pharmacological study using a selective agonist of the EP3 receptor 102 in which intracerebroventricular administration of agonist before ischemia worsened infarct volume in a mouse model of transient focal ischemia 103. These conflicting data need to be interpreted in light of potential limitations both for genetic knockout modeling, which can be associated with compensatory effects to make up for loss of a critical protein, and pharmacologic strategies, where there can be off-target effects. In addition, in the case of the EP3 receptor in particular, genetic deletion and pharmacologic activation may not necessarily be complementary. The murine EP3 receptor consists of three distinct isoforms derived by alternative splicing of the carboxy terminus; these isoforms differ in downstream signaling pathways, desensitization, and constitutive activity 104-108. Thus, genetic deletion of EP3 results in total ablation of all three isoforms whereas administration of EP3 agonist may activate one or more isoforms depending on the cellular expression patterns of the EP3 isoforms, the brain penetration of the EP3 agonist, as well as the constitutive signaling properties and desensitization kinetics of the various isoforms.
In models of neuroinflammation, in particular the canonical LPS model of innate immunity, deletion of the EP3 receptor does not appear to alter levels of lipid peroxidation, as measured by F2-isoprostanes and F4-neuroprostanes or contribute significantly to oxidative inflammatory stress (T. Montine, personal communication). Its function in other models of inflammatory neurodegeneration, such as models of Familial ALS and AD are not yet known. It is important to note the relatively low abundance of EP3 receptor in brain regions typically involved in these models. Previous in situ hybridization studies 109 as well as immunostaining 110-112 of the EP3 receptor show limited expression in forebrain structures, but higher expression in thalamic, hypothalamic, and brain stem structures. However, EP3 expression may be dynamically regulated within these models in specific cell types, as suggested by studies demonstrating induction of EP3 expression in glial cells 113, 114.
D. The EP4 receptor
The EP4 receptor is positively coupled to cAMP production. In forebrain, the EP4 receptor is expressed basally in neurons and at low levels in endothelial cells 66. An expanding literature documents pro-survival effects of GPCRαs signaling, and of the EP4 receptor in particular, suggesting that the EP4 receptor, like its related EP2 receptor, may function to confer neuroprotection in excitotoxic or hypoxic paradigms. Anti-apoptotic effects of EP4 have been demonstrated in non-CNS models including gastric and intestinal injury 115-117, myocardial injury 118, and endothelial injury 119. In the CNS, in an vivo model of striatal excitotoxicity, pharmacological activation of EP4 showed protection 120, and in organotypic hippocampal slices, pharmacologic stimulation with selective EP4 agonist rescues CA1 pyramidal neurons death in vitro (Liang, Taniguchi, et al., unpublished data). Anti-apoptotic effects of EP4 in non-neuronal paradigms that may be relevant to neuronal EP4 protection involve direct effects of PKA activation 116, increased PI3-kinase mediated AKT phosphorylation 121, increased phosphorylation of BAD 122, 123, reduced Bax translocation to mitochondria 124, and increases in anti-apoptotic survivin 125, bcl-2 126, and inhibitor of apoptosis proteins (IAPs 127).
In addition to a neuronal EP4-mediated protective effect, the endothelial EP4 receptor mediates physiologic vasodilation in a number of vascular beds 128, 129. PGE2 signaling via endothelial EP4 receptors 130 can result in activation of endothelial NOS (eNOS) and NO-mediated relaxation of smooth muscle 131, 132. The EP4 as well as EP2 receptors contribute to the systemic vasodepressor response to PGE2 administration in mice 133. In human post-mortem samples of cerebral vessels, the EP4 receptor can induce vasodilation 134. An emerging concept in cerebrovascular research is the importance of a viable and functional neurovascular unit, which comprises the intricate complex of endothelial cells, astrocytes, neurons, pericytes, smooth muscle cells, and perivascular microglia. As with the other EP receptors, the specific distribution of the EP4 receptor in these cell types is not yet fully determined, much less the dynamics of expression of the EP receptors in these cells in different models of neurological disease. The proper functioning of the neurovascular unit ensures physiologic coupling of cerebral blood flow and neuronal activity. This coupling has been elegantly demonstrated in studies by Iadecola and colleagues where vasodilation in response to neuronal activity is dependent on COX-2 activity and prostaglandin signaling 135. Given the role of EP receptors in vasodilation or vasoconstriction in peripheral vasculature, it would be reasonable to hypothesize that these receptors may play an important role in modulating cerebral blood flow dynamics, for example in models of cerebral ischemia. The endothelium and cerebral vasculature play a critical role in cerebral ischemia, where reactive vasoconstriction in response to hypoxia are believed to enhance brain injury 136. The EP4 receptor, along with the EP2 receptor, appear to be induced in endothelium after ischemia and during reperfusion, suggesting a function of these vasodilatory receptors in cerebral blood flow in a model of transient focal cerebral ischemia 66. In addition, the importance of the endothelium and its function within the neurovascular unit is now emerging in disease models not typically associated with cerebrovascular injury, for example models of AD 137-142 and ALS 143.
In the periphery, the EP4 receptor has also been demonstrated to influence the inflammatory response in a context-dependent fashion. Anti-inflammatory actions of EP4 via inhibition of toxic cytokines are confirmed in models of inflammatory bowel disease 144-146, degenerative arthritis 147, atherosclerosis 148-150, and septic shock 151. The prostanoid PGE1, which can bind the EP2-4 receptors, blocks induction of intercellular adhesion molecule (ICAM) expression in various models of inflammation 152-154, an effect that is mediated via the EP4 receptor 152, 154, 155. These findings support a potential anti-inflammatory effect of EP4 in brain diseases characterized by an inflammatory response. Indeed, in unpublished studies, macrophage and microglial receptor activation appear important in modulating the CNS innate immune response in a model of peripheral LPS administration (Shi et al., unpublished data).
In summary, the PGE2 EP receptors can mediate toxic or pro-survival effects in models of neurological disease, depending on the specific injury context, and depending on the cell type(s) in which they are expressed. Further studies are required to understand the dynamics of EP receptor expression in ischemic, excitotoxic, and inflammatory models of neurologic disease, where selected EP signaling pathways can elicit neuroprotective or neurotoxic effects, pro- or anti-inflammatory effects, or vasodilatory/vasoconstrictive effects. The net effect of a particular EP signaling cascade will be dependent on the injury context, which involves different cell types and differing timing of receptor activation and signaling. Despite its complexity, because prostaglandins signal through G-protein coupled receptors, which represent the bulk of therapeutic targets, the EP receptors may offer a promising new targets in CNS therapeutics.
Acknowledgments
Funding support was provided by the American Federation for Aging Research, the Packard Center for ALS Research, the Muscular Dystrophy Association, the March of Dimes, the Alzheimer's Association, the Department of Defense PR043148, NINDS, and NIA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11(2):371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 2.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–96. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87(6):2851–7. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen S, Fusco FR, Yrjanheikki J, et al. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci U S A. 1997;94(12):6500–5. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem. 1996;66(1):6–13. doi: 10.1046/j.1471-4159.1996.66010006.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann WE, Andreasson KI, Isakson PC, Worley PF. Cyclooxygenases and the central nervous system. Prostaglandins. 1997;54:601–24. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 7.Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem Int. 2007;51(24):112–20. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17(8):2746–55. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama M, Uchimura K, Zhu RL, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95(18):10954–9. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dore S, Otsuka T, Mito T, et al. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54(2):155–62. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- 11.Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol (Berl) 2001;101(1):2–8. doi: 10.1007/s004010000251. [DOI] [PubMed] [Google Scholar]
- 12.Hoozemans JJ, Veerhuis R, Rozemuller AJ, Arendt T, Eikelenboom P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol Dis. 2004;15(3):492–9. doi: 10.1016/j.nbd.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998;87(2):319–24. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- 14.Montine TJ, Sidell KR, Crews BC, et al. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53(7):1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 15.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10 1:S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 16.in t' Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345(21):1515–21. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 17.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–32. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 18.Teismann P, Vila M, Choi DK, et al. COX-2 and neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2003;991:272–7. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- 19.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63(9):901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 20.Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS--a dual role? Exp Neurol. 2004;187(1):1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Candelario-Jalil E, Fiebich BL. Cyclooxygenase inhibition in ischemic brain injury. Curr Pharm Des. 2008;14(14):1401–18. doi: 10.2174/138161208784480216. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14(14):1443–51. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewett SJ, Bell SC, Hewett JA. Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol Ther. 2006;112(2):335–57. doi: 10.1016/j.pharmthera.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18(3):315–21. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- 25.Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102(3):577–86. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 27.Andersohn F, Schade R, Suissa S, Garbe E. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs and the risk of ischemic stroke: a nested case-control study. Stroke; a journal of cerebral circulation. 2006;37(7):1725–30. doi: 10.1161/01.STR.0000226642.55207.94. [DOI] [PubMed] [Google Scholar]
- 28.Chen LC, Ashcroft DM. Do selective COX-2 inhibitors increase the risk of cerebrovascular events? A meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2006;31(6):565–76. doi: 10.1111/j.1365-2710.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 29.Abraham NS, El-Serag HB, Hartman C, Richardson P, Deswal A. Cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment Pharmacol Ther. 2007;25(8):913–24. doi: 10.1111/j.1365-2036.2007.03292.x. [DOI] [PubMed] [Google Scholar]
- 30.Egan KM, Lawson JA, Fries S, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306(5703):1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 31.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50(5):470–9. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 32.Rudic RD, Brinster D, Cheng Y, et al. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res. 2005;96(12):1240–7. doi: 10.1161/01.RES.0000170888.11669.28. [DOI] [PubMed] [Google Scholar]
- 33.Candelario-Jalil E, Slawik H, Ridelis I, et al. Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: accumulation in Purkinje cells of the cerebellum. J Mol Neurosci. 2005;27(3):303–10. doi: 10.1385/JMN:27:3:303. [DOI] [PubMed] [Google Scholar]
- 34.Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12(2):225–9. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka Y, Furuyashiki T, Yamada K, et al. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A. 2005;102(44):16066–71. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitaoka S, Furuyashiki T, Nishi A, et al. Prostaglandin E2 acts on EP1 receptor and amplifies both dopamine D1 and D2 receptor signaling in the striatum. J Neurosci. 2007;27(47):12900–7. doi: 10.1523/JNEUROSCI.3257-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough L, Wu L, Haughey N, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24(1):257–68. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxcity. Brain Res. 1994;663:237–44. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- 39.Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C. Protection by prostaglandins from glutamate toxicity in cortical neurons. Neurochem Int. 1994;24(4):395–8. doi: 10.1016/0197-0186(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 40.Carlson NG. Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res. 2003;71:71–88. doi: 10.1002/jnr.10465. [DOI] [PubMed] [Google Scholar]
- 41.Gendron TF, Brunette E, Tauskela JS, Morley P. The dual role of prostaglandin E(2) in excitotoxicity and preconditioning-induced neuroprotection. Eur J Pharmacol. 2005;517(12):17–27. doi: 10.1016/j.ejphar.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Manabe Y, Anrather J, Kawano T, et al. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55(5):668–75. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad AS, Saleem S, Ahmad M, Dore S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89(1):265–70. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- 44.Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29(1):66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P, Qian L, Chou T, Iadecola C. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis. 2008;29(3):543–51. doi: 10.1016/j.nbd.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White ES, Atrasz RG, Dickie EG, et al. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32(2):135–41. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson NG, Rojas MA, Black JD, et al. Microglial inhibition of neuroprotection by antagonists of the EP1 prostaglandin E2 receptor. J Neuroinflammation. 2009;6:5. doi: 10.1186/1742-2094-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleem S, Li RC, Wei G, Dore S. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85(11):2433–40. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jadhav V, Jabre A, Lin SZ, Lee TJ. EP1- and EP3-receptors mediate prostaglandin E2-induced constriction of porcine large cerebral arteries. J Cereb Blood Flow Metab. 2004;24(12):1305–16. doi: 10.1097/01.WCB.0000139446.61789.14. [DOI] [PubMed] [Google Scholar]
- 50.Purdy KE, Arendshorst WJ. EP(1) and EP(4) receptors mediate prostaglandin E(2) actions in the microcirculation of rat kidney. Am J Physiol Renal Physiol. 2000;279(4):F755–64. doi: 10.1152/ajprenal.2000.279.4.F755. [DOI] [PubMed] [Google Scholar]
- 51.Carrasco E, Casper D, Werner P. PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J Neurosci Res. 2007;85(14):3109–17. doi: 10.1002/jnr.21425. [DOI] [PubMed] [Google Scholar]
- 52.Carrasco E, Casper D, Werner P. Dopaminergic neurotoxicity by 6-OHDA and MPP+: differential requirement for neuronal cyclooxygenase activity. J Neurosci Res. 2005;81(1):121–31. doi: 10.1002/jnr.20541. [DOI] [PubMed] [Google Scholar]
- 53.Bilak M, Wu L, Wang Q, et al. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann Neurol. 2004;56(2):240–8. doi: 10.1002/ana.20179. [DOI] [PubMed] [Google Scholar]
- 54.Zhu P, Genc A, Zhang X, Zhang J, Bazan NG, Chen C. Heterogeneous expression and regulation of hippocampal prostaglandin E2 receptors. J Neurosci Res. 2005;81(6):817–26. doi: 10.1002/jnr.20597. [DOI] [PubMed] [Google Scholar]
- 55.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7(6):643–50. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 56.Burks SR, Wright CL, McCarthy MM. Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience. 2007;146(3):1117–27. doi: 10.1016/j.neuroscience.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5(1):34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 58.Harvey RJ, Depner UB, Wassle H, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304(5672):884–7. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 59.Reinold H, Ahmadi S, Depner UB, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115(3):673–9. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25(43):9858–70. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J Neurosci. 2006;26(40):10209–21. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savonenko A, Munoz P, Melnikova T, Wang Q, Liang X, Breyer RM, Montine TJ, Kirkwood A, Andreasson K. Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Exp Neurol. doi: 10.1016/j.expneurol.2009.01.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57(5):758–61. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad AS, Zhuang H, Echeverria V, Dore S. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J Neurotrauma. 2006;23(12):1895–903. doi: 10.1089/neu.2006.23.1895. [DOI] [PubMed] [Google Scholar]
- 65.Carrasco E, Werner P, Casper D. Prostaglandin receptor EP2 protects dopaminergic neurons against 6-OHDA-mediated low oxidative stress. Neurosci Lett. 2008;441(1):44–9. doi: 10.1016/j.neulet.2008.05.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Liang X, Wang Q, Breyer RM, McCullough L, Andreasson K. Misoprostol, an anti-ulcer agent and PGE(2) receptor agonist, protects against cerebral ischemia. Neurosci Lett. 2008;438(2):210–5. doi: 10.1016/j.neulet.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kennedy CR, Zhang Y, Brandon S, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5(2):217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 68.Wu L, Wang Q, Liang X, Andreasson K. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neurosci Lett. 2007;421(3):253–8. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52(1):70–7. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- 70.Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J Neuroinflammation. 2004;1(1):20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83(2):463–70. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 72.Liang X, Wang Q, Hand T, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J Neurosci. 2005;25(44):10180–7. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin J, Shie FS, Liu J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang X, Wang Q, Shi J, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008;64(3):304–14. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Rivest S. Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur J Neurosci. 1999;11(8):2651–68. doi: 10.1046/j.1460-9568.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 76.Akaogi J, Yamada H, Kuroda Y, Nacionales DC, Reeves WH, Satoh M. Prostaglandin E2 receptors EP2 and EP4 are up-regulated in peritoneal macrophages and joints of pristane-treated mice and modulate TNF-alpha and IL-6 production. J Leukoc Biol. 2004;76(1):227–36. doi: 10.1189/jlb.1203627. [DOI] [PubMed] [Google Scholar]
- 77.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73(6):756–63. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 78.Hubbard NE, Lee S, Lim D, Erickson KL. Differential mRNA expression of prostaglandin receptor subtypes in macrophage activation. Prostaglandins Leukot Essent Fatty Acids. 2001;65(56):287–94. doi: 10.1054/plef.2001.0327. [DOI] [PubMed] [Google Scholar]
- 79.Kubo S, Takahashi HK, Takei M, et al. E-prostanoid (EP)2/EP4 receptor-dependent maturation of human monocyte-derived dendritic cells and induction of helper T2 polarization. J Pharmacol Exp Ther. 2004;309(3):1213–20. doi: 10.1124/jpet.103.062646. [DOI] [PubMed] [Google Scholar]
- 80.Fennekohl A, Sugimoto Y, Segi E, Maruyama T, Ichikawa A, Puschel GP. Contribution of the two Gs-coupled PGE2-receptors EP2-receptor and EP4-receptor to the inhibition by PGE2 of the LPS-induced TNFalpha-formation in Kupffer cells from EP2-or EP4-receptor-deficient mice. Pivotal role for the EP4-receptor in wild type Kupffer cells. J Hepatol. 2002;36(3):328–34. doi: 10.1016/s0168-8278(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 81.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223(2):120–32. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 82.Treffkorn L, Scheibe R, Maruyama T, Dieter P. PGE2 exerts its effect on the LPS-induced release of TNF-alpha, ET-1, IL-1alpha, IL-6 and IL-10 via the EP2 and EP4 receptor in rat liver macrophages. Prostaglandins Other Lipid Mediat. 2004;74(14):113–23. doi: 10.1016/j.prostaglandins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Largo R, Diez-Ortego I, Sanchez-Pernaute O, et al. EP2/EP4 signalling inhibits monocyte chemoattractant protein-1 production induced by interleukin 1beta in synovial fibroblasts. Ann Rheum Dis. 2004;63(10):1197–204. doi: 10.1136/ard.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noguchi K, Iwasaki K, Shitashige M, et al. Downregulation of lipopolysaccharide-induced intercellular adhesion molecule-1 expression via EP2/EP4 receptors by prostaglandin E2 in human fibroblasts. Inflammation. 2001;25(2):75–81. doi: 10.1023/a:1007110304044. [DOI] [PubMed] [Google Scholar]
- 85.Timoshenko AV, Lala PK, Chakraborty C. PGE2-mediated upregulation of iNOS in murine breast cancer cells through the activation of EP4 receptors. Int J Cancer. 2004;108(3):384–9. doi: 10.1002/ijc.11575. [DOI] [PubMed] [Google Scholar]
- 86.Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G. Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia. 1997;19:152–60. [PubMed] [Google Scholar]
- 87.Fassbender K, Walter S, Kuhl S, et al. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. Faseb J. 2004;18(1):203–5. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 88.Hoshino T, Nakaya T, Homan T, et al. Involvement of prostaglandin E2 in production of amyloid-beta peptides both in vitro and in vivo. J Biol Chem. 2007;282(45):32676–88. doi: 10.1074/jbc.M703087200. [DOI] [PubMed] [Google Scholar]
- 89.Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166(4):1163–72. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173(1):559–65. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 91.Boillee S, Yamanaka K, Lobsiger CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–92. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 92.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10(5):608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drachman DB, Frank K, Dykes-Hoberg M, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52(6):771–8. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 96.Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28(1):37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 98.Vasilache AM, Andersson J, Nilsberth C. Expression of PGE2 EP3 receptor subtypes in the mouse preoptic region. Neurosci Lett. 2007;423(3):179–83. doi: 10.1016/j.neulet.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 99.Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–3. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 100.Ushikubi F, Segi E, Sugimoto Y, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395(6699):281–4. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 101.Saleem S, Kim YT, Maruyama T, Narumiya S, Dore S. Reduced acute brain injury in PGE(2) EP3 receptor-deficient mice after cerebral ischemia. J Neuroimmunol. 2009;208(12):87–93. doi: 10.1016/j.jneuroim.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cao J, Shayibuzhati M, Tajima T, Kitazawa T, Taneike T. In vitro pharmacological characterization of the prostanoid receptor population in the non-pregnant porcine myometrium. Eur J Pharmacol. 2002;442(12):115–23. doi: 10.1016/s0014-2999(02)01489-9. [DOI] [PubMed] [Google Scholar]
- 103.Ahmad M, Ahmad AS, Zhuang H, Maruyama T, Narumiya S, Dore S. Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J Neuroimmunol. 2007;184(12):172–9. doi: 10.1016/j.jneuroim.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bilson HA, Mitchell DL, Ashby B. Human prostaglandin EP3 receptor isoforms show different agonist-induced internalization patterns. FEBS Lett. 2004;572(13):271–5. doi: 10.1016/j.febslet.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 105.Hasegawa H, Katoh H, Yamaguchi Y, Nakamura K, Futakawa S, Negishi M. Different membrane targeting of prostaglandin EP3 receptor isoforms dependent on their carboxy-terminal tail structures. FEBS Lett. 2000;473(1):76–80. doi: 10.1016/s0014-5793(00)01508-8. [DOI] [PubMed] [Google Scholar]
- 106.Hasegawa H, Negishi M, Katoh H, Ichikawa A. Two isoforms of prostaglandin EP3 receptor exhibiting constitutive activity and agonist-dependent activity in Rho-mediated stress fiber formation. Biochem Biophys Res Commun. 1997;234(3):631–6. doi: 10.1006/bbrc.1997.6655. [DOI] [PubMed] [Google Scholar]
- 107.Hasegawa H, Negishi M, Ichikawa A. Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J Biol Chem. 1996;271(4):1857–60. doi: 10.1074/jbc.271.4.1857. [DOI] [PubMed] [Google Scholar]
- 108.An S, Yang J, So SW, Zeng L, Goetzl EJ. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33(48):14496–502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 109.Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. Distribution of the EP3 prostaglandin E(2) receptor subtype in the rat brain: relationship to sites of interleukin-1-induced cellular responsiveness. J Comp Neurol. 2000;428(1):5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura K, Li YQ, Kaneko T, Katoh H, Negishi M. Prostaglandin EP3 receptor protein in serotonin and catecholamine cell groups: a double immunofluorescence study in the rat brain. Neuroscience. 2001;103(3):763–75. doi: 10.1016/s0306-4522(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol. 2000;42:543–69. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 112.Nakamura K, Kaneko T, Yamashita Y, et al. Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett. 1999;260(2):117–20. doi: 10.1016/s0304-3940(98)00962-8. [DOI] [PubMed] [Google Scholar]
- 113.Waschbisch A, Fiebich BL, Akundi RS, et al. Interleukin-1 beta-induced expression of the prostaglandin E-receptor subtype EP3 in U373 astrocytoma cells depends on protein kinase C and nuclear factor-kappaB. J Neurochem. 2006;96(3):680–93. doi: 10.1111/j.1471-4159.2005.03599.x. [DOI] [PubMed] [Google Scholar]
- 114.Slawik H, Volk B, Fiebich B, Hull M. Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochem Int. 2004;45(5):653–60. doi: 10.1016/j.neuint.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 115.Hatazawa R, Tanigami M, Izumi N, Kamei K, Tanaka A, Takeuchi K. Prostaglandin E(2) stimulates VEGF expression in primary rat gastric fibroblasts through EP4 receptors. Inflammopharmacology. 2007;15(5):214–7. doi: 10.1007/s10787-007-1595-z. [DOI] [PubMed] [Google Scholar]
- 116.Hoshino T, Tsutsumi S, Tomisato W, Hwang HJ, Tsuchiya T, Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J Biol Chem. 2003;278(15):12752–8. doi: 10.1074/jbc.M212097200. [DOI] [PubMed] [Google Scholar]
- 117.Jiang GL, Nieves A, Im WB, Old DW, Dinh DT, Wheeler L. The prevention of colitis by E Prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration. J Pharmacol Exp Ther. 2007;320(1):22–8. doi: 10.1124/jpet.106.111146. [DOI] [PubMed] [Google Scholar]
- 118.Xiao CY, Yuhki K, Hara A, et al. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109(20):2462–8. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 119.Rao R, Redha R, Macias-Perez I, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282(23):16959–68. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 120.Ahmad AS, Ahmad M, de Brum-Fernandes AJ, Dore S. Prostaglandin EP4 receptor agonist protects against acute neurotoxicity. Brain Res. 2005;1066(12):71–7. doi: 10.1016/j.brainres.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 121.Liou JY, Ellent DP, Lee S, et al. Cyclooxygenase-2-derived prostaglandin e2 protects mouse embryonic stem cells from apoptosis. Stem Cells. 2007;25(5):1096–103. doi: 10.1634/stemcells.2006-0505. [DOI] [PubMed] [Google Scholar]
- 122.Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67(5):2015–21. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sugiura H, Liu X, Togo S, et al. Prostaglandin E(2) protects human lung fibroblasts from cigarette smoke extract-induced apoptosis via EP(2) receptor activation. J Cell Physiol. 2007;210(1):99–110. doi: 10.1002/jcp.20825. [DOI] [PubMed] [Google Scholar]
- 124.Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114(11):1676–85. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baratelli F, Krysan K, Heuze-Vourc'h N, et al. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol. 2005;78(2):555–64. doi: 10.1189/jlb.1004569. [DOI] [PubMed] [Google Scholar]
- 126.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–6. [PubMed] [Google Scholar]
- 127.Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A. 2003;100(15):8921–6. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108(1):25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35(5):1129–34. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 130.Hristovska AM, Rasmussen LE, Hansen PB, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50(3):525–30. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- 131.Dumont I, Hou X, Hardy P, et al. Developmental regulation of endothelial nitric oxide synthase in cerebral vessels of newborn pig by prostaglandin E(2) J Pharmacol Exp Ther. 1999;291(2):627–33. [PubMed] [Google Scholar]
- 132.Najarian T, Marrache AM, Dumont I, et al. Prolonged hypercapnia-evoked cerebral hyperemia via K(+) channel- and prostaglandin E(2)-dependent endothelial nitric oxide synthase induction. Circ Res. 2000;87(12):1149–56. doi: 10.1161/01.res.87.12.1149. [DOI] [PubMed] [Google Scholar]
- 133.Audoly LP, Ma L, Feoktistov I, de Foe SK, Breyer MD, Breyer RM. Prostaglandin E-prostanoid-3 receptor activation of cyclic AMP response element-mediated gene transcription. J Pharmacol Exp Ther. 1999;289(1):140–8. [PubMed] [Google Scholar]
- 134.Davis RJ, Murdoch CE, Ali M, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141(4):580–5. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20(2):763–70. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 137.Iadecola C. Atherosclerosis and neurodegeneration: unexpected conspirators in Alzheimer's dementia. Arterioscler Thromb Vasc Biol. 2003;23(11):1951–3. doi: 10.1161/01.ATV.0000102660.99744.85. [DOI] [PubMed] [Google Scholar]
- 138.Iadecola C, Zhang F, Niwa K, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2(2):157–61. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 139.Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24(3):334–42. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- 140.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64(2):168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 142.Study, N.G.M.R.C.C.F.a.A. Pathological correlates of late-onset dementia in a multicentre, community-based population in England Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 143.Zhong Z, Deane R, Ali Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11(4):420–2. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoang B, Trinh A, Birnbaumer L, Edwards RA. Decreased MAPK- and PGE2-dependent IL-11 production in Gialpha2-/- colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1511–9. doi: 10.1152/ajpgi.00307.2006. [DOI] [PubMed] [Google Scholar]
- 145.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109(7):883–93. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nitta M, Hirata I, Toshina K, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunology. 2002;56(1):66–75. doi: 10.1046/j.1365-3083.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 147.Fushimi K, Nakashima S, You F, Takigawa M, Shimizu K. Prostaglandin E2 downregulates TNF-alpha-induced production of matrix metalloproteinase-1 in HCS-2/8 chondrocytes by inhibiting Raf-1/MEK/ERK cascade through EP4 prostanoid receptor activation. J Cell Biochem. 2007;100(3):783–93. doi: 10.1002/jcb.21099. [DOI] [PubMed] [Google Scholar]
- 148.Minami M, Shimizu K, Okamoto Y, et al. Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J Biol Chem. 2008;283(15):9692–703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Takayama K, Sukhova GK, Chin MT, Libby P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ Res. 2006;98(4):499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- 150.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277(46):44147–54. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 151.Sakamoto A, Matsumura J, Mii S, Gotoh Y, Ogawa R. A prostaglandin E2 receptor subtype EP4 agonist attenuates cardiovascular depression in endotoxin shock by inhibiting inflammatory cytokines and nitric oxide production. Shock (Augusta, Ga. 2004;22(1):76–81. doi: 10.1097/01.shk.0000129338.99410.5d. [DOI] [PubMed] [Google Scholar]
- 152.Takahashi HK, Iwagaki H, Tamura R, et al. Differential effect of prostaglandins E1 and E2 on lipopolysaccharide-induced adhesion molecule expression on human monocytes. Eur J Pharmacol. 2005;512(23):223–30. doi: 10.1016/j.ejphar.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi HK, Xue D, Iwagaki H, et al. Prostaglandin E1-initiated immune regulation during human mixed lymphocyte reaction. Clin Immunol. 2005;115(1):85–92. doi: 10.1016/j.clim.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 154.Takahashi HK, Iwagaki H, Tamura R, et al. Unique regulation profile of prostaglandin e1 on adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. J Pharmacol Exp Ther. 2003;307(3):1188–95. doi: 10.1124/jpet.103.056432. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi HK, Iwagaki H, Yoshino T, et al. Prostaglandin E(2) inhibits IL-18-induced ICAM-1 and B7.2 expression through EP2/EP4 receptors in human peripheral blood mononuclear cells. J Immunol. 2002;168(9):4446–54. doi: 10.4049/jimmunol.168.9.4446. [DOI] [PubMed] [Google Scholar]