Abstract
The intestinal epithelium maintains a state of controlled inflammation despite continuous contact with Gram-negative commensal bacteria and lipopolysaccharide (LPS) on its luminal surface. Recognition of LPS by the TLR4/MD-2 complex results in proinflammatory gene expression and cytokine secretion in intestinal epithelial cells (IEC). We have shown that IEC express low levels of MD-2 and TLR4 and are poorly responsive to LPS. In this study, we did a comprehensive analysis to understand the immune-mediated and epigenetic mechanisms by which IEC down-regulate MD-2 expression. Expression of MD-2 and TLR4 mRNA was examined in human inflammatory bowel disease and intestinal epithelial cell lines (T84, HT-29, Caco-2). Nuclear factor-κB transcriptional activation was used as a measure of LPS responsiveness. Intestinal epithelial cellsin patients with IBD exhibited increased expression of MD-2 and TLR4 mRNA. Lipopolysaccharide responsiveness in IEC was polarized to the basolateral membrane. Bisulfite sequencing of the MD-2 promoter demonstrated methylation of CpG dinucleotides. Inhibition of methylation by 5-azacytidine and histone deactylation by trichostatin A, two forms of epigenetic silencing, resulted in increased mRNA expression of MD-2 in IEC. These results demonstrate various molecular mechanisms by which IEC down-regulate MD-2 and thereby protect against dysregulated inflammation to commensal bacteria in the intestinal lumen.
Keywords: MD-2, Toll-like Receptor 4 (TLR4), Intestinal Epithelial Cells, Lipopolysaccharide, Methylation, Histone deacetylation
INTRODUCTION
The intestinal epithelium serves as a protective barrier contiguous with a myriad of bacteria and bacterial products along its apical surface and the mucosal immune system along its basolateral surface.1, 2 However, the epithelium is not an inert barrier as intestinal epithelial cells (IEC) are active participants in the innate immune response to bacterial pathogens, capable of pro-inflammatory gene expression, cytokine secretion, and recruitment of inflammatory cells to the site of epithelial injury.3-5 Despite the high concentration of diverse luminal bacteria continuously in contact with the epithelium, IEC tightly regulate their response to commensal bacteria, maintaining a controlled state of inflammation. Thus, the intestinal epithelium is intricately involved in a fine balance co-existing with commensal bacteria and defending against invading pathogens.
In contrast, human idiopathic inflammatory bowel disease, i.e. ulcerative colitis and Crohn’s disease, is characterized by chronic intestinal inflammation in the absence of a specific pathogen. Animal and human models of inflammatory bowel disease have demonstrated the requirement of nonpathogenic, commensal bacteria and Th1 and Th17 cytokines for the initiation of chronic inflammation.6-9 Furthermore, chronic intestinal inflammation is a risk factor for malignancies of the small and large intestine and a clear link has been shown between inflammatory bowel disease and colorectal cancer.10-12 Studies involving animal models of colitis have implicated intestinal bacteria as a requisite for colitis associated cancer (CAC).13, 14 These findings suggest that human inflammatory bowel disease and possibly CAC may result from dysregulated inflammation in response to the normal intestinal flora due to aberrant host-microbial interactions. A better understanding of the interplay between bacteria and the intestinal epithelium may lead to improved strategies for maintaining normal host-microbial interactions in patients with IBD.
In recent years, many studies have evaluated the mechanisms involved in IEC resistance to chronic inflammation in the presence of Gram-positive and Gram-negative bacteria that constitute the normal intestinal flora. Specifically, we have focused our efforts on the role of toll-like receptor 4 (TLR4) signaling in the intestine to better understand the relationship between IEC and bacteria during inflammatory states. Others have shown that patients with inflammatory bowel disease have increased expression of TLR4 and we have found that increased TLR4 expression is required for epithelial proliferation after injury and for the development of colitis-associated cancer.15-18 In regards to TLR4 signaling, we have shown that IEC are poorly responsive to lipopolysaccharide (LPS), an immunostimulatory component of the Gram-negative bacterial cell wall.19, 20 Lipopolysaccharide is a pro-inflammatory pathogen-associated molecular pattern responsible for systemic effects resulting in septic shock.21 Recognition of LPS begins with its transfer to CD14 via the opsonin LPS-binding protein.22, 23 The LPS/CD14 interaction stimulates the pattern recognition receptor TLR4, the sole sensor for LPS, via MD-2 mediated recognition of LPS and activation of TLR4.24, 25 MD-2, an essential co-receptor, binds to the extracellular domain of TLR4 resulting in NF-κB activation and induction of IRF-3 resulting in secretion of pro-inflammatory cytokines.26-31
We have previously demonstrated using three intestinal epithelial cell lines that IEC respond poorly to purified, protein-free LPS as measured by NF-κB activation and IL-8 secretion and express low levels of TLR4 and MD-2.19, 20 In addition, we have described that expression of both TLR4 and MD-2 in IEC reconstitutes LPS responsiveness whereas expression of either gene individually is not sufficient to induce LPS responsiveness.19 Finally, we and others have shown that MD-2 expression is induced by Th1 cytokines, specifically IFN-γ mediated MD-2 promoter induction, via a STAT dependent mechanism.20, 32
Previous studies have attempted to elucidate the mechanisms by which IEC regulate LPS mediated TLR4 activation, yet relatively little is known about the molecular mechanisms involved in intestinal epithelial cell regulation of MD-2. A number of studies have described the importance of MD-2 in LPS mediated activation of TLR4. MD-2 knockout mice are phenotypically similar to TLR4 knockout mice exhibiting decreased responsiveness to LPS.33 The extracellular domain of TLR4 is unable to bind LPS without MD-2, which directly interacts with LPS and serves as the ligand binding component of the TLR4/MD-2 complex.34-40 In addition, TLR4 surface expression and LPS recognition are mediated by MD-2.33 Lipopolysaccharide signaling requires ligand induced TLR4 oligomerization on the surface of cells. This clustering of TLR4 on the cell surface is regulated by MD-2 expression.41 Recently, Park et al. have determined the crystal structure of the TLR4-MD-2-LPS complex and demonstrated that LPS binding to MD-2 induces a multimer composed of two copies of the TLR4-MD-2-LPS complex. 42 These findings depict the crucial role MD-2 plays in TLR4 signaling and LPS responsiveness and suggests that MD-2 is an integral molecule in the inflammatory response to Gram-negative bacteria.
Taken together, these findings highlight the importance of MD-2 signaling in LPS responsiveness and demonstrate that both TLR4 and MD-2 are required by intestinal epithelial cells to activate pro-inflammatory cytokines in response to Gram-negative bacteria. Thus, down-regulation of one or both of the components of the LPS signaling TLR4/MD-2 complex in IEC may serve as a mechanism by which the intestinal epithelium maintains a state of controlled inflammation towards commensal Gram-negative bacteria and their products. Therefore, a careful analysis of MD-2 regulation in IEC is warranted and in this study, we have taken a comprehensive approach to examining various ways in which MD-2 itself is regulated in IEC. Our findings may lead to novel approaches to manipulate host-microbial interactions in patients with IBD.
METHODS AND MATERIALS
Cells and reagents
Intestinal epithelial cell lines Caco-2, HT-29, and T84 were obtained from ATCC (Rockville, MD). Subconfluent monolayers of these cell lines were kept in a humidified incubator at 37°C with 5% CO2. T84 were cultured on 12-mm Transwell polycarbonate membranes (Costar 3401) and maintained in DMEM/F-12 (Invitrogen) with 5% Pen/Strep, 5% l-glutamine, supplemented with 5% fetal bovine serum (FBS) as previously described.43 T84 cells were used between passages 16 and 35.44 Caco-2 were maintained in minimum essential medium (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 0.1 mm nonessential amino acids, 1 mm sodium pyruvate, and 5% Pen/Strep. HT-29 were maintained in McCoy’s 5A medium supplemented with 10% FBS and 5% Pen/Strep. Immortalized human dermal endothelial cells (HMEC)45 were cultured in MCDB-131 medium supplemented with 10% FBS, 2 mm glutamine, and 100 μg/ml penicillin and streptomycin in 24-well plates, and used between passages 10 and 14, as described previously.45-47 Highly purified, phenol-water-extracted Escherichia coli K235 LPS (<0.008% protein), which was prepared according to the method of McIntire et al. 48 was obtained from Stefanie N. Vogel (Uniformed Services University of the Health Sciences, Bethesda, MD).49, 50 The purity of this LPS preparation has been previously demonstrated.48, 51, 52 5-Azacytidine and trichostatin A (TSA) were purchased from Sigma Chemical Company (St. Louis, MO). Cells were treated with one of the following regimens: 5-azacytidine (0, 2, 10, 20 μM) daily for 4 days, trichostatin A 100 or 500 ng/ml for 24 h, or the vehicle control. Cells were then harvested for RNA. GAPDH was used as an internal control.
QRT-PCR analysis
Total RNA was isolated from T84, Caco-2, HT-29, and HMEC with a Qiagen kit following manufacturer’s instruction and treated with RNase free DNase I. The MMLV Preamplification system (Life Technologies) was used for the reverse transcription reaction. PCR amplification was performed with Taq polymerase (Perkin-Elmer) for 38 cycles at 95°C for 45 s, 54°C for 45 s, and 72°C for 1 min (for TLR2 and TLR4) as described earlier,47 and for 38 cycles at 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s (for MD-2). The TLR4 oligonucleotide primers used for RT-PCR were described earlier.47 The oligonucleotide primers for MD-2 were as follows: forward, GAAGCTCAGAAGCAGTATTGGGTC; reverse, GGTTGGTGTAGGATGACAAACTCC. GAPDH primers were obtained from Clontech and used as per manufacturer’s instructions. ImageJ 1.41 software was used as a densitometer to quantify intensity of PCR products.
MD-2 promoter studies
The MD-2 gene is located on the minus strand of chromosome 8. GenBank was searched for human MD-2 and yielded two accession numbers which were used to create primers to amplify a 1013-bp sequence (−1 kb) upstream of the ATG start site as previously described:20 primer1, GCTTTACAAATGCAAAGAGGATCAG (same primer for −1 kb and deletion mutants), −1 kb = primer2 reverse, CATGGCCTGTTAGGAATCTGGT; −728 deletion mutant = GTTGAGCACACACACAC. T84 cells were transfected with −1 kB-MD-2 pGL3 or deletion mutants. After transfection (24 h), T84 cells were stimulated with IFN-γ 40ng/ml for 5 h and lysed for luciferase measurements.
Transient gene expression assays
T84 cells were plated at a density of 150,000 cells/well in 12-well transwell plates 24 h before transfection. Cells were transfected the following day with Fugene 6 transfection reagent (Roche Biomedical Laboratories, Burlington, NC) as per manufacturer’s instructions and as described earlier.46, 47 The reporter gene ELAM-NF-κB-luciferase (0.4 μg), pCDNA3 empty vector (0.3–0.6 μg), Flag-tagged wild-type human TLR4 (0.3 μg) or human MD-2 cDNA (0.3 μg) constructs were cotransfected as indicated in the figure legend. After 16 h transfection, cells were stimulated for 5 h with LPS (50 ng/ml) to the apical or basolateral well. Cells were then lysed in 200 μl of reporter lysis buffer (Promega, Madison, WI), and luciferase activity was measured with a Promega firefly luciferase kit on a Wallac 1450 Microbeta liquid scintillation counter (PerkinElmer, Foster City, CA). Transfection efficiency was determined by assaying for β-galactosidase activity and data are normalized with β-galactosidase activity using a colorimetric method (Promega) as previously described.46 Data shown are mean ± SD of three or more independent experiments, and are reported as fold-induction in relative luciferase activity over cells transfected with a control vector.
Bisulfite-treated genomic DNA sequencing
Genomic DNA was isolated from cell lines and human biopsied specimens. Genomic DNA (10 ug) was chemically modified by sodium bisulfite treatment using the EZ DNA Methylation kit (Zymo Research, Orange, CA, USA) converting unmethylated cytosines to uracils while methylated cytosines remained unchanged. Following bisulfate treatment, the treated DNA was amplified via PCR using the following primer sequences: forward, GGAGGTTAACAGCAGGTGAGCGAGCATTAC; reverse, GTAGTGCATGCCTGTAATCCCAGCTATTC carried out at an annealing temperature of 62°C. The resulting DNA products were cloned using the TOPO TA Cloning Kit (Invitrogen). The cloned PCR products were then sequenced using an automated sequencer (Applied Biosystems, Foster City, CA, USA).
Laser capture microscopy
Frozen sections derived from human intestinal resections were obtained under the auspices of Cedars-Sinai Medical Center IRB 1465. The tissue used for this study included uninvolved areas of intestine from patients with inflammatory bowel disease or colon cancer. Slides were gently fixed in 100% ethanol followed by a light hematoxylin and eosin staining. An Arcturus laser capture microscope was used to microdissect the tissue. Briefly, intestinal epithelial cells were identified based on appearance and location, microdissected, and captured on a microcentrifuge cap. The lamina propria (LP) was separately microdissected and captured from each intestinal specimen. Photo documentation was obtained before and after dissection. Total RNA was made by incubating cells at −80 °C overnight in lysis buffer containing 50 mm Tris-HCl, pH 8.0, 100 mm NaCl, 5 mm MgCl2, 0.5% Triton X-100, 1 mm dithiothreitol, and 1000 units/ml RNase inhibitor. After lysis and centrifugation, total RNA was followed directly by reverse transcription using random hexamers and Superscript II (Invitrogen). The cDNA generated was amplified as described above.
Statistical analysis
Student’s t tests, standard deviation, and standard errors were performed using the statistics package within Microsoft Excel. Data were analyzed using Kruskal-Wallis one-way analysis of variance followed by Mann-Whitney U multiple comparison test. P-Values were considered statistically significant when <0.05. Statistically significant differences are denoted as *P < 0.05.
RESULTS
Intestinal epithelial cells in human inflammatory bowel disease express increased levels of MD-2 and TLR4
We have previously demonstrated that MD-2 expression is low in both normal human colonic epithelial cells and intestinal epithelial cell lines and others have shown that IEC from normal human intestinal biopsies express low levels of TLR4.15, 19, 20 Previous reports describe increased expression of TLR4 in IBD patients but differ in regards to which cells exhibit increased TLR4 expression, namely IEC versus lamina propria cells.15, 16 We hypothesized that expression of MD-2 is normally down-regulated in intestinal epithelial cells and increases in response to inflammation in patients with inflammatory bowel disease. We examined MD-2 and TLR4 mRNA expression in both intestinal epithelial cells and lamina propria cells from the small intestine and colon in patients with inflammatory bowel disease and controls. We utilized laser capture microscopy to microdissect intestinal epithelial cells and lamina propria cells from intestinal tissue from patients undergoing surgical resections for cancer, diverticulitis, or inflammatory bowel disease. Using quantitative real-time PCR to examine MD-2 and TLR4 mRNA expression, we found significantly increased levels of MD-2 and TLR4 in both intestinal epithelial cells and lamina propria cells from patients with active ulcerative colitis or Crohn’s disease (Fig. 1). By contrast, samples from normal colon or patients with diverticulitis did not demonstrate an increase in TLR4 or MD-2 expression. These data support the hypothesis that intestinal epithelial cells normally down-regulate TLR4 and MD-2 expression but that levels are increased in individuals with inflammatory bowel disease. In addition, these findings demonstrate that both IEC and LP cells have increased TLR4 mRNA expression in IBD and suggest that differences in the underlying inflammatory response between IBD and other forms of inflammation may result in the differential expression of TLR4 and MD-2.
Fig. 1.
MD-2 and TLR4 mRNA expression are increased in human inflammatory bowel disease. Quantitative expression of MD-2 and TLR4 by human colonic epithelial cells. Colonic epithelial cells and lamina propria cells were isolated from sections of small bowel or colon as indicated. In each case, lamina propria and epithelial cell samples were matched. Expression of MD-2 and TLR4 mRNA were analyzed by real-time PCR using TaqMan probes. β-Actin was used as an internal control for all reactions. THP-1 cDNA was used as a positive control for an LPS-responsive cell line (not shown). Each reaction was performed in triplicate. Top Panel demonstrates results for MD-2 and bottom panel for TLR4. MD-2 mRNA expression was significantly higher in inflammatory bowel disease versus other tissues in both the epithelial cells, p=0.02 and lamina propria cells, p=0.02.
IFN-γ is one of the critical cytokines upregulated in the inflamed mucosa of IBD patients and clinical trials have shown that blocking IFN-γ is an effective therapeutic strategy.53 We have previously identified that the transcriptional regulation element of the human MD-2 gene is located within the first kilobase upstream (−1 kb) of the start codon and that this region is responsive to IFN-γ.20 Based on our previous data, we have developed deletion mutants of the MD-2 promoter and tested the ability of these mutants to direct luciferase expression in response to IFN-γ. Our data demonstrate that loss of the −1000bp and −700bp region of the promoter resulted in absence of IFN-γ inducibility of the MD-2 promoter (Fig. 2). Basal promoter activity, however, was not lost. These data suggest that the region between −700 and −1000bp of the MD-2 promoter contains the binding sites for IFN-γ inducible factors and offers an explanation for the observed increased expression in IBD.
Fig. 2.
Cloning and characterization of the −1 kb fragment of the MD-2 promoter using deletion mutants. Top panel demonstrates cloning of deletion mutants within the −1 kb fragment of the MD-2 gene promoter using primers flanked by the arrows. Bottom panel demonstrates transfection of T84 cells with −1 kb-MD-2 pGL3 or deletion mutants as indicated. The day following transfection, T84 cells were stimulated with IFN-γ and cells lysed for luciferase and β-galactosidase measurements. Reporter gene activation was significantly higher in cells transfected with −1 kb-MD-2 pGL3 or the mutants compared with the empty pGL3 vector control (data not shown). Stimulation with IFN-γ significantly increased −1 kb-MD-2 pGL3 reporter gene activation in T84 cells. This IFN-γ-dependent activation was abrogated with deletion of the −728 to −1 kb region. This graph is one experiment representative of three with similar findings and was performed in triplicate. Error bars indicate standard deviation.
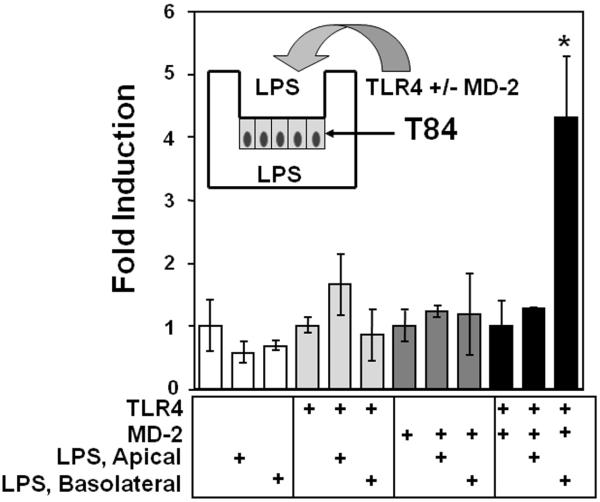
Lipopolysaccharide responsiveness is polarized to the basolateral membrane in intestinal epithelial cells
We have shown that intestinal epithelial cells are poorly responsive to LPS but that transgenic expression of TLR4 and MD-2 restores LPS-responsiveness.19 Another way in which TLR signaling may be regulated in the intestine is through distinct polarization. We hypothesized that recognition of LPS by intestinal epithelial cells is polarized to the basolateral membrane, a surface not normally exposed to bacteria. To test this hypothesis, T84 cells were transfected with an NF-κB reporter gene, ELAM-NF-κB luciferase, and co-transfected with TLR4, MD-2, or both. T84 cells grown in transwells form a polarized monolayer. Cells were then exposed to either apical or basolateral LPS. Experiments were performed when trans-epithelial resistance (TER) was > 2000 Ω-cm2 such that the monolayer was impermeable to LPS. Our data demonstrate that expression of TLR4 and MD-2 restores LPS responsiveness and this responsiveness is localized to the basolateral membrane (Fig. 3). These data suggest that expression of TLR4, MD-2 or both is polarized to the basolateral membrane in intestinal epithelial cells. Thus, in addition to the level of TLR4 and MD-2 protein expression, the location of protein expression in intestinal epithelial cells may control its function.
Fig. 3.
The response to LPS is polarized to the basolateral membrane of T84 cells. T84 were transfected with ELAM-NF-κB-luciferase (0.4 μg) and co-transfected with 0.3 μg of MD-2, TLR4 or both as indicated. Cells were then cultured until TER>2000 Ω-cm2. Lipopolysaccharide 50 ng/ml was added to the apical or basolateral well as indicated. Cells were exposed to LPS for 5 h and lysed for luciferase activity. The data are expressed as fold-induction of relative light units when compared with transfection of the vector control. Only basolateral addition of LPS in cells expressing both TLR4 and MD-2 resulted in NF-κB activation. These data are one representative experiment of three independent experiments performed in triplicate and error bars indicate standard deviation.
Methylation of CpG’s occurs within the MD-2 promoter and its first exon in intestinal epithelial cells
Normal colonic epithelial cells and intestinal epithelial cell lines express low levels of MD-2 mRNA. Methylation of cytosine residues that are followed by guanosines, CpG dinucleotides, in promoter elements is a well documented epigenetic mechanism by which eukaryotic cells silence transcription and regulate gene expression.54, 55 We hypothesized that methylation of the MD-2 gene may be responsible for its decreased expression in the intestinal epithelium. To test this hypothesis, we examined methylation of the MD-2 gene directly using bisulfite labeling of genomic DNA isolated from intestinal epithelial cells. In bisulfite labeling, unmethylated cytosines are changed to uracils whereas methylated cytosines are unchanged.
We examined cytosine methylation within the first kilobase upstream (−1 kb) of the start codon and within the first exon of the MD-2 gene (Fig. 4A). This region is most likely to contain methylated cytosines resulting in transcriptional repression.54, 55 Figure 4B demonstrates the sequence obtained after amplification and cloning of untreated and bisulfite-treated genomic DNA from T84 cells. Similar results were found with Caco-2 cells. None of the cytosines located in CpG dinucleotides were changed to thymidine consistent with gene methylation of these CpG dinucleotides. Whereas other cytosines not followed by guanosines were changed to thymidine. These data demonstrate that CpG dinucleotides within the MD-2 promoter are methylated.
Fig. 4.
Methylation of human MD-2 promoter site. A. Location of CpG dinucleotides in MD-2 gene promoter and first exon and PCR strategy. The diagram indicates the sites of CpG within the MD-2 promoter and first exon. Also shown is the location of the sense and anti-sense primers used to amplify genomic DNA from intestinal epithelial cells. B. Bisulfite Sequence of MD-2 Promoter in T84 cells. Upper sequence is untreated genomic DNA and lower sequence is bisulfite treated. CpG dinucleotides are underlined and highlighted. Cytosines which are not methylated are converted to thymidine and are highlighted. Similar results were obtained for Caco-2 and using exonic primers.
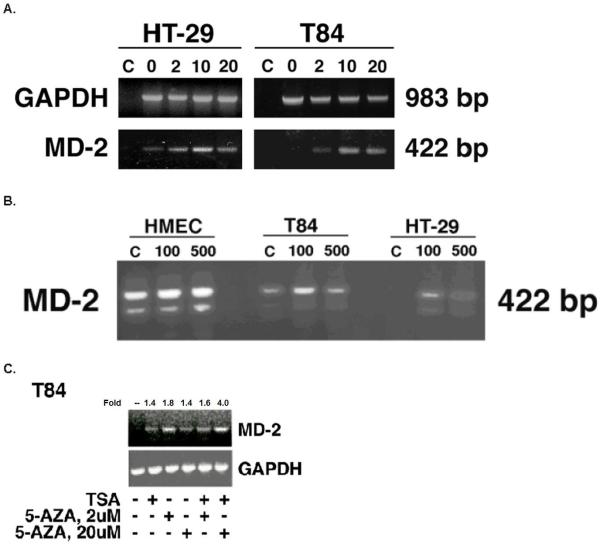
Inhibition of methylation and histone deacetylation increases expression of MD-2 in intestinal epithelial cells
Given that the MD-2 promoter is methylated in intestinal epithelial cell lines, we hypothesized that additional epigenetic mechanisms may be involved in down-regulating MD-2 gene expression. Histone acetylation facilitates engagement of the transcription machinery.56, 57 In contrast, histone deacetylases remove acetyl groups from histone resulting in chromatin condensation and inhibition of RNA transcription.58 We hypothesized that changes in the methylation or histone acetylation states of the MD-2 gene will result in changes in the level of MD-2 mRNA expression. To test this hypothesis, we measured MD-2 mRNA expression via RT-PCR in intestinal epithelial and endothelial cell lines after inhibition of methylation or histone deacetylation. 5-Azacytidine, a specific inhibitor of de novo gene methylation, increased expression of MD-2 mRNA in T84 and HT-29 cells (Fig. 5A). No increase in GAPDH, a housekeeper gene, was found with addition of 5-azacytidine, supporting the specificity of its effect on methylated genes. Trichostatin A, an inhibitor of histone deacetylases, increased expression of MD-2 mRNA but not TLR4 nor GAPDH (Fig. 5B). In addition, trichostatin A had no effect on MD-2 or TLR4 mRNA expression in endothelial cells which express high endogenous levels of TLR4 and MD-2.
Fig. 5.
Inhibition of methylation and histone deacetylation increases expression of MD-2. A. Inhibition of methylation increases expression of MD-2 mRNA in intestinal epithelial cell lines. Intestinal epithelial cells as indicated were treated with increasing concentrations of 5-azacytidine. GAPDH was used as an internal control and was not increased with demethylation. Results demonstrate that expression of MD-2 mRNA is increased after inhibiting methylation with 5-azacytidine. C refers to negative control. B. Inhibition of histone deacetylases increases expression of MD-2 mRNA in intestinal epithelial cell lines. Intestinal epithelial cells as indicated were treated with trichostatin A. HMEC were used as a positive control for MD-2 expression. GAPDH was used as an internal control and was not increased with trichostatin A. Results demonstrate that expression of MD-2 mRNA is increased after inhibiting histone deacetylases. Figure shows one experiment representative of three with similar findings. C. Inhibition of methylation and histone deacetylation increases expression of MD-2 mRNA in intestinal epithelial cell lines. T84 cells were treated with two concentrations of 5-azacytidine (0, 2, 20 μM) daily for 4 days or the vehicle control. On the last day, cells were exposed to trichostatin A 100 ng/ml for 24 h as indicated. Cells were harvested for RNA on the fifth day. GAPDH was used as an internal control. ImageJ 1.41 software was used as a densitometer to quantify intensity of PCR products. Fold induction compared with unstimulated cells is shown. Figure shows one experiment representative of three with similar findings. Results demonstrate that expression of MD-2 mRNA is increased after inhibiting methylation and histone deacetylases.
To determine whether inhibition of methylation in combination with histone deacetylation additively suppress MD-2 gene expression in intestinal epithelial cells, we examined the effect of combined trichostatin A and 5-azacytidine on MD-2 mRNA expression. Treatment of T84 cells with 5-azacytidine increased MD-2 mRNA expression. In combination with trichostatin A, 5-azacytidine resulted in increased MD-2 mRNA expression that was approximately 3-fold compared with trichostatin A treatment alone (Fig. 5C). These data demonstrate that methylation and histone deacetylation play a role in the tonic inhibition of MD-2 gene expression by intestinal epithelial cells and that their combined effect is synergistic.
DISCUSSION
Our findings provide new insight into how intestinal epithelial cells regulate the dual responsibilities of maintaining a state of controlled inflammation in response to commensal bacteria yet are ready to secrete pro-inflammatory cytokines and recruit inflammatory cells to areas of pathogen invasion and intestinal injury. Elucidating the mechanisms of TLR4 and MD-2 regulation in intestinal epithelial cells may aid in understanding the intricate relationship between bacteria, the intestinal epithelium, and the innate and adaptive immune systems. As others have shown that patients with inflammatory bowel disease have increased expression of TLR4 and we have previously found that increased TLR4 expression is required for epithelial proliferation after injury and for the development of CAC, our results provide a better understanding of the role of IEC in the pathogenesis of inflammatory bowel disease and the progression to colitis associated cancer.15-18 In this study, we demonstrate that TLR4 and MD-2 expression are increased in both IEC and lamina propria cells in patients with human inflammatory bowel disease and we present immune-mediated and epigenetic mechanisms by which IEC down-regulate MD-2 expression.
Inflammatory bowel disease is characterized by acute and chronic inflammation in the absence of a recognized pathogen due to aberrant host-microbial interactions resulting in Th1/Th17 cytokine production and inflammation to nonpathogenic, commensal bacteria.6-8 We have previously demonstrated that MD-2 expression is low in both normal human colonic epithelial cells and intestinal epithelial cell lines.19, 20 Others have shown that IEC from normal human intestinal biopsies express low levels of TLR4 and that the continuous presence of bacterial components results in a state of IEC hyporesponiveness and decreased TLR4 surface expression.15, 59 If the normal intestinal epithelium remains immunologically silent to commensal flora and expresses low levels of MD-2 and TLR4, it is plausible that in inflammatory bowel disease, expression of MD-2 and TLR4 is increased resulting in a heightened state of bacterial recognition. We demonstrate that compared to normal intestinal epithelium, MD-2 and TLR4 mRNA expression are significantly increased in IEC from small intestine and colon in patients with Crohn’s disease or ulcerative colitis. In addition, we found that both IEC and lamina propria cells in patients with IBD express increased levels of TLR4. This finding accounts for the discrepancies between previous reports as to which cells are responsible for the increased TLR4 expression observed in IBD patients. Interestingly, samples from patients with diverticulitis exhibited low expression levels of MD-2 and TLR4 mRNA, comparable to normal intestine suggesting these phenomena may be unique to IBD and further emphasizing the importance of better understanding the regulation of these molecules for possible therapeutic strategies for IBD.
Intestinal epithelial cells remain immunologically silent to commensal bacteria and LPS on the luminal surface, yet are able to respond to a potential pathogen. One way in which the intestinal epithelium may determine the appropriate immune response is the location of bacteria. TLR5, another member of the TLR family, is expressed in a polarized fashion on the basolateral membrane of IEC and the response to its ligand is likewise polarized.60, 61 We hypothesized that recognition of LPS by IEC occurs predominantly along the basolateral membrane, a surface not normally exposed to bacteria. This would make teleological sense since the majority of interactions between the intestinal epithelium and commensal bacteria occur along the apical surface of the epithelium, while non-pathogenic commensal organisms and pathogens that traverse the epithelial barrier come in contact with the basolateral surface of the violated epithelium. Thus, the intestinal epithelium may have evolved mechanisms to suppress recognition of commensal bacteria and their products on the luminal surface while retaining the ability to activate pro-inflammatory genes in response to invading pathogens. Our data demonstrate that expression of TLR4 and MD-2 restores LPS responsiveness in intestinal epithelial cells and this responsiveness is polarized to the basolateral membrane in the human colonic adenocarcinoma T84 cell line. These findings suggest expression of TLR4, MD-2 or both is polarized to the basolateral membrane and that in addition to molecular mechanisms of regulating TLR4 and MD-2 expression, the location of protein expression may control its function. This finding is of interest given the normal location of bacteria along the apical surface of intestinal epithelium and may provide a mechanism by which IEC avoid chronic inflammation in response to luminal bacteria.
Since normal colonic epithelial cells and intestinal epithelial cell lines express low levels of MD-2 and are LPS unresponsive, we used T-84 and HT-29 intestinal epithelial cell lines to ask whether changes in methylation or histone acetylation states, two forms of epigenetic silencing, changed the level of MD-2 expression. Intestinal epithelial cell lines offer the opportunity to examine regulation of MD-2 since they recapitulate the expression pattern in the normal colon.62 Using bisulfite sequencing we determined that the CpG dinucleotides in the MD-2 promoter are indeed methylated. We then examined the effects of inhibition of methylation and histone deacetylation in the MD-2 promoter region. We found that inhibition of either methylation or histone deacetylation increased expression of MD-2 mRNA whereas inhibition of methylation in combination with histone deacetylation resulted in a synergistic effect with a fourfold induction in MD-2 mRNA expression. In contrast, cells with inherently high levels of MD-2 expression such as endothelial cells did not demonstrate increased MD-2 mRNA expression following inhibition of histone deacetylase. Our data demonstrate that CpG dinucleotides within the MD-2 promoter are methylated and inhibition of methylation and histone deacetylation, two epigenetic mechanisms for regulating gene expression, increased MD-2 expression. Taken together, these findings demonstrate that methylation and histone deacetylation play a critical role in the tonic inhibition of MD-2 gene expression and suggest that this phenomenon may be unique to intestinal epithelial cells or cells with inherently low levels of MD-2 expression.
Our studies highlight the complex process by which the intestinal epithelium co-exists with the commensal flora. On the one hand, innate immune signaling is needed for repair but excessive signaling can culminate in malignancy. Increased MD-2 and TLR4 expression seen in IBD may be a primary defect involved in the pathogenesis of the disease or may serve to potentiate chronic inflammation lowering the threshold for progression to colitis associated cancer. By understanding the immunological and epigenetic mechanisms that regulate MD-2 and TLR4 expression in intestinal epithelial cells, pathways can be targeted in inflammatory bowel disease and colitis-associated cancer to dampen deleterious responses.
Acknowledgements
Supported by NIH grant 3R56AI05226607S1 (MTA), NCI grant 5R01CA137869-2 (MTA), and Career Development Award from CCFA (MF).
References
- 1.Ismail A, Hooper L. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;289:G779–784. doi: 10.1152/ajpgi.00203.2005. [DOI] [PubMed] [Google Scholar]
- 2.Dunne C. Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm Bowel Dis. 2001;7:136–145. doi: 10.1097/00054725-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Hu L, Hickey T. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun. 2005;73:4437–4440. doi: 10.1128/IAI.73.7.4437-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol. 1999;277:C351–358. doi: 10.1152/ajpcell.1999.277.3.C351. [DOI] [PubMed] [Google Scholar]
- 5.Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: Epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veltkamp C, Tonkonogy S, De Jong Y, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology. 2001;120:900–913. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald T, Pettersson S. Bacterial regulation of intestinal immune responses. Inflamm Bowel Dis. 2000;6:116–122. doi: 10.1097/00054725-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rath HC. Role of commensal bacteria in chronic experimental colitis: lessons from the HLA-B27 transgenic rat. Pathobiology. 2002;70:131–138. doi: 10.1159/000068144. [DOI] [PubMed] [Google Scholar]
- 9.Zenewicz L, Yancopoulos G, Valenzuela D, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunter M, Stolzenberg-Solomon R, Cross A, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 11.Itzkowitz S, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 12.Jess T, Loftus EV, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–836. doi: 10.1111/j.1572-0241.2007.01070.x. Epub 2007 Jan 2011. [DOI] [PubMed] [Google Scholar]
- 13.Engle SJ, Ormsby I, Pawlowski S, et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 14.Kado S, Uchida K, Funabashi H, et al. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–2398. [PubMed] [Google Scholar]
- 15.Cario E, Podolsky D. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 17.Fukata M, Michelsen K, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 18.Fukata M, Chen A, Vamadevan A, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. Epub 2007 Sep 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abreu MT, Vora P, Faure E, et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 20.Abreu MT, Arnold ET, Thomas LS, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–20437. doi: 10.1074/jbc.M110333200. Epub 22002 Mar 20428. [DOI] [PubMed] [Google Scholar]
- 21.Aderem A, Ulevitch R. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 22.Ulevitch R, Tobias P. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 23.Medzhitov R, Janeway CJ. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 24.Teghanemt A, Widstrom R, Gioannini T, Weiss J. Isolation of monomeric and dimeric secreted MD-2. Endotoxin.sCD14 and Toll-like receptor 4 ectodomain selectively react with the monomeric form of secreted MD-2. J Biol Chem. 2008;283:21881–21889. doi: 10.1074/jbc.M800672200. Epub 22008 Jun 21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollnes TE, Christiansen D, Brekke OL, Espevik T. Hypothesis: Combined Inhibition of Complement and CD14 as Treatment Regimen to Attenuate the Inflammatory Response. Current Topics in Complement II. 2008;632:253–263. [PubMed] [Google Scholar]
- 26.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beutler B. TLR4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 28.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 29.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziarski R, Wang Q, Miyake K, Kirschning C, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 31.Reimer T, Brcic M, Schweizer M, Jungi T. Poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008;83:1249–1257. doi: 10.1189/jlb.0607412. Epub 2008 Feb 1245. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Hisamatsu, Podolsky D. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. Epub 2002 Jun 2010. [DOI] [PubMed] [Google Scholar]
- 34.Mancek M, Pristovsek P, Jerala R. Identification of LPS-binding peptide fragment of MD-2, a toll-receptor accessory protein. Biochem Biophys Res Commun. 2002;292:880–885. doi: 10.1006/bbrc.2002.6748. [DOI] [PubMed] [Google Scholar]
- 35.Lenoir C, Sapin C, Broquet A, et al. MD-2 controls bacterial lipopolysaccharide hyporesponsiveness in human intestinal epithelial cells. Life Sci. 2008;82:519–528. doi: 10.1016/j.lfs.2007.12.007. Epub 2007 Dec 2017. [DOI] [PubMed] [Google Scholar]
- 36.Visintin A, Latz E, Monks B, Espevik T, Golenbock D. Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J Biol Chem. 2003;278:48313–48320. doi: 10.1074/jbc.M306802200. Epub 42003 Sep 48315. [DOI] [PubMed] [Google Scholar]
- 37.Hyakushima N, Mitsuzawa H, Nishitani C, et al. Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signaling. J Immunol. 2004;173:6949–6954. doi: 10.4049/jimmunol.173.11.6949. [DOI] [PubMed] [Google Scholar]
- 38.Akash iS, Saitoh S, Wakabayashi Y, et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. Epub 2003 Sep 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain V, Halle A, Halmen K, et al. Phagocytosis and intracellular killing of MD-2 opsonized gram-negative bacteria depend on TLR4 signaling. Blood. 2008;111:4637–4645. doi: 10.1182/blood-2007-11-126862. Epub 2008 Jan 4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visintin A, Iliev DB, Monks BG, Halmen KA, Golenbock DT. MD-2. Immunobiology. 2006;211:437–447. doi: 10.1016/j.imbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Saitoh S, Tanimura N, et al. Regulatory roles for MD-2 and TLR4 in ligand-induced receptor clustering. J Immunol. 2006;176:6211–6218. doi: 10.4049/jimmunol.176.10.6211. [DOI] [PubMed] [Google Scholar]
- 42.Park B, Song D, Kim H, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009 doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 43.Abreu M, Palladino A, Arnold E, Kwon R, McRoberts J. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology. 2000;119:1524–1536. doi: 10.1053/gast.2000.20232. [DOI] [PubMed] [Google Scholar]
- 44.Dharmsathaphorn K, McRoberts J, Mandel K, Tisdale L, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 45.Ades E, Candal F, Swerlick R, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F, Kirschning C, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 47.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 48.McIntire F, Sievert H, Barlow G, Finley R, Lee A. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- 49.Qureshi N, Takayama K, Sievert T, et al. Novel method for the purification and characterization of lipopolysaccharide from Escherichia coli D31m3. Prog Clin Biol Res. 1995;392:151–160. [PubMed] [Google Scholar]
- 50.Hirschfeld M, Ma Y, Weis J, Vogel S, Weis J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 51.Hogan M, Vogel S. Inhibition of macrophage tumoricidal activity by glucocorticoids. J Immunol. 1988;140:513–519. [PubMed] [Google Scholar]
- 52.Hogan M, Vogel S. Lipid A-associated proteins provide an alternate “second signal” in the activation of recombinant interferon-gamma-primed, C3H/HeJ macrophages to a fully tumoricidal state. J Immunol. 1987;139:3697–3702. [PubMed] [Google Scholar]
- 53.Van den Brande J, Koehler T, Zelinkova Z, et al. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut. 2007;56:509–517. doi: 10.1136/gut.2006.105379. Epub 2006 Nov 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada N, Nishida Y, Tsutsumida H, et al. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer. 2009;100:344–351. doi: 10.1038/sj.bjc.6604845. Epub 2009 Jan 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bird A. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 56.Sterner D, Berger S. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth S, Denu J, Allis C. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 58.Khochbin S, Kao H. Histone deacetylase complexes: functional entities or molecular reservoirs. FEBS Lett. 2001;494:141–144. doi: 10.1016/s0014-5793(01)02327-4. [DOI] [PubMed] [Google Scholar]
- 59.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. Journal of Immunology. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 61.Eaves-Pyles T, Murthy K, Liaudet L, et al. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. Journal of Immunology. 2001;166:1248–1260. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 62.Abreu M, Thomas L, Arnold E, et al. TLR signaling at the intestinal epithelial interface. J Endotoxin Res. 2003;9:322–330. doi: 10.1179/096805103225002593. [DOI] [PubMed] [Google Scholar]