Abstract
Many Serratia marcescens strains produce the red pigment prodigiosin, which has antimicrobial and anti-tumor properties. Previous reports suggest that cyclic AMP (cAMP) is a positive regulator of prodigiosin production. Supporting this model, the addition of glucose to growth medium inhibited pigment production in rich and minimal media. Unexpectedly, we observed highly elevated levels of prodigiosin production in isogenic strains with mutations in genes involved in cAMP production (cyaA and crr) and in cAMP-dependent transcriptional signaling (crp). Multicopy expression of the Escherichia coli camp phosphodiesterase gene, cpdA, also conferred a striking increase in prodigiosin production. Exogenous cAMP decreased both pigment production and pigA-lacZ transcription in the wild-type (WT) strain, and pigA-lacZ transcription was significantly increased in a crp mutant relative to WT. Suppressor and epistasis analysis indicate that the hyperpigment phenotype was dependent upon pigment biosynthetic genes (pigA, pigB, pigC, pigD and pigM). These experiments establish cAMP as a negative regulator of prodigiosin production in S. marcescens.
Keywords: cAMP, Catabolite repression, Pigment, Prodigiosin
1. Introduction
S. marcescens is a Gram-negative bacterium that is well known for its red pigment prodigiosin. This pigment has a tri-pyrrole structure and may have a role in competition for environmental niches, as it has antimicrobial activity, reviewed by [11, 36]. Reports indicate that prodigiosin could function as an anti-cancer compound [24, 27]. Prodigiosin has also been correlated with hydrophobicity-mediated bacterial adhesion and aerosol-based dispersion [3, 28].
The cyclic nucleotide 3′,5′-cyclic AMP (cAMP) is a signaling molecule in prokaryotes that has a global impact upon gene expression through binding with the transcription factor, cAMP-receptor protein (CRP), which can positively and negatively regulate expression of target genes [2, 6, 19]. Cellular levels of cAMP are positively controlled by adenylate cyclase activity, which is inhibited by glucose [2]. Bacteria import glucose using the phosphoenolpyruvate phosphotransferase system (PTS). One of the PTS components, coded for by the crr gene, is enzyme IIAGlc. When glucose is limiting, enzyme IIAGlc becomes phosphorylated and activates adenylate cyclase (CyaA) to generate cAMP. Conversely, when glucose is abundant, enzyme IIAGlc does not become phoshphorylated or activate CyaA, leading to lower intracellular levels of cAMP [2, 21]. Levels of cellular cAMP can be further modulated by the action of a cAMP-phosphodiesterase, which hydrolyzes cAMP, yielding 5′-AMP [17].
Previous studies suggest a positive role for cAMP in regulation of prodigiosin production. One report showed that exogenous glucose inhibited pigment [4]. Another report, by Winkler and colleagues, described strains with unmapped mutations that lacked cAMP-phosphodiesterase and adenylate cyclase activity, and briefly mentioned that these strains were colorless and that pigment could be restored with exogenous cAMP, though no data was shown [40]. Similarly, a transposon mutation in a predicted CyaB-like class IV adenylate cyclase gene was reported in another Serratia species, and was reported to confer a ~30% decrease in prodigiosin production, but its potential role as an adenylate cyclase was not addressed [9]. Together, these led to the model that cAMP was a positive regulator of pigment production.
Genetic studies in Serratia sp. ATCC 39006 have shown that pigment production has a large number of regulators including PigP, PigQ, PigR, PigS, PigV, PigX, and Rap [9]. Other reported prodigiosin modulating factors include different carbon sources [8, 12, 14], phosphate [32], quorum sensing [5, 9, 25, 35], temperature [1, 37], ATP [14], and most likely cyclic-di-GMP [10].
Recently, S. marcescens cAMP-associated genes have been identified and mutated [18]. Strains with mutation of cyaA and crr exhibited a severe reduction in intracellular camp levels, and strains with crp mutations were unresponsive to exogenous cAMP [18, 33]. One unreported phenotype of these mutations was a striking increase in red pigment production, raising the question of whether cAMP positively regulates prodigiosin production as previously proposed. Here we provide evidence that cAMP negatively regulates prodigiosin production.
2. Materials and methods
2.1. Bacterial strains and growth conditions
All strains and plasmids are listed in Table 1. All bacteria were grown in LB (0.5% yeast extract, 1% tryptone, 0.5% NaCl). LB broth was supplemented with adenosine 3′, 5′-cyclic monophosphate (A9501, Sigma-Aldrich, Inc.) or glucose where noted, with the supplement dissolved in LB medium, which was subsequently filter-sterilized. M63 medium supplemented with casein amino acids (0.08% w/v), glucose (0.4-4.0% w/v) and agar (1.5% w/v) was used for one pigment experiment. All experiments were performed at 30°C. The antibiotics used were gentamicin (10 μg/ml), kanamycin (100 μg/ml), and tetracycline (10 μg/ml). The strain CHASM (compost heap- acquired S. marcescens) was isolated from a compost pile, as a red-pigmented colony on Pseudomonas isolation agar (Biomérieux, Lyon, France), which also selects for S. marcescens. This red-pigmented strain was observed to be a Gram-negative rod that was cytochrome-oxidase-negative (BD diagnostics systems, Franklin Lakes NJ). An API 20 E (Biomérieux, Lyon, France) test strip was inoculated with a bacterial suspension (as per manufacturer's instructions) and the resulting profile compared to the API 20 E analytical profile index was consistent with the identified red bacteria being S. marcescens. K904 is a pigmented ocular clinical isolate of S. marcescens from the University of Pittsburgh Eye Center.
Table 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| S. cerevisiae | ||
| InvSc1 | MATa/MATa leu2/leu2 trp1-289/trp1-289 ura3-52/ura3-52 his3-Δ1/his3-Δ1 | Invitrogen |
| E. coli | ||
| SM10 λpir | thi thr leu tonA lacY supE recA::RP4-2Tc::Mu pir | [23] |
| S17-1 λpir | thi pro hsdR hsdM+ ΔrecA RP4-2::TcMu-Km::Tn7 pir | [23] |
| JM3000 cpdA::kan | cpdA null mutation | E. coli Genetic Stock Center |
| S. marcescens | ||
| CMS376 WT | PIC strain number 3611 | Presque Isle Cultures |
| CMS737 | fimC-4 | [18] |
| CMS641 | crp-1 fimC-2 | [18] |
| CMS524 | cyaA-2 (transposon mutation) | [18] |
| CMS772 | crr-1 (insertion mutation) | [18] |
| CMS786 | crp-23 (transposon mutation) | This study |
| CMS592B | pigB::Tnmariner | This study |
| CMS613 | crp-1 (insertion mutation) | [18] |
| CMS794 | crp-1 pigB::Tnmariner | This study |
| CMS1687 | crp-Δ4 (deletion mutation) | This study |
| CHASM | pigmented environmental isolate | This study |
| K904 | pigmented ocular clinical isolate | This study |
| Nima | ATCC#29632 | Pryce Haddix |
| CMS1812 | Nima crp-1 | This study |
| Plasmids | ||
| pMQ117 | oripSC101ts, aacC1, oriT, Plac-lacZa, URA3, CEN6/ARSH4 | [30] |
| pMQ118 | nptII, rpsL, oriT, oriR6K, URA3, CEN6/ARSH4 | [30] |
| pMQ124 | oriiColE1, PBAD-lacZa, oripRO1600, orip15a, oriT, URA3, CEN6/ARSH4 | [30] |
| pMQ131 | oripBBR1, aphA-3, oriT, URA3, CEN6/ARSH4 | [30] |
| pStvZ3 | oriR6K lacZ nptII promoter probe, oriT, URA3, CEN6/ARSH4 | This study |
| pMQ236 | pMQ118 with I-SceI site | [30] |
| pMQ240 | pMQ117 + I-SceI meganuclease, oriT | [30] |
| pRMQS157/pcyaA | pMQ131 + cyaA | [18] |
| pRMQS166/pcrp | pMQ131 with crp ORF | This study |
| pEJK2/pcrp-Δ4 | pRMQS166 with crp-Δ4 | This study |
| pEJK3/pcpdA | pMQ131 + cpdA | This study |
| pEJK4/pcpdA-1 | pMQ131 + cpdA-1 (S70F,G80S) | This study |
| pRMQS242 | pMQ124 + His8-crp | This study |
| pRMQS260 | pMQ236 + crp-Δ4 | This study |
| pRMQS268 | pStvZ3 + pigA internal fragment | This study |
2.2. Mutagenesis and plasmid construction
All plasmids were introduced into S. marcescens by conjugation using either strain SM10 λpir or S17-1 λpir. Briefly, 0.5 ml of overnight cultures from donor and acceptor strains were concentrated by centrifugation, heat-shocked at 42°C for 15 min and plated in small pools on LB agar. After 10-24 h, the entire pool was streaked to single colonies on LB agar with tetracycline to select against donor Escherichia coli and either kanamycin or gentamicin.
Oligonucleotide primer sequences are available on request. Chromosomal DNA and plasmid DNA were isolated with commercial kits (Achieve pure DNA cell/tissue, 5 Prime; GenElute Plasmid, Sigma). DNA was amplified using a high-fidelity polymerase (Phusion, New England Biolabs) and cloned using yeast in vivo cloning [29]. All vectors used were previously described [29, 30]. The crp open reading frame (ORF) of S. marcescens was cloned into pMQ131 (oripBBR1) using yeast in vivo recombination [29] and placed under transcriptional control of the E. coli Plac promoter, generating pRMQS166 or “pcrp”. A mutation was made in the crp gene of pRMQS166, by digestion with Sal1, followed by end polishing using the End-IT kit (Epicenter, Madison, WI, USA), and ligation with T4 DNA ligase (NEB, Beverly MA). The resulting construct, pEJK2 (pcrp-Δ4), had a 4 base pair deletion in the crp gene starting at base pair 217 of the 630 base pair open reading frame; this allele is labeled as crp-Δ4. The cyaA and cpdA genes were cloned into the pMQ131 vector [30] and expressed from the E. coli Plac promoter, yielding pcyaA and pcpdA.
Mariner transposon mutations were generated as previously described using pBT20 [31]. The crp-1, crr-1 and fimC-4 were previously generated [18, 31] and constructed by introduction of plasmid pMQ118 with an internal fragment of these genes, designed to recombine with the chromosomal respective gene, yielding a disruption of the gene. Mutations were verified using PCR.
2.2.1. Allelic replacement of crp
The crp-Δ4 allele from pEJK2 was cloned into the allelic replacement vector pMQ236 [30], containing a I-SceI meganuclease site for counterselection. After recombination of the pMQ236 + crp-Δ4 plasmid into the chromosome, a temperature-sensitive I-SceI meganuclease-expressing plasmid, pMQ240, was introduced into the merodiploid strain [30]. The I-SceI meganuclease introduces a double-stranded break in the pMQ236 backbone integrated into the chromosome and thereby selects for either restoration of the crp WT gene or replacement of the WT crp gene with the crp-Δ4 allele. Mutations were verified by diagnostic PCR.
The pStvZ3 plasmid was generated as a chromosomal promoter probe to assess transcription of the gene of interest. The full-length lacZ gene from pYC2-lacZ (Invitrogen) was introduced into pMQ118 by in vivo cloning. The pStvZ3 plasmid, which is non-replicative in S. marcescens, was introduced into various strains by conjugation creating chromosomal lacZ fusions at pigA. A 611 bp internal fragment of pigA was cloned into pStvZ3. Integration of this plasmid creates a transcriptional lacZ fusion with pigA and causes disruption of pigA at base pair 626.
An N-terminal 8-histidine tag (His8) was added to the crp gene and cloned under control of the PBAD promoter on a high-copy ColE1 shuttle vector, pMQ124 using yeast in vivo recombination [30].
2.3. Prodigiosin production assays
Single colonies were inoculated in 5 ml of LB medium ± antibiotics ± cAMP and incubated 16-18 h on a rotary shaker (TC-7, New Brunswick). Prodigiosin was extracted from centrifuged cell pellets with acidified ethanol and levels were determined by measuring absorbance at 534 nm, based upon the method of Slater et. al. [32]. Absorbance was read (Beckman DU-70 spectrophotometer) at 534 nm to measure extracted prodigiosin and 600 nm to measure culture turbidity using a 1 cm2 cuvette, and the ratio was determined. Extracted red pigment had a maximum absorbance at ~535 nm in acidic conditions as expected for prodigiosin (data not shown, [16]). A similar approach was used with M63 agar except that 4 independent cultures of WT and crp-Δ4 were grown up in LB to saturation, then subcultured and grown to logarithmic phase. Ten μl of each were spotted onto M63 agar plates with different concentrations of glucose (0.2-4.0%). These were incubated at 30°C for 16-19 h, cells were scraped from the plate and cell concentration was measured at A600 nm. Cells were harvested by centrifugation; prodigiosin was extracted and measured at A534 nm. The experiment was repeated three times on different days with similar results.
2.4. Phosphodiesterase and ß-galactosidase (ß-gal) assays
For phosphodiesterase (PDE) activity assays, JM3000 cpdA::kan with the appropriate plasmid was grown overnight in LB medium with kanamycin, normalized to A600 nm = 1.0; 1 ml of culture medium was pelleted by centrifugation and washed with Tris-Cl reaction buffer (50 mM Tris-Cl, 1 mM MnCl2, pH 8.5). The cells were resuspended in 0.75 ml Tris-Cl reaction buffer and sonicated on ice until clear. The lysate was centrifuged at 16100 × g for 15 min at 4°C and the protein concentration of supernatants was determined by the Bradford assay. PDE activity from 100 μg of protein was measured using the chromogenic phosphodiesterase substrate bis(p-nitrophenyl) phosphate (bis-pNPP, Sigma, product number N3002) at a final concentration of 5 mM in Tris-Cl reaction buffer. Reactions were incubated at 37°C for 15 min and yellow color resulting from bis-pNPP hydrolysis was measured at A=410 nm with a plate reader (Biotek Synergy 2, Winooski VT).
For ß-gal assays, cultures were grown overnight in LB medium with kanamycin at 30°C, then subcultured (1:100) two times and grown to an A600 of 0.1 in order to synchronize cultures in early exponential growth phase. After growth to a desired optical density, culture aliquots were pelleted, washed with Z-buffer, and ß-galactosidase (ß-gal) activity was determined [22]. Lysates were prepared by sonication in Z-buffer and were clarified by centrifugation at 16,100 g for 5 min. Protein concentration from supernatants was determined by Bradford analysis, and the same amount of protein from each sample in a given experiment was added to microtiter plate wells and the volume adjusted to 100 μl with Z-buffer. ONPG (25μl at 4mg/ml) was added as a substrate and allowed to incubate until a yellow color developed. A410 readings were taken with a plate reader.
2.5. Protein purification and EMSA analysis
The His8-CRP protein was generated using PBAD-expressed His8-CRP in E. coli. An overnight culture of E. coli harboring the PBAD-His8-crp vector, pRMQS242, was subcultured 1:100 in 250 ml of fresh LB with gentamicin and grown to an A600 of 0.4 at 30°C. L-arabinose was added to a final concentration of 0.2%, and incubation was continued for 4 h to induce expression of His8-CRP. Bacteria were then harvested by centrifugation (4,000 × g for 15 min, 4°C), resuspended in B-Per lysis solution (Pierce) with 1X Halt protease Inhibitor Cocktail (Pierce), and lysed on ice by sonication (ten bursts of 10s at ~20W). The lysate was then centrifuged (4,000 × g for 15 min, 4°C) and His 8-CRP purified with IMAC (Pierce HisPur Cobalt Spin Column, Rockford, IL, USA). The purified protein was verified as CRP by immunoblotting with a monoclonal anti-CRP antibody (NeoClone, Madison, WI, USA). The purity of CRP was determined to be >90% as visualized by a Coomassie-stained, SDS-12% polyacrylamide gel electrophoresis (PAGE) and analyzed with Image J software (NIH).
Crude lysates were prepared by growing bacteria overnight in LB broth. Cells (4 ml) were harvested and washed twice with Tris-buffered saline, pH 8 (TBS), and resuspended in TBS (0.75 ml) with HALT protease inhibitor cocktail (Pierce). Cell suspensions were sonicated until cleared and centrifuged for 10 min at 16,100 × g at 4°C. Protein concentration was determined from supernatants and used directly in some EMSA reactions.
Labeled DNA amplicons were made with a 5′-biotinylated oligonucleotide primer (Integrated DNA Technologies, Skokie, IL, USA), gel-purified and verified by sequencing. A commercial EMSA kit was employed as specified by the manufacturer (Lightshift Chemiluminescent EMSA kit, Pierce, Rockford IL, USA), using biotinylated target DNA (1-3 ng), purified 8-histidine-tagged CRP (≥50 ng) and poly-dIdC (500 ng), cAMP (500 μM) and non-labeled competitor DNA (0-600 ng) as specified, in a 20 μl reaction. A 10 μl aliquot of the reaction was separated on 5% PAGE, TBE gel (Bio-Rad) with TBE running buffer containing 500 μM cAMP.
2.6. Statistical analysis
GraphPad Prism and Microsoft Excel software was used to determine statistical significance using one-way ANOVA with Tukey's post-test and Student's T-tests as noted.
3. Results
3.1. Glucose inhibits prodigiosin production by S. marcescens
A previous report showed that exogenous glucose inhibited pigment production by S. marcescens, leading to the hypothesis that cAMP is a positive regulator of pigment production, as glucose inhibits cAMP production [4]. Inconsistently, we noted that a cyaA mutant strain exhibited a strikingly hyperpigmented phenotype, suggesting a negative role for cAMP in regulation of pigment production, as this strain was previously shown to be deficient in intracellular cAMP levels (Table 2). We tested whether glucose also inhibited pigment production in S. marcescens strain PIC3611 and found that glucose conferred nearly complete inhibition of wild-type pigment production in LB liquid (Table 2 and Fig. 1).
Table 2.
Effect of glucose, cAMP and mutations upon prodigiosin production.
| Straina | Mediumb | Prodigiosinc |
|---|---|---|
| WT | LB | 0.14 ± 0.02 |
| WT | LB + glucose (2% w/v) | <0.01 |
| crp-23 | LB | 0.88 ± 0.18 |
| crp-23 | LB + glucose (2% w/v) | <0.01 |
| WT | M63 + glucose (0.4% w/v) | 0.47±0.11 |
| WT | M63 + glucose (2% w/v) | 0.16±0.03* |
| WT | M63 + glucose (4% w/v) | 0.16±0.02* |
| crp-Δ4 | M63 + glucose (0.4% w/v) | 1.20±0.23 |
| crp-Δ4 | M63 + glucose (2% w/v) | 0.23±0.06* |
| crp-Δ4 | M63 + glucose (4% w/v) | 0.17±0.07* |
| cyaA-2 | LB | 0.63 ± 0.03 |
| crr-1 | LB | 0.48 ± 0.01 |
| crp-Δ4 | LB | 0.92 ± 0.16 |
| WT + pMQ131 (vector) | LB | 0.13 ± 0.03 |
| WT + pcyaA | LB | 0.15 ± 0.02 |
| cyaA-2 + pMQ131 (vector) | LB | 0.46 ± 0.04 |
| cyaA-2 + pcyaA | LB | 0.11 ± 0.02 |
| crp-23 + pMQ131 (vector) | LB | 1.19 ± 0.22 |
| crp-23 + pcrp | LB | <0.01 |
| crp-23 + pcrp-Δ4 | LB | 1.03 ± 0.03 |
| pigB::Tnmariner | LB | <0.01 |
| crp-1 pigB::Tnmariner | LB | <0.01 |
| Nima | LB | 0.30 ± 0.03 |
| Nima crp-1 | LB | 3.93 ± 0.59 |
| Nima | LB + cAMP (10 mM) | 0.05 ± 0.01 |
| K904 | LB | 0.33 ± 0.06 |
| K904 | LB + cAMP (10 mM) | 0.13 ± 0.01 |
| CHASM | LB | 0.06 ± 0.01 |
| CHASM | LB + cAMP (10 mM) | 0.02 ± 0.01 |
All strains in CMS376 background unless noted. All experiments were performed in LB liquid medium.
Experiments with LB were done in liquid medium, M63 were with solid medium.
A534/A600, measured at 16-18 h; for LB experiments, a representative experiment is shown with the average of 4 independent biological replicates per data point ± one standard deviation. For M63 experiments, data is an average of 8 independent replicates from two separate experiments per strain ± one standard deviation.
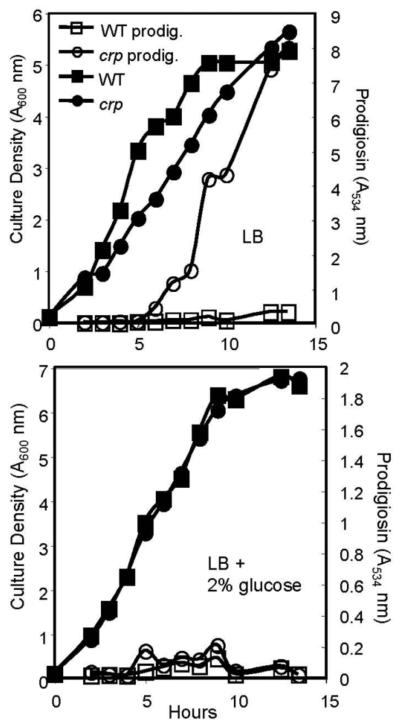
Fig. 1.
Mutation of crp confers a hyperpigment phenotype and prodigiosin production is inhibited by glucose. (Top) Growth and prodigiosin production by WT (fimC-4) and an isogenic crp (crp-1 fimC-2) mutant strain in LB medium. The fimC mutation is used to maintain a homogenous culture, as crp mutants aggregate and form robust biofilms on test tubes that could complicate growth analysis. The average of 4 independent replicates per strain are shown. (Bottom) Growth and prodigiosin production by WT (fimC-4) and an isogenic crp (crp-1 fimC-2) mutant strain in LB medium supplemented with 2% glucose. The averages of 4 independent replicates per strain are shown. These experiments were performed on different days with the same trend.
If glucose inhibits prodigiosin production through reduction of bacterial cAMP levels, then glucose should have no effect upon crp mutants, because the CRP protein is required to respond to cAMP. Mutation of crp in our strain background eliminated the effect of exogenous cAMP on fimbriae production and expression of flhD [18] [33], supporting the notion that this strain is unresponsive to cAMP as a second messenger. We observed that crp mutants had a hyperpigment phenotype that was highly sensitive to glucose in LB liquid (Table 2, Fig. 1). This suggests that glucose inhibits prodigiosin production in a cAMP-independent manner.
The glucose effect was also tested in defined minimal medium to ensure it was not an artifact of working with LB medium. There was undetectable prodigiosin production in liquid minimal medium (data not shown); however, pigment production was observed on M63 agar plates. A statistically significant decrease in pigment production was observed for both WT and crp-Δ4 mutants grown on M63 agar when glucose levels were increased (Table 2). These results support the model showing that glucose inhibits pigment production in a cAMP-independent manner, and suggest that previous studies misinterpreted the glucose effect on prodigiosin as being due to altered cAMP levels.
3.2. Prodigiosin production is dependent upon cAMP-related genes: cyaA, crr and crp
Mutation of genes involved in production of cAMP (crr, cyaA) or cAMP-dependent transcriptional signaling (crp) conferred a dramatic increase in red pigment production compared to the wild type (Table 2). We performed experiments to quantify the role of these catabolite repression system genes in pigment production. As noted above for CyaA, the Crr protein of S. marcescens is also required for cAMP production in our strain background, as mutants have a severe reduction in intracellular cAMP levels [18]. Prodigiosin levels from stationary phase cultures grown in LB medium were found to be elevated >400% in the cyaA strain over wild-type levels (Table 2). This hyperpigment phenotype was complemented by the wild-type cyaA gene on a multicopy plasmid (cyaA-2 + pcyaA) (Table 2). A similar trend was observed with different crp mutant alleles (crp-Δ4 and crp-23) (Table 2). We were able to complement the hyperpigment phenotype of crp mutants with the wild-type crp gene in trans (crp-23 + pcrp), but not by the same plasmid with a 4 bp deletion in the crp gene (crp-23+ pcrp-Δ4) (Table 2). Furthermore, multicopy expression of the wild-type crp gene in the crp-23 mutant (crp-23 + pcrp), but not multicopy expression of a mutant crp allele (crp-23 + pcrp-Δ4) confers pigment levels lower than the wild-type, suggesting that multicopy expression of the functional crp gene inhibits prodigiosin production (Table 2).
Further supporting the idea that inactivation of the cAMP-dependent catabolite repression system activates prodigiosin production, we were able to efficiently screen for random transposon-induced mutations in crp, crr, and cyaA genes based upon their hyper-red colony phenotype, e.g. crp-23 (Table 2, data not shown). Random transposon-induced suppressor mutations of the crp hyperpigment phenotype mapped to prodigiosin biosynthetic genes pigB, pigC, pigD and pigM, confirming our spectrophotometric analysis (Materials and methods section) indicating that the hyperpigmented phenotype of crp mutants was due to elevated levels of prodigiosin rather than some other red pigment (Table 2 and data not shown). The use of multiple mutant alleles and complementation analysis provides strong genetic evidence in support of the hypothesis that prodigiosin is inhibited by cAMP in our strain background.
Insertion mutagenesis of crp in the S. marcescens strain Nima (ATCC#29632), which has been extensively used to study prodigiosin production [14, 38], led to a similar increase in red pigment production, indicating that CRP's impact is not limited to our laboratory strain background (Table 2).
3.3. Prodigiosin production is inhibited by exogenous cAMP
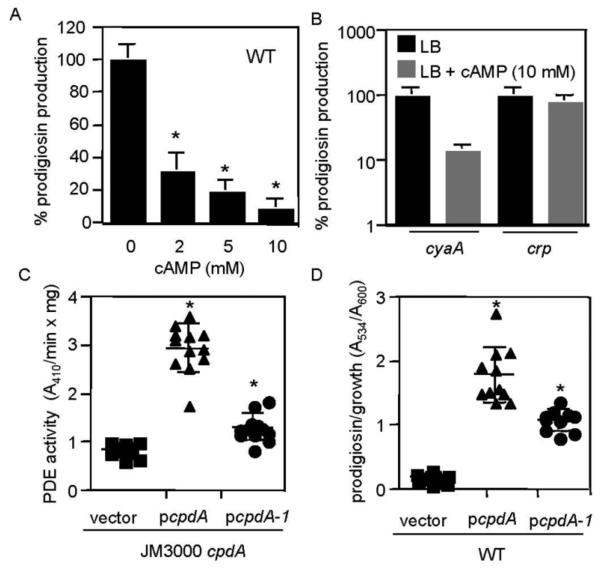
If cAMP negatively regulates prodigiosin production, then growth of bacteria in medium supplemented with cAMP should yield reduced pigment production. A cAMP dose-dependent decrease in pigment production by the WT strain was measured (Fig. 2A). Exogenous cAMP reversed the hyperpigment phenotype of cyaA mutants (Fig. 2B) and inhibited prodigiosin production in pigmented S. marcescens strains Nima, K904 and CHASM (Table 2), suggesting a conserved regulatory mechanism among diverse strains.
Fig. 2.
Exogenous cAMP inhibits prodigiosin production, whereas multicopy expression of a bona fide cAMP-PDE gene confers a hyperpigment phenotype. (A) Exogenous cAMP in LB medium elicits a dose-dependent reduction in prodigiosin production from the WT strain. Prodigiosin levels normalized by culture density are shown as a percentage of WT without exogenous cAMP. This experiment shows the average of 11-12 independent replicates per cAMP concentration from three experiments performed on different days. (B) Prodigiosin levels with respect to culture density in the presence or absence of cAMP (10 mM) in LB medium. Prodigiosin production by the cyaA, but not crp mutant is sensitive to exogenous cAMP. This experiment shows the average of 6 independent replicates per genotype; the experiment was performed two times on different days. (C) Phosphodiesterase (PDE) activity from crude lysates of a cpdA mutant of E. coli with an empty pBBR1-based vector, the vector expressing WT cpdA from E. coli (pcpdA), or a mutant version (pcpdA-1) using bis-pNPP as a substrate. The WT cpdA gene confers PDE activity, whereas the pcpdA-1 mutant confers an intermediate phenotype, indicating partial function. The data are from 10-12 independent replicates from two separate experiments performed on different days. Experiments were performed in LB medium. (D) Prodigiosin production by WT S. marcescens bearing an empty pBBR1-based vector, the vector expressing WT cpdA from the E. coli Plac promoter (pcpdA), or a partial-function mutant version (pcpdA-1). The cpdA gene confers a hyperpigment phenotype to the WT, and the pcpdA-1 mutant confers an intermediate phenotype. The data are from n≥10 independent replicates from two separate experiments performed on different days. Experiments were performed in LB medium. Asterisk = statistically significant difference from prodigiosin levels achieved without cAMP (p<0.05) by one-way ANOVA with the Tukey post-test. Error bars = one standard deviation.
Mutation of crp was predicted to render pigment production unresponsive to exogenous cAMP, since CRP is required for cAMP-induced signal transduction. Consistently, we observed no significant change (p=0.46) in prodigiosin levels from the crp mutant grown with cAMP at 10 mM (Fig. 2B). Because the crp mutant was unaffected, we conclude that the effect of cAMP on pigment production was through the cAMP-CRP signal transduction pathway rather than an unknown physiological effect from the exogenous cAMP. Growth of WT, cyaA and crp mutants is not decreased by exogenous cAMP [18] (data not shown).
3.4. Multicopy expression of a bona fide cAMP phosphodiesterase gene increases prodigiosin production
The E. coli cAMP phosphodiesterase gene, cpdA, codes for a protein that hydrolyzes cAMP into 5′-AMP [17]. We predicted that multicopy expression of a known cAMP phosphodiesterase gene would increase pigment production if cAMP negatively regulates prodigiosin production. To do this, we cloned the cpdA gene from E. coli and placed it under the control of the E. coli Plac promoter on a medium-copy pBBR1-based plasmid (pcpdA). To ensure that multicopy expression of this gene conferred phosphodiesterase (PDE) activity, an E. coli cpdA::kan mutant was transformed using pcpdA, and phosphodiesterase activity was measured from the resulting crude lysates. A significant increase in PDE activity was observed in cells bearing multiple copies of cpdA compared to the same strain with the empty vector (pMQ131) (Fig. 2C). We found that multicopy expression of cpdA in WT S. marcescens conferred a significant increase in pigment production (Fig. 2D), without altering growth (data not shown).
A mutant version of cpdA (cpdA-1) was fortuitously made through PCR errors, with S70F and G80S changes. Multicopy expression of this mutant version conferred an intermediate amount of phosphodiesterase activity upon E. coli (Fig. 2C). Multicopy expression of the mutant cpdA-1 allele in WT S. marcescens conferred a moderate significant increase in pigment production (Fig. 2D), correlating PDE activity with pigment production. Another mutant strain with only an S70F mutation was found and had no difference in PDE activity (data not shown); from this result we infer that a G80S change deleteriously impacts CpdA's PDE activity.
3.5. Control of pigA transcription by cAMP-CRP
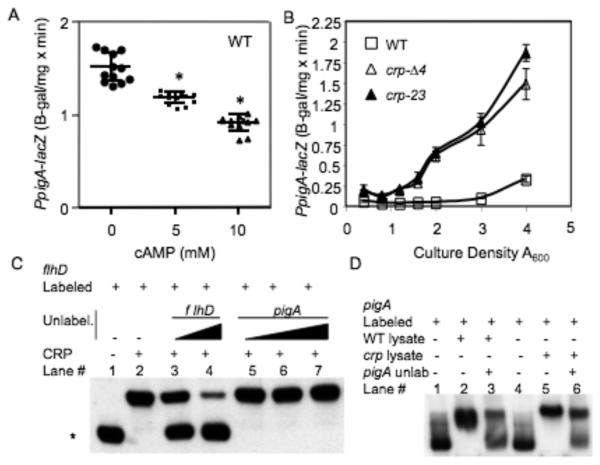
If cAMP inhibits pigment production at the transcriptional level, then exogenous cAMP would be expected to produce a decrease in expression of the prodigiosin biosynthetic operon. To test this prediction, we generated a chromosomal pigA-lacZ transcriptional reporter (pigA is the first gene in the prodigiosin biosynthetic operon), grew bacteria to early log phase, split the cultures and added cAMP to various concentrations. Bacteria were harvested at A600 nm = 5.5 (~12 h) in early stationary phase when pigA transcript levels are predicted to be highest, and β-gal levels were determined. Similar to its effect on pigment production, exogenous cAMP reduced wild-type pigA-lacZ expression in a significant dose-dependent manner (Fig. 3A).
Fig. 3.
A chromosomal pigA-lacZ fusion is regulated by CRP and exogenous cAMP. (A) Exogenous cAMP in LB medium elicits a dose-dependent reduction in chromosomal pigA-lacZ ß-galactosidase activity at A600=5.5 in a WT background. This experiment shows the average of 12 independent replicates per cAMP concentration, performed on two different days. (B) ß-galactosidase activity as expressed from the chromosomal pigA promoter with respect to culture density in LB medium. This experiment shows the average of 3 independent replicates per genotype, the experiment was performed two times on different days with similar results. Experiments were performed in LB medium. (C) Competitive EMSA to determine the ability of unlabeled DNA to compete with His8-CRP-flhD interactions. Biotin-labeled flhD promoter DNA (1 ng) was incubated with His8-CRP at 0 ng (lane 1) or 50 ng (lanes 2-7) per reaction. Unlabeled promoter fragments of flhD (lanes 3-4) and pigA (lanes 5-7) were incubated in the specified binding reactions at 0 ng (lanes 1-2), 50 ng (lane 3), 200 ng (lanes 4 - 5), 400 ng (lane 6) or 600 ng (lane 7). The asterisk indicates the migration of unbound labeled-flhD promoter DNA, signifying successful competitive inhibition of His8-CRP-flhD promoter interactions (lanes 3-4). (D) EMSA analysis with crude lysates. Biotin-labeled pigA promoter DNA (2 ng, lanes 1-6) was incubated with crude lysates from WT (lanes 2-3, 10 μg of protein) or crp-23 cultures (lanes 5-6, 8 μg of protein). Lanes 1 and 4 have no protein added. Unlabeled pigA promoter DNA (1100 ng) was added (lanes 3 and 6) to compete for binding. Asterisk = statistically significant difference from prodigiosin levels achieved without cAMP (p<0.05) by one-way ANOVA with the Tukey post-test. Error bars = one standard deviation.
In the absence of exogenous cAMP, the wild-type strain with the pigA-lacZ construct exhibited a culture density-dependent increase in ß-galactosidase activity, in agreement with previous reports (Fig. 3B) [8, 10]. In crp mutant strains, the amount of pigA-lacZ-associated ß-galactosidase activity was ~5-fold higher (A600=4) than WT levels, (Fig. 3B). Two different crp mutant alleles were used to support the notion that the phenotype was a result of crp mutation.
A previous report speculated that CRP may regulate pigment production, as a predicted CRP-binding site was detected upstream of the pigment biosynthetic operon in Serratia sp. ATCC 39006 [8]. The ATCC 39006 species has the pigment biosynthetic operon located in a different genomic context compared to several analyzed strains of S. marcescens [15]. We found that the sequence of the pigA promoter from our strain is highly similar to the reported pigment operon promoter from S. marcescens strain 274 [15], and neither have a predicted CRP binding site within 400 base pairs of the start codon. Although there was no consensus CRP binding site in the pigA promoter region, we performed EMSA assays with purified CRP to determine whether CRP directly binds the pigA promoter. We used the S. marcescens flhD promoter region as a putative positive control, as we had previously shown that flhD expression is positively regulated by cAMP and CRP [33], and the flhD promoter region has a predicted CRP binding site [13]. CRP bound DNA upstream of the flhD open in vitro (lane 2, Fig. 3C), whereas DNA upstream of pigA was not bound (data not shown). As an alternative method to test for CRP binding to pigA, we attempted to titrate out CRP-flhD promoter binding with excess unlabeled pigA promoter DNA. CRP-flhD promoter interactions could be inhibited by an excess of unlabelled flhD DNA (lane 3-4, Fig. 3C); however, an excess of unlabelled pigA promoter DNA was unable to compete for CRP-flhD promoter interactions (lane 5-7, Fig. 3C). Lastly, EMSA reactions performed with crude lysates from both WT and a crp-23 mutant strain both conferred gel shifts of the pigA promoter, supporting the idea that the pigA promoter can be bound under our experimental conditions and that the gel shift occurs in the absence of CRP (lane 2,5, Fig. 3D). Together these experiments support the model indicating that cAMP-CRP negatively regulates the prodigiosin biosynthetic operon in an indirect manner, rather than by directly binding to the pigA promoter.
4. Discussion
Here we present a model in which cAMP-CRP negatively regulates prodigiosin production through transcriptional control of the pigment biosynthetic operon. In support of this model, (1) mutation in genes known to be required for full levels of intracellular cAMP in our strain background conferred a large increase in pigment production, and this hyperpigment phenotype could be suppressed by exogenous cAMP; (2) null mutation of the crp gene, which is necessary for the cell to respond to cAMP, conferred a significant increase in prodigiosin, and this hyperpigment phenotype could not be reversed by exogenous cAMP; (3) multicopy expression of a functional but not a null mutant version of the crp gene reduced pigment production; (4) multicopy expression of a functional bona fide cAMP-PDE gene conferred a significant increase in pigment production; (5) expression of the first gene of the pigment biosynthetic operon (pigA) is inhibited by exogenous cAMP, and increased in crp mutants. The absence of a CRP binding site in the S. marcescens pigA promoter and the negative EMSA studies suggest that CRP indirectly regulates pigment biosynthetic operon expression. This may not be surprising, given the large number of pigment regulators described in Serratia sp. ATCC 39006 and S. marcescens that likely serve as intermediate regulators, reviewed by Williamson, et al. [39].
Previous reports have suggested a positive regulatory role for cAMP in Serratia prodigiosin production. One report showed that glucose severely repressed prodigiosin production in S. marcescens and that this inhibition could be reduced by theophylline [4], which is a potent inhibitor of purified cAMP phosphodiesterase activity in vitro [26]. While these are interesting observations, it was not clear whether the exogenous addition of glucose or theophylline had effects on multiple regulatory systems that could lead to altered pigment production. Data presented here also support the hypothesis that glucose inhibits prodigiosin production, but suggest that this is a cAMP-independent phenomenon, as glucose also inhibits pigment production in a crp mutant. We are in the process of finding and characterizing genes in this pathway using random mutagenesis.
Another report more strongly suggests that cAMP is a positive regulator of prodigiosin production [40]. In that report, a S. marcescens strain lacking adenylate cyclase (CyaA) and cAMP phosphodiesterase activity exhibited reduced pigment production and this deficiency could be rescued by exogenous cAMP, though these findings were not quantified [40]. The differences between the previous study and the current one could be due to strain differences, which are common amongst S. marcescens strains [20, 31, 33]. Phenotypes from the strain used in the former study [40] were not complemented nor were the mutations mapped, so these effects could result from unknown mutations elsewhere in the chromosome. The lack of adenylate cyclase activity in the strain used in the former study [40] could stem from mutation of the cyaA gene, the crr gene or other possible regulators of cAMP production and pigment. The data presented here show a negative role for cAMP in pigment production by four different strains (Table 2) using defined isogenic strains with mutations in catabolite repression genes to establish this role.
A recent report by Haddix and coworkers provides evidence that prodigiosin has a role in energy spilling [14]. Given the known role of cAMP in the starvation response of E. coli, it follows that cAMP production and prodigiosin production would be inversely regulated, as it would be counterproductive to activate an energy spilling mechanism (prodigiosin production) under starvation conditions (elevated cAMP levels). Together, these data support a teleological argument for why cAMP would be directly tied to prodigiosin production under both favorable environmental conditions and starvation conditions. The role of cAMP in inhibition of prodigiosin has been observed in another common soil microorganism, Streptomyces coelicolor, suggesting a conserved role [34].
Prodigiosin has potential as a therapeutic anti-cancer agent [27]. Several studies have reported methods to increase prodigiosin production by alteration of media constituents or by cloning of the prodigiosin biosynthetic operon [7, 12] [15, 35]. Here we have characterized a significant increase in pigment production by mutation of catabolite repression system components, suggesting that this pathway could be exploited in large-scale production of prodigiosin.
Acknowledgements
We acknowledge Pryce Haddix for helpful discussion and the kind gift of strain Nima. This work was supported by the Campbell Laboratory of Ophthalmic Microbiology, the Eye and Ear Foundation of Pittsburgh, a career development award from Research to Prevent Blindness (R.S.), and an NEI Core Grant for Vision Research, EY08098.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blizzard JL, Peterson GE. Selective inhibition of proline-induced pigmentation in washed cells of Serratia marcescens. J. Bacteriol. 1963;85:1136–1140. doi: 10.1128/jb.85.5.1136-1140.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol. Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger SR, Bennett JW. Droplet enrichment factors of pigmented and nonpigmented Serratia marcescens: possible selective function for prodigiosin. Appl. Environ. Microbiol. 1985;50:487–490. doi: 10.1128/aem.50.2.487-490.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements-Jewery S. The reversal of glucose repressed prodigiosin production in Serratia marcescens by the cyclic 3′5′-adenosine monophosphate inhibitor theophylline. Experientia. 1976;32:421–422. doi: 10.1007/BF01920771. [DOI] [PubMed] [Google Scholar]
- 5.Coulthurst SJ, Kurz CL, Salmond GP. luxS mutants of Serratia defective in autoinducer-2-dependent ‘quorum sensing’ show strain-dependent impacts on virulence and production of carbapenem and prodigiosin. Microbiology. 2004;150:1901–1910. doi: 10.1099/mic.0.26946-0. [DOI] [PubMed] [Google Scholar]
- 6.Crasnier M. Cyclic AMP and catabolite repression. Res. Microbiol. 1996;147:479–482. doi: 10.1016/0923-2508(96)84002-2. [DOI] [PubMed] [Google Scholar]
- 7.Dauenhauer SA, Hull RA, Williams RP. Cloning and expression in Escherichia coli of Serratia marcescens genes encoding prodigiosin biosynthesis. J. Bacteriol. 1984;158:1128–1132. doi: 10.1128/jb.158.3.1128-1132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fineran PC, Everson L, Slater H, Salmond GP. A GntR family transcriptional regulator (PigT) controls gluconate-mediated repression and defines a new, independent pathway for regulation of the tripyrrole antibiotic, prodigiosin, in Serratia. Microbiology. 2005;151:3833–3845. doi: 10.1099/mic.0.28251-0. [DOI] [PubMed] [Google Scholar]
- 9.Fineran PC, Slater H, Everson L, Hughes K, Salmond GP. Biosynthesis of tripyrrole and beta-lactam secondary metabolites in Serratia: integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. Mol. Microbiol. 2005;56:1495–1517. doi: 10.1111/j.1365-2958.2005.04660.x. [DOI] [PubMed] [Google Scholar]
- 10.Fineran PC, Williamson NR, Lilley KS, Salmond GP. Virulence and prodigiosin antibiotic biosynthesis in Serratia are regulated pleiotropically by the GGDEF/EAL domain protein, PigX. J. Bacteriol. 2007;189:7653–7662. doi: 10.1128/JB.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber NN. Prodigiosin-like pigments. CRC Crit. Rev. Microbiol. 1975;3:469–485. doi: 10.3109/10408417509108758. [DOI] [PubMed] [Google Scholar]
- 12.Giri AV, Anandkumar N, Muthukumaran G, Pennathur G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Micro. 2004;4 doi: 10.1186/1471-2180-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Givskov M, Eberl L, Christiansen G, Benedik MJ, Molin S. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol. Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 14.Haddix PL, Jones S, Patel P, Burnham S, Knights K, Powell JN, LaForm A. Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. J. Bacteriol. 2008;190:7453–7463. doi: 10.1128/JB.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris AK, Williamson NR, Slater H, Cox A, Abbasi S, Foulds I, Simonsen HT, Leeper FJ, Salmond GP. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology. 2004;150:3547–3560. doi: 10.1099/mic.0.27222-0. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard R, Rimington C. The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens) Biochem. J. 1950;46:220–225. doi: 10.1042/bj0460220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura R, Yamanaka K, Ogura T, Hiraga S, Fujita N, Ishihama A, Niki H. Identification of the cpdA gene encoding cyclic 3′,5′-adenosine monophosphate phosphodiesterase in Escherichia coli. J. Biol. Chem. 1996;271:25423–25429. doi: 10.1074/jbc.271.41.25423. [DOI] [PubMed] [Google Scholar]
- 18.Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RM. The cAMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl. Environ. Microbiol. 2008;74:3461–3470. doi: 10.1128/AEM.02733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 20.Labbate M, Zhu H, Thung L, Bandara R, Larsen MR, Willcox MDP, Givskov M, Rice SA, Kjelleberg S. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J. Bacteriol. 2007;189:2702–2711. doi: 10.1128/JB.01582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy S, Zeng GQ, Danchin A. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene. 1990;86:27–33. doi: 10.1016/0378-1119(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 22.Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 23.Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1988;170:2575. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaner B, Navarro S, Pique M, Vilaseca M, Martinell M, Giralt E, Gil J, Perez-Tomas R. Prodigiosin from supernatent of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br. J. Pharmacol. 2000;131:585–593. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morohoshi T, Shiono T, Takidouchi K, Kato M, Kato N, Kato J, Ikeda T. Inhibition of Quorum Sensing in Serratia marcescens AS-1 by the Synthetic Analogs of N-Acylhomoserine Lactone. Appl. Environ. Microbiol. 2007 doi: 10.1128/AEM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabayashi T, Ide M. Cyclic 3′,5′-nucleotide phosphodiesterase of Serratia marcescens. Biochim. Biophys. Acta. 1970;220:116–123. doi: 10.1016/0005-2744(70)90235-4. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Tomas R, Montaner B, Llagostera E, Soto-Cerrato V. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem. Pharmacol. 2003;66:1447–1452. doi: 10.1016/s0006-2952(03)00496-9. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg M, Blumberger Y, Judes H, Bar-Ness R, Rubinstein E, Mazor Y. Cell surface hydrophobicity of pigmented and nonpigmented clinical Serratia marcescens strains. Infect. Immun. 1986;51:932–935. doi: 10.1128/iai.51.3.932-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol. 2006;72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanks RM, Kadouri DE, MacEachran DP, O'Toole GA. New yeast recombineering tools for bacteria. Plasmid. 2009;62:88–97. doi: 10.1016/j.plasmid.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanks RM, Stella NA, Kalivoda EJ, Doe MR, M. ODD, Lathrop KL, Guo FL, Nau GJ. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J. Bacteriol. 2007;189:7262–7272. doi: 10.1128/JB.00859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slater H, Crow M, Everson L, Salmond GP. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol. Microbiol. 2003;47:303–320. doi: 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- 33.Stella NA, Kalivoda EJ, O'Dee DM, Nau GJ, Shanks RM. Catabolite repression control of flagellum production by Serratia marcescens. Res. Microbiol. 2008;159:562–568. doi: 10.1016/j.resmic.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Süsstrunk U, Pidoux J, Taubert S, Ullmann A, Thompson CJ. Pleiotropic effects of cAMP on germination, antibiotic biosynthesis and morphological development in Streptomyces coelicolor. Mol. Microbiol. 1998;30:33–46. doi: 10.1046/j.1365-2958.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomson NR, Crow MA, McGowan SJ, Cox A, Salmond GP. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 2000;36:539–556. doi: 10.1046/j.1365-2958.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- 36.Vining LC. Functions of secondary metabolites. Annu Rev Microbiol. 1990;44 doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- 37.Williams RP, Gott CL, Qadri SM, Scott RH. Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. J. Bacteriol. 1971;106:428–443. doi: 10.1128/jb.106.2.438-443.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RP, Green JA, Rappo-Port DA. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J. Bacteriol. 1956;71:115–120. doi: 10.1128/jb.71.1.115-120.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson NR, Fineran PC, Leeper FJ, Salmond GP. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 2006;4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 40.Winkler U, Scholle H, Bohne L. Mutants of Serratia marcescens lacking cyclic nucleotide phosphodiesterase activity and requiring cyclic 3′,5′-AMP for the utilization of various carbohydrates. Arch. Microbiol. 1975;104:189–196. doi: 10.1007/BF00447323. [DOI] [PubMed] [Google Scholar]