Abstract
The use of animals lacking genes or expressing genes under the control of cell-specific promoters has significantly increased our knowledge of the genetic and molecular basis of physiopathology, allowing testing of functional hypotheses and validation of biochemical and pharmacologic approaches in order to understand cell function. However, with unexpected frequency, gene knockout animals and, more commonly, animal models of transgenesis give experimental support to even opposite conclusions on gene function. Here we summarize what we learned on the role of cyclooxygenase 2 (COX-2) in liver and revise the results obtained in 3 independent models of mice expressing a COX-2 transgene specifically in the hepatocyte. Upon challenge with pro-inflammatory stimuli, the animals behave very differently, some transgenic models having a protective effect but others enhancing the injury. In addition, one transgene exerts differential effects on normal liver physiology depending on the transgenic animal model used.
Keywords: Cyclooxygenase 2, Prostaglandins, Liver diseases, Apoptosis, Inflammation, Animal models
INTRODUCTION
Bioactive lipids, including prostaglandins and thromboxanes - collectively known as prostanoids - are involved in many physiopathological processes ranging from vascular function to gastric mucosa integrity, inflammation and development/progression of various types of oncologic processes including colorectal cancer[1-5]. These prostanoids are synthesized from arachidonic acid by the sequential action of cyclooxygenase (COX) and a specific prostaglandin or thromboxane synthase. Two isoforms of COX exist: COX-1 that is constitutively expressed in almost all tissues and is responsible for the homeostatic synthesis of prostanoids, and COX-2 that is expressed upon response to cell stressors, such as pro-inflammatory cytokines, growth factors and hormones[2,6-8]. Whereas COX-1 exhibits a modest but continuous synthesis of prostanoids, COX-2 is involved in the high throughput synthesis of these bioactive lipids under pathological conditions. Mice lacking COX-1, COX-2 or both isoenzymes have been generated and these animals are fully viable despite the observation of alterations in fertility (the COX-2 deficient females are sterile) and the appearance of nephropathies during aging[2,9,10]. In adult liver, the expression of COX-2 under rapid response to a pro-inflammatory challenge is almost restricted to the non-hepatocyte cell population. However, under chronic pro-inflammatory conditions hepatocytes express this isoenzyme and the contribution of the increased synthesis of prostanoids to liver pathology is a current subject of research[11-13]. To better approach the study of COX-2 expression as an early cause of liver pathology, various groups engineered mice that expressed this isoenzyme specifically in the hepatocytes. The results obtained using these animal models are the subject of this review and highlight the need to interpret the data from animal models with a certain caution.
GENE TARGETING IN LIVER INJURY AND REGENERATION
The development of targeted animal models to answer key biological questions in some cases requires appraisal to understand why animals lacking the same gene but generated under different genetic backgrounds or gene-deletion strategies result in different and sometimes even opposite physiopathological conclusions. One case is liver regeneration after partial hepatectomy where only a reduced number of genes appeared to be essential for the survival following resection of two-thirds of the liver[14-19]. Cumulative studies in this area identified about 70 genes whose expression increased following partial hepatectomy; interestingly, despite the large number of signals controlling the early steps of regeneration only a handful have been described to play a critical role in the successful outcome of the process. Models in which liver regeneration is impaired after partial hepatectomy are animals deficient of insulin-like growth factor-1-binding protein, TAB2 (transforming growth factor-β-activated kinase 1-binding protein 2)-a transforming growth factor-activated kinase-1 interacting protein involved in the early response to interleukin-1β (IL-1β), or animals overexpressing transforming growth factor-β (TGF-β)[20-22]. Examples of relevant genes for regeneration are those controlling commitment to proliferation or inhibiting apoptosis, such as c-met, pdk1, p75ntr (the neurotrophin receptor in stellate cells), and gadd45b[23-26]. Previous studies reported delayed regeneration and sometimes increased death in animals lacking il-6, stat-3, cox-2 or nos-2[2,9,27-29], but further studies notably found an attenuated impact of the deficiencies in these genes in terms of liver mass recovery and survival. One extreme example is the requirement of caveolin-1 for regeneration that in one model has been described to be “a gene essential for liver regeneration”[30], whereas in the commercially available caveolin-1-deficient mice this gene appears to be absolutely “dispensable” with the peculiarity of an accelerated regeneration and, therefore, being a positive condition for a rapid liver mass recovery[31]. The reasons for these discrepancies lie with the different genetic backgrounds of both mice strains. Indeed, in those caveolin-1-deficient animals that died after partial hepatectomy, there was partial restoration of the liver mass but they died at day 4-5 post-surgery, a situation that could be overcome after administration of glucose, suggesting that a metabolic problem was the most likely defect in these animals rather than deficient cell replication and growth[30]. Indeed, in addition to the caveolin-1-deficient mice, there are also gene-targeted animals that exhibit an accelerated early proliferation and liver mass recovery, among them animals deficient in pai-1, timp-1, ikk2 or socs-3[32-35]. Finally, there are a few models that, despite being unable to restore liver cellularity because of deficient hepatocyte proliferation/division, show liver growth and fully restored hepatic function through an hypertrophic response with multinucleated and polyploid hepatocytes[36].
COX-2 TARGETING IN LIVER
As previously mentioned, prostaglandin (PGs) synthesis in mammals is carried out by the expression of 2 forms of cyclooxygenase. COX-1 is constitutively expressed in most tissues and has a narrow specificity for substrates, preferentially using arachidonic acid and releasing PGs that are involved in the physiological action of these lipid mediators. However, for expression of COX-2, the inducible enzyme, in various tissues, a high throughput synthesis of PGs occurs both from arachidonic acid and other polyunsaturated fatty acids. These PGs are involved in the regulation of physiopathological responses as diverse as inflammation, tumor development and progression, and cell growth[1,3].
One interesting observation in the liver is that normal adult hepatocytes, either in primary culture or in vivo, fail to express COX-2 upon challenge with pro-inflammatory stimuli, including toll-like receptor ligands and combinations of tumor necrosis factor-α (TNF-α), IL-1β and interferon-γ (IFN-γ). This lack of inducibility by pro-inflammatory mediators occurs in adult hepatocytes, but not in hepatocytes from fetal or early newborn animals or in hepatic-derived stable cell lines[2,11,37]. Previous studies indicated that this behavior resulted from the presence of elevated levels of CCAT/enhancer binding protein-α (C/EBP-α), a transcription factor that is highly expressed in the adult hepatocyte and that interferes with the commitment of the cells to proliferate[9]. This absence of COX-2 expression has also been observed in in vivo models of sepsis, where the production of PGs in the liver is accomplished by the expression of COX-2 in non-hepatocyte cells, mostly Kupffer cells and infiltrating macrophages[11,13]. These observations reinforce the role of liver infiltration by circulating inflammatory cells in the release of transcellular mediators, such as PGs. Despite lipopolysaccharide (LPS) or a pro-inflammatory challenge failing to induce the expression of COX-2 in hepatocytes, liver regeneration after partial hepatectomy promotes a rapid expression of COX-2 and synthesis of PGs[11] that contribute to the regeneration onset as deduced by the impaired recovery observed after administration of selective COX-2 inhibition with COX inhibitors or from animals lacking the COX-2 gene[2]. Indeed, COX-2-deficient animals exhibited a full recovery of liver mass and function after partial hepatectomy with a delayed early commitment to proliferation[2,9,11,38]. The simultaneous absence of COX-2 and other genes relevant for liver regeneration, such as nitric oxide synthase-2 resulted in an impaired liver mass recovery after partial hepatectomy leading to animal death[39,40]. Dual deficiencies of COX-2 and other genes relevant for liver function and regeneration may help to identify targets critical for hepatocyte survival.
COX-2 TRANSGENESIS AND LIVER INJURY
More intriguing are the models of COX-2 transgenesis that lead to different end-responses without a clear reason. One example came recently when 3 groups engineered mice specifically expressing COX-2 in hepatocytes in order to investigate the role of this inducible enzyme on liver physiopathology. As previously mentioned, hepatocytes only express low levels of COX-1, the constitutive COX enzyme that is responsible for PGE2 synthesis measured in primary cultures of hepatocytes. However, hepatocytes fail to express COX-2 after onset of inflammation as do typically inflammation-responsive cells, such as Kupffer and stellate cells, macrophages, astrocytes and microglial cells[2,9]. Interestingly, in the case of hepatocytes, only under time-dependent progression is COX-2 expressed as a result of the drop in C/EBPα levels, among other conditions[9]. Thus, ectopic expression of COX-2 in hepatocytes constitutes an unphysiological condition ideal for evaluating the role of PGs in liver pathogenesis. Recently, the COX-2 gene has been expressed under the control of different specific promoters: apolipoprotein E[38,41], transthyretin[42,43] or the albumin-enhancer promoter[44,45], all 3 models giving a high liver-specificity in the expression of the transgene. On analysis, after the selection of the founder colonies, it is remarkable to observe the different intrahepatic levels of PGE2 reached under each model, as summarized in Table 1. This is despite the observation of a robust expression of the transgene by Western blotting analysis in the 3 models. More tantalizing are the effects upon LPS/D-galactosamine (D-GalN) challenge of the transgenic animals: whereas in the third model[44], the expression of the COX-2 transgene notably enhanced the injury, in the first model[41] a clear protection in terms of transaminases release and histological integrity of the tissue was observed. Perhaps the genetic background of the animals was also playing a role in view of the absence of liver apoptosis in the wild-type animals of the third model (C57BL/6) after LPS/D-GalN treatment, whereas those of a C57BL/6XDBA background (first model) exhibited a significant apoptotic response, previously described by many authors. This apoptosis was prevented after the expression of the COX-2 transgene, and was lost upon pharmacological inhibition of COX-2 with selective inhibitors. However, when animal models #1 and #3 were confronted with the Fas/FasL challenge using Jo2 as the stimulus, a very potent protection against liver injury and animal death was observed in those animals that carried the COX-2 transgene, through a mechanism that involved Src/epidermal growth factor receptor signaling[38,45]. Finally, in a methionine and choline-deficient diet model MCD/CCl4-induced injury, COX-2 transgenesis failed to exhibit any significant protection on liver injury (animal model #2 and reference 43).
Table 1.
Summary of metabolic patterns and liver responses in transgenic mice with a liver-specific expression of the COX-2 gene
| Model #1[38,41] | Model #2[42,43] | Model #3[44,45] | |
| PGE2 WT vs TG | 45 vs 1752 | 30 vs 5502 | 22 vs 581 |
| Challenge | |||
| LPS/D-GalN | Protection | ND | Sensitization |
| MCD/CCl4 | ND | Irrelevant | ND |
| Jo2 (FasL) | Protection | ND | Protection |
| Transaminases after challenge | |||
| WT/TG (UI/L) | 3750/625 | 500/500 | 400/1600 |
| Infiltration3 | No | Yes | ND |
| Fibrosis3 | No | No | No |
| Hepatitis3 | No | Yes | ND |
| HCC-induction3 | ND | ND | ND |
pg/mg of liver;
pg/mg of protein;
In animals aged 12-mo. COX-2: Cyclooxygenase 2; ND: Not determined; PG: Prostaglandin; LPS: Lipopolysaccharide; D-GalN: D-galactosamine; MCD: Methionine and choline-deficient diet model; HCC: Hepatocellular carcinoma; WT: Wild type; TG: COX-2 transgenic mice.
The consequences of transgene expression over time also exhibited different patterns among the animal models: whereas at 12 mo mice of the first model did not exhibit histopathological symptoms of cell infiltration or fibrosis, the animals of model #2 developed significant inflammatory cell infiltration and hepatitis through a mechanism that appears to involve a persistent activation of nuclear factor-κB. As there is a continuous activity of COX-2 in the hepatocyte in both models, with elevated PGE2 synthesis, it can be concluded that other factors are required for the development of infiltration and spontaneous hepatitis. Interestingly, COX-2 does not appear to mediate the development of liver fibrosis since similar results were observed in wild-type, COX-2 knockout and COX-2 transgenic mice in an experimental model of induction of liver fibrosis[43].
At present, there are cumulative studies supporting the proliferative and antiapoptotic role of PGs in different models of liver failure as well as after ischemia/reperfusion injury[46,47]. This protective role has been observed even in other tissues like cardiomyocytes[48,49]. Moreover, it is known that PGE2 inhibits T-cell proliferation, and exerts a suppressant effect on type-1 immune responses in macrophages, drastically inhibiting the production of Th1 cytokines, such as IFN-γ and TNF-α and upregulating IL-10[4,7].
In conclusion, experiments on transgenesis need to take into account the biological activity of the expressed protein. In the case of COX-2, the availability of substrate for this enzyme (arachidonic acid) appears to be a rate-limiting step in the synthesis of PGs. Therefore, different levels of protein expression might result in similar levels of PG synthesis because of substrate restrictions for COX-2. In addition, the time of activation of the ectopic promoter of the transgene, usually after or during the perinatal transition restricts the influence of COX-2 in the embryonic steps of development, but effects on early postnatal development cannot be ruled out among the different COX-2 transgenic models considered. This is in addition to the contribution of the distinct genetic backgrounds used in these animal models. Accordingly, caution must be exercised in deducing conclusions in view of the arbitrary insertion of the transgene and the fact that the biological repercussions of this genetic event may have unexpected effects in the transcriptome, in addition to the specific actions of the protein encoded by the transgene. Scientists are innocent players in this scenario and their work should be well considered, although filtered by evidence coming from ancillary physiopathological data.
COX-2 TRANSGENESIS AND LIVER CARCINOGENESIS
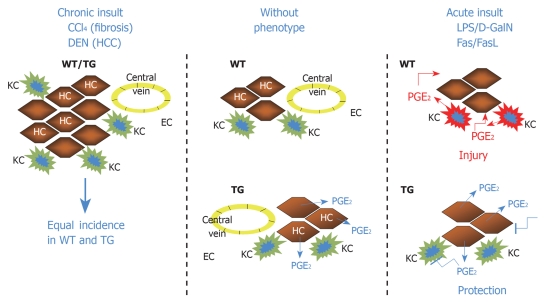
Despite the constitutive presence of COX-2 in hepatocytes in the mice[38,41-45], they failed to spontaneously develop hepatocellular tumors, and only hepatitis and fibrosis was observed in model #2[42,43]. This is interesting because COX-2 has been frequently associated with the presence of hepatocellular tumors (but in the “healthy” portion of the liver) and exacerbated COX-1 and COX-2 expression are frequently observed in hepatoma cell lines[13]. In addition, targeting of the COX enzymes or the PG receptors (EP1-4) contribute to antiproliferative effects in these cultured cells[50,51]. Tissue-specific constitutive expression of COX-2 has been reported as a positive factor for the development of carcinogenesis. In mice expressing the enzyme in mammary glands, the continuous release of PGE2 has been reported to favor angiogenesis and development per se of tumorigenic foci in the mammary gland[52,53]; these data were corroborated by pharmacological approaches based on COX-2 inhibition[54]. In addition to this, constitutive expression of COX-2 under the control of the keratin 5 promoter markedly sensitized skin to carcinogens; for example, under these conditions the sole challenge of DMBA, without further requirement of phorbol ester administration or other skin tumor promoters was sufficient to induce epidermal hyperplasia and frequent dysplastic lesions in the skin of the transgenic animals[55,56]. In the gastrointestinal tract, constitutive coexpression of COX-2 and microsomal PGE synthase (mPGES-1; the enzyme that is coupled to COX-2 and directs the prostaglandin synthesis towards PGE2) under the control of the cytokeratin 19 promoter (that targets the expression of these transgenes in the epithelial cells of gastric mucosa), resulted in animals developing hyperplasia and tumors in the stomach glandular anatomy through a process in which the contribution of infiltrating inflammatory cells, mainly macrophages plays a relevant role[57,58]. Stable expression of COX-2 in hepatocyte-like cells induced proliferation, with an increase in the proportion of cells in S-phase[59]. Interestingly, the basal protein levels of pJNK (phosphorylated c-jun-NH2-kinase) and p53, were greater in COX-2 expressing cells and were induced treatment with diethylnitrosamine (DEN). However, animals of model #1, challenged with DEN did not show an increased sensitivity, compared to the parental strain, in developing hepatic tumors during the following 12 mo of treatment (preliminary results). A schematic representation of these actions of COX-2 in hepatocytes and livers is summarized in Figure 1.
Figure 1.
Schematic representation of the effects of transgenic expression of cyclooxygenase 2 (COX-2) in hepatocytes. The transgene protects against acute liver injury, but fails to alter the response to hepatocarcinogens, such as diethylnitrosamine (DEN). KC: Kupffer cell; HC: Hepatocyte; EC: Endothelial cell; HCC: Hepatocellular carcinoma; WT: Wild type; TG: COX-2 transgenic mice.
CONCLUSION
The data reported in this review suggest that COX-2 expression provides a continuous supply of bioactive lipids that protect the liver against acute injury. However, attention should be paid to ensure that the substrate for COX-2 is available, since arachidonic acid mobilization requires the activation of phospholipase A2 isoenzymes, a process that is not usually accomplished under in vivo conditions. A noteworthy point is that COX-2 may use other unsaturated fatty acids as substrates, releasing molecules that can activate various nuclear receptors, such as peroxisome proliferator-activated receptors. In addition to this, the fact that COX-2 is not expressed in hepatocytes under pro-inflammatory conditions restricts PG synthesis to other hepatic cells, such as Kupffer cells and activated macrophages. However, in the course of progression of hepatocellular carcinomas, it cannot be excluded that there is a transient expression of COX-2 in transformed cells, as observed in many cell lines derived from hepatocellular carcinomas that express this enzyme either constitutively or after pro-inflammatory induction. Finally, the data from the models of COX-2 transgenesis in hepatocytes support the idea that by itself, COX-2 appears not to contribute to development of tumors in the full life-span of these animals.
Footnotes
Supported by Grant BFU2008-02161 and SAF2007-60551 from MICINN, S-BIO-0283/2006 from Comunidad de Madrid and FIS-RECAVA RD06/0014/0025. RECAVA and CIBERehd are funded by the Instituto de Salud Carlos III
Peer reviewer: Dr. Nagarajan Perumal, Compliance Veterinarian, Center for Life Science, IACUC OFFICE, National University of Singapore, 117456, Singapore
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
References
- 1.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 2.Martín Sanz P, Hortelano S, Bosca L, Casado M. Cyclooxygenase 2: understanding the pathophysiological role through genetically altered mouse models. Front Biosci. 2006;11:2876–2888. doi: 10.2741/2016. [DOI] [PubMed] [Google Scholar]
- 3.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Funk CD. A novel genetic model of selective COX-2 inhibition: comparison with COX-2 null mice. Prostaglandins Other Lipid Mediat. 2007;82:77–84. doi: 10.1016/j.prostaglandins.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Daikoku T, Tranguch S, Trofimova IN, Dinulescu DM, Jacks T, Nikitin AY, Connolly DC, Dey SK. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006;66:2527–2531. doi: 10.1158/0008-5472.CAN-05-4063. [DOI] [PubMed] [Google Scholar]
- 7.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 8.Takasu S, Tsukamoto T, Cao XY, Toyoda T, Hirata A, Ban H, Yamamoto M, Sakai H, Yanai T, Masegi T, et al. Roles of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 expression and beta-catenin activation in gastric carcinogenesis in N-methyl-N-nitrosourea-treated K19-C2mE transgenic mice. Cancer Sci. 2008;99:2356–2364. doi: 10.1111/j.1349-7006.2008.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callejas NA, Boscá L, Williams CS, DuBOIS RN, Martín-Sanz P. Regulation of cyclooxygenase 2 expression in hepatocytes by CCAAT/enhancer-binding proteins. Gastroenterology. 2000;119:493–501. doi: 10.1053/gast.2000.9374. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Dey SK. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat. 2005;77:84–102. doi: 10.1016/j.prostaglandins.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Casado M, Callejas NA, Rodrigo J, Zhao X, Dey SK, Boscá L, Martín-Sanz P. Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. FASEB J. 2001;15:2016–2018. doi: 10.1096/fj.01-0158fje. [DOI] [PubMed] [Google Scholar]
- 12.Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006;12:5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannitrapani L, Ingrao S, Soresi M, Florena AM, La Spada E, Sandonato L, D'Alessandro N, Cervello M, Montalto G. Cyclooxygenase-2 expression in chronic liver diseases and hepatocellular carcinoma: an immunohistochemical study. Ann N Y Acad Sci. 2009;1155:293–299. doi: 10.1111/j.1749-6632.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 14.Christophi C, Harun N, Fifis T. Liver regeneration and tumor stimulation--a review of cytokine and angiogenic factors. J Gastrointest Surg. 2008;12:966–980. doi: 10.1007/s11605-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 15.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int. 2008;28:3–11. doi: 10.1111/j.1478-3231.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- 17.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 19.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leu JI, Crissey MA, Craig LE, Taub R. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation. Mol Cell Biol. 2003;23:1251–1259. doi: 10.1128/MCB.23.4.1251-1259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leu JI, Crissey MA, Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J Clin Invest. 2003;111:129–139. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanjo H, Takeda K, Tsujimura T, Ninomiya-Tsuji J, Matsumoto K, Akira S. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol. 2003;23:1231–1238. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, Akira S, Todo S. The survival pathways phosphatidylinositol-3 kinase (PI3-K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49:204–214. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- 25.Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K, Christiansen PA, Anders RA, Franzoso G. Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest. 2008;118:1911–1923. doi: 10.1172/JCI33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 27.Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978–980. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hortelano S, Dewez B, Genaro AM, Díaz-Guerra MJ, Boscá L. Nitric oxide is released in regenerating liver after partial hepatectomy. Hepatology. 1995;21:776–786. [PubMed] [Google Scholar]
- 29.Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, Enrich C, Parton RG, Pol A. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 31.Mayoral R, Fernández-Martínez A, Roy R, Boscá L, Martín-Sanz P. Dispensability and dynamics of caveolin-1 during liver regeneration and in isolated hepatic cells. Hepatology. 2007;46:813–822. doi: 10.1002/hep.21746. [DOI] [PubMed] [Google Scholar]
- 32.Malato Y, Sander LE, Liedtke C, Al-Masaoudi M, Tacke F, Trautwein C, Beraza N. Hepatocyte-specific inhibitor-of-kappaB-kinase deletion triggers the innate immune response and promotes earlier cell proliferation during liver regeneration. Hepatology. 2008;47:2036–2050. doi: 10.1002/hep.22264. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 34.Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91–103. doi: 10.1084/jem.20070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, Niwa M, Akita K, Yamada Y, et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33:569–576. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- 36.Minamishima YA, Nakayama K, Nakayama K. Recovery of liver mass without proliferation of hepatocytes after partial hepatectomy in Skp2-deficient mice. Cancer Res. 2002;62:995–999. [PubMed] [Google Scholar]
- 37.Martín-Sanz P, Callejas NA, Casado M, Díaz-Guerra MJ, Boscá L. Expression of cyclooxygenase-2 in foetal rat hepatocytes stimulated with lipopolysaccharide and pro-inflammatory cytokines. Br J Pharmacol. 1998;125:1313–1319. doi: 10.1038/sj.bjp.0702196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casado M, Mollá B, Roy R, Fernández-Martínez A, Cucarella C, Mayoral R, Boscá L, Martín-Sanz P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 39.Hortelano S, Zeini M, Casado M, Martín-Sanz P, Boscá L. Animal models for the study of liver regeneration: role of nitric oxide and prostaglandins. Front Biosci. 2007;12:13–21. doi: 10.2741/2045. [DOI] [PubMed] [Google Scholar]
- 40.Zeini M, Hortelano S, Través PG, Martín-Sanz P, Boscá L. Simultaneous abrogation of NOS-2 and COX-2 activities is lethal in partially hepatectomised mice. J Hepatol. 2004;40:926–933. doi: 10.1016/j.jhep.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Mayoral R, Mollá B, Flores JM, Boscá L, Casado M, Martín-Sanz P. Constitutive expression of cyclo-oxygenase 2 transgene in hepatocytes protects against liver injury. Biochem J. 2008;416:337–346. doi: 10.1042/BJ20081224. [DOI] [PubMed] [Google Scholar]
- 42.Yu J, Hui AY, Chu ES, Cheng AS, Go MY, Chan HL, Leung WK, Cheung KF, Ching AK, Chui YL, et al. Expression of a cyclo-oxygenase-2 transgene in murine liver causes hepatitis. Gut. 2007;56:991–999. doi: 10.1136/gut.2006.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Wu CW, Chu ES, Hui AY, Cheng AS, Go MY, Ching AK, Chui YL, Chan HL, Sung JJ. Elucidation of the role of COX-2 in liver fibrogenesis using transgenic mice. Biochem Biophys Res Commun. 2008;372:571–577. doi: 10.1016/j.bbrc.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 44.Han C, Li G, Lim K, DeFrances MC, Gandhi CR, Wu T. Transgenic expression of cyclooxygenase-2 in hepatocytes accelerates endotoxin-induced acute liver failure. J Immunol. 2008;181:8027–8035. doi: 10.4049/jimmunol.181.11.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology. 2009;50:834–843. doi: 10.1002/hep.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45:159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 47.Wu T. Cyclooxygenase-2 in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:28–44. doi: 10.1016/j.ctrv.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inserte J, Molla B, Aguilar R, Través PG, Barba I, Martín-Sanz P, Boscá L, Casado M, Garcia-Dorado D. Constitutive COX-2 activity in cardiomyocytes confers permanent cardioprotection Constitutive COX-2 expression and cardioprotection. J Mol Cell Cardiol. 2009;46:160–168. doi: 10.1016/j.yjmcc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Cusimano A, Foderà D, D'Alessandro N, Lampiasi N, Azzolina A, Montalto G, Cervello M. Potentiation of the antitumor effects of both selective cyclooxygenase-1 and cyclooxygenase-2 inhibitors in human hepatic cancer cells by inhibition of the MEK/ERK pathway. Cancer Biol Ther. 2007;6:1461–1468. doi: 10.4161/cbt.6.9.4629. [DOI] [PubMed] [Google Scholar]
- 51.Cusimano A, Foderà D, Lampiasi N, Azzolina A, Notarbartolo M, Giannitrapani L, D'Alessandro N, Montalto G, Cervello M. Prostaglandin E2 receptors and COX enzymes in human hepatocellular carcinoma: role in the regulation of cell growth. Ann N Y Acad Sci. 2009;1155:300–308. doi: 10.1111/j.1749-6632.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 52.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 54.Narko K, Zweifel B, Trifan O, Ristimäki A, Lane TF, Hla T. COX-2 inhibitors and genetic background reduce mammary tumorigenesis in cyclooxygenase-2 transgenic mice. Prostaglandins Other Lipid Mediat. 2005;76:86–94. doi: 10.1016/j.prostaglandins.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neufang G, Furstenberger G, Heidt M, Marks F, Müller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci USA. 2001;98:7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Martínez A, Mollá B, Mayoral R, Boscá L, Casado M, Martín-Sanz P. Cyclo-oxygenase 2 expression impairs serum-withdrawal-induced apoptosis in liver cells. Biochem J. 2006;398:371–380. doi: 10.1042/BJ20060780. [DOI] [PMC free article] [PubMed] [Google Scholar]