Abstract
AIM: To assess expression of matrix metalloproteinases 2 (MMP2) and MMP9 in gastric cancer, superficial gastritis and normal mucosa, and to measure metalloproteinase activity.
METHODS: MMP2 and MMP9 mRNA expression was determined by quantitative real-time polymerase chain reaction. Normalization was carried out using three different factors. Proteins were analyzed by quantitative gelatin zymography (qGZ).
RESULTS: 18S ribosomal RNA (18SRNA) was very highly expressed, while hypoxanthine ribosyltransferase-1 (HPRT-1) was moderately expressed. MMP2 was highly expressed, while MMP9 was not detected or lowly expressed in normal tissues, moderately or highly expressed in gastritis and highly expressed in cancer. Relative expression of 18SRNA and HPRT-1 showed no significant differences. Significant differences in MMP2 and MMP9 were found between cancer and normal tissue, but not between gastritis and normal tissue. Absolute quantification of MMP9 echoed this pattern, but differential expression of MMP2 proved conflictive. Analysis by qGZ indicated significant differences between cancer and normal tissue in MMP-2, total MMP-9, 250 and 110 kDa bands.
CONCLUSION: MMP9 expression is enhanced in gastric cancer compared to normal mucosa; interpretation of differential expression of MMP2 is difficult to establish.
Keywords: Gastric cancer, Superficial gastritis, Matrix metalloproteinases, Quantitative real-time polymerase chain reaction, Quantitative zymography
INTRODUCTION
The matrix metalloproteinase (MMP) family is a group of 24 zinc-dependent endopeptidases in humans that degrade components of extracellular matrix (ECM), and are noteworthy due to their involvement in a great number of physiological and pathological processes, including stomach diseases such as gastritis and gastric cancer[1-3]. Hence MMPs, besides their ability to degrade ECM, participate in regulating growth, angiogenesis, invasion, immune response, survival and epithelial mesenchymal transition[3]. As a result proteolytic parameters may be suitable as prognosis tools in gastric cancer.
The group of gelatinases comprises MMP-2 (gelatinase A) and MMP-9 (gelatinase B), both of which cleave proteins, solubilize pericellular matrix components such as chemokines, shed cellular ectodomains and have been implicated in angiogenesis stimulation by means of integrin-ανβ[4]. Both MMP-2 and MMP-9 gelatinolytic activities can be studied by means of quantitative gelatin zymography (qGZ)[5]. These proteases are synthesized predominantly by stromal cells rather than cancer cells, and it has been proposed that both contribute to cancer progression[3]. Studies show that high levels of MMP2 and/or MMP9 have a significant correlation with gastric cancer invasion[6,7] and moreover are associated with poor prognosis[7,8]. This is important; hence both proteases could participate during invasion into the gastric wall and metastasis, which are key clinical parameters in defining patient treatment in gastric cancer.
Quantitative real-time polymerase chain reaction (qRT-PCR) is a highly specific and sensitive technique for gene expression analysis[9]; it can provide the quantification of transcripts in many different tissues and cell lines for a limited number of genes, and is particularly suitable when availability of cells is limited, such as in microdissected or biopsy tissue studies[10,11]. Gene expression analysis is increasingly important in the study of complex regulatory networks and in the understanding of disease pathogenesis. Multiple assays based on qRT-PCR have been developed for diagnosis, prognosis and monitoring of chronic and infectious diseases[12,13]. In these assays, it is essential to relate gene expression to quantity of tissue analyzed. Ideally, the RNA target employed as an endogenous control gene should be expressed at a similar level between tissue samples at all stages of development and remain unaffected by experimental treatments[14]. In general, housekeeping genes are selected to normalize for the variability between samples, which can occur due to constitutive expression. Unfortunately, there is no single RNA molecule for which expression is constant in all biological conditions and tissues samples[14,15]. Therefore, the constant expression of an endogenous control gene, selected for each particular set of experimental samples, requires testing and validation before reliable data can be obtained. In this study we used qRT-PCR to assess genetic expression of two commonly used housekeeping genes: 18S ribosomal RNA (18SRNA) and hypoxanthine ribosyltransferase-1 (HPRT-1), and two members of the MMP family, MMP2 and MMP9, in two related gastric diseases: gastritis and cancer[16]. The variances of each gene and their correlation coefficients were analyzed. In addition, we used qGZ[5] to determine the metalloproteinase activity of MMP-2 and MMP-9.
MATERIALS AND METHODS
Ethics
This study was given approval by the Hospital Ethics Committee which, from April 2007 to April 2008, permitted the recruitment of patients undergoing upper gastrointestinal endoscopy. This study complied with the code of ethics of the World Medical Association (Helsinki Declaration of 1964, as revised in 2002).
Clinical samples and histological analysis
Antral gastrointestinal biopsies from 28 patients were analyzed: 11 normal (7 females, 4 males: mean age 52 ± 14.86 years; range 28-72 years); 11 cases of superficial gastritis (9 females, 2 males: mean age 52 ± 12.56 years; range 29-72 years), and 6 cases of advanced gastric cancer (3 females, 3 males: mean age 58 ± 8.73 years; range 49-69 years). All gastrointestinal endoscopies were performed using an EVIS EXERA Video Gastroscope Olympus GIF-Q145 (Olympus, Wendenstr, HRB, DE). Biopsy specimens were collected from each patient for routine histological examination and for RNA/protein isolation.
RNA isolation
Antral gastric biopsy specimens were collected in phosphate buffered saline (PBS) solution and immediately immersed in a tissue stabilization solution (RNAlater®, Applied Biosystems, Foster City, CA, US). Tissues were homogenized in 1 mL of TRI reagent (Molecular Research Center, INC, Cincinnati, OH, US). RNA isolation was performed following the TRI reagent protocol. gDNA was digested using DNase I (Applied Biosystems). Quality and quantity of RNA were established by measuring the optical density of each sample at 260 and 280 nm.
Reverse transcription
RNA was pre-incubated with random primers and the reverse transcription reaction was performed at 42°C for 60 min. Expression levels of 18SRNA, HPRT-1, MMP2 and MMP9 were assessed by qRT-PCR in 28 antral gastric mucosa tissue samples: 11 normal, 11 cases of superficial gastritis and 6 cases of advanced gastric cancer.
qRT-PCR
The qRT-PCR was performed using ABI PRISM 7500 Real-time PCR System as described by Nuttall et al[17]. All qRT-PCR reagents, plates, optical adhesives covers, primer and probe for 18SRNA, HPRT-1, MMP2 and MMP9 were from Applied Biosystems (assay ID: 4308329, Hs99999909_m1, Hs00234579_m1 and Hs00234422_m1, respectively). Standard curves were prepared for each gene by serial dilution. The number of PCR cycles, termed cycle threshold (CT), at which amplification entered the exponential phase was determined and this number was used as an indicator of the amount of target RNA in each sample. CT values were used to classify genetic expression as very high (CT ≤ 25), high (CT = 26-30), moderate (CT = 31-35), low (CT = 36-39) or not detected (CT = 40) as validated by Nuttall et al[17]. The absolute quantity of the clinical samples was determined from comparison with the standard curve divided by three different factors: 18SRNA CT values; HPRT-1 CT values and geometric mean of 18SRNA/HPRT-1 CT values.
Zymography
From the homogenized samples, the lower organic phase was transferred into fresh tubes and proteins were isolated following the protocol of the supplier (TRI reagent, Research Organic). Proteins were normalized and electrophoresed under non-reducing conditions. The gQZ was performed as described by Peake et al[5]. Electrophoresis was performed using 5% polyacrylamide stacking gel and 10% resolving polyacrylamide co-polymerized with 1 mg/mL gelatin. MMP-2/MMP-9, human zymography standards (Millipore, Billerica, MA, US), were simultaneously loaded onto the gel. Gels were run in standard Tris-glycine-SDS running buffer. Gelatin gels were washed overnight by gentle shaking at room temperature in rinse buffer [50 mmol/L Tris-HCl pH 8.0, 5 mmol/L CaCl2 and 2.5% (v/v) Triton X-100], incubated in 50 mmol/L Tris-HCl pH7.5, 5 mmol/L CaCl2 for 18 h. Parallel gels of gelatin zymography were incubated in buffers containing 10 mmol/L EDTA to inhibit metalloproteinase activity. Gelatinolytic activity appeared as a clear band over a blue background. Using a 170-8170 Molecular Imager Gel Doc XR System and Quantity one software (Bio-Rad) images taken at the same magnification were quantified by densitometry, on the basis of their contour quantity after background subtraction. The arbitrary densitometry units were correlated with a standard curve prepared by serial dilutions of human recombinant gelatinases across a linear range (0.039-1.25 ng/mL). A total of 19 samples were randomly selected for qGZ studies: 7 of 11 normal gastric mucosa; 7 of 11 gastritis cases, and 5 of 6 gastric cancer samples.
Statistical analysis
Statistical analyses were carried out using Sigma Stat (SPSS Inc.). CT values were expressed as mean ± SD and the variances of each gene were calculated. A Spearman correlation was used to compare 18SRNA and HPRT-1 expression patterns between normal, gastritis and cancer biopsies. A positive correlation coefficient r > 0.5 with P < 0.05 was considered significant. A Kruskal-Wallis test was used to examine differences observed, in both qRT-PCR and qGZ, between normal and gastritis tissue samples, and between normal and cancer tissue samples, where P < 0.05 was considered significant.
RESULTS
Relative expression of 18SRNA, HPRT-1, MMP2 and MMP9
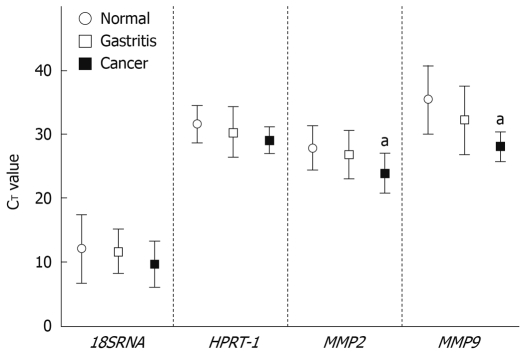
The qRT-PCR analysis showed that in all samples the endogenous levels of 18SRNA and HPRT-1 were very high (CT ≤ 25) and moderate (CT = 31-35), respectively. MMP2 was highly expressed (CT = 26-30) in all samples. MMP9 was either not detected (CT = 40, 3 samples) or lowly expressed in normal tissues (CT = 36-39, 8 samples), while in gastritis it was not detected in 1 sample, moderately expressed in 5, and highly expressed in the remaining 5. In cancer samples, MMP9 was moderately and highly expressed in 2 and 4 samples respectively. Using a Kruskal-Wallis test we found, as expected, for 18SRNA and HPRT-1 no significant differences between relative expression in gastric cancer and normal tissue, and between gastritis and normal tissue. For MMP2 (P = 0.039) and MMP9 (P = 0.018) there were significant differences between cancer and normal tissue samples (Figure 1).
Figure 1.
Relative mRNA expression of 18S ribosomal RNA (18SRNA), hypoxanthine ribosyltransferase-1 (HPRT-1), matrix metalloproteinases 2 (MMP2) and MMP9 in three different groups of gastric mucosa tissues. Error bars represent the mean ± SD. aP < 0.05 vs normal condition.
The mean CT value across all clinical samples of the endogenous control genes tested was 18.48 (SD 3.05) for 18SRNA and 32.92 (SD 2.49) for HPRT-1. HPRT-1 was the gene with the lowest CT variance (5.98) in comparison with 18SRNA (8.96). Analysis by Spearman rank correlation showed a high correlation between genetic expression of the 18SRNA and HPRT-1 genes in normal, gastritis and cancer clinical samples (r = 0.800, P < 0.001, data not shown).
Absolute quantification of MMP2 and MMP9 transcripts using different normalization factors
We subsequently determined the absolute mRNA levels of MMP2 and MMP9 using 18SRNA, HPRT-1 and the geometric mean of 18SRNA/HPRT-1 CT values as normalization factors. We believed that the geometric mean of 18SRNA/HPRT-1 would indicate the central tendency of the expression of these commonly used endogenous genes in gastric mucosa tissue samples. In accordance with relative expression, we found significant differences (P = 0.039) for MMP2 between gastric cancer and normal tissue, but not between gastritis and normal tissue, using HPRT-1 as the normalization factor (data not shown). However, in the estimation of absolute expression of MMP2 employing 18SRNA and the geometric mean of 18SRNA/HPRT-1 as normalization factors, there were no significant differences found between gastric cancer and normal tissue, which is in discordance with the relative expression data (data not shown). However, the P value for absolute expression of MMP2 using geometric mean of 18SRNA/HPRT-1 was marginally significant (P = 0.063). In agreement with the relative expression data, there was a significant difference for MMP9 between cancer and normal tissue, but not between gastritis and normal tissue, using all normalization factors (data not shown).
Gelatinase activity in gastritis, gastric cancer and gastric normal mucosa biopsies
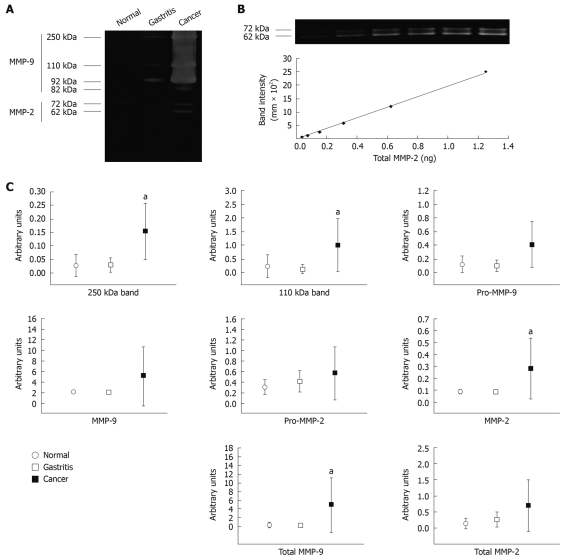
Samples chosen for qGZ studies were analyzed by densitometry of each of the six bands activity: 250 kDa reported as MMP-9 homodimer[18]; 110 kDa reported as MMP-9/lipocalin-2[19]; 92 kDa (Pro-MMP-9); 82 kDa (MMP-9); 72 kDa (Pro-MMP-2) and 62 kDa (MMP-2), see representative test samples in Figure 2A. Total MMP-9 was calculated by adding the 250, 110, 92 and 82 kDa band activities, and total MMP-2 by adding 82 and 72 kDa band activities. Arbitrary values for each individual band, total MMP-2 and total MMP-9 were determined from the standard curve; see representative standard curve generated from human recombinant MMP-2 on Figure 2B.
Figure 2.
Validation of quantitative gelatin zymography (qGZ) of human mucosal biopsy homogenates. A: Representative zymogram of tested samples; B: Representative standard curve generated from human recombinant MMP-2 across linear range of 0.039-1.25 ng/mL, used to generate arbitrary units from densitometric data; C: Differential gelatinase activity of each of the six bands activity detected in normal (open circle), gastritis (open square) and gastric cancer (close square). Error bars represent the mean ± SD. aP < 0.05 vs normal condition.
Using a Kruskal-Wallis test, we found no significant differences between gastritis and normal tissue in all bands. In addition, no significant differences were found for Pro-MMP-9, MMP-9, Pro-MMP-2 total MMP-2, between cancer and normal tissue (data not shown). However, significant differences were found between cancer and normal tissue for 250 kDa (P = 0.01), 110 kDa (P = 0.01), MMP-2 (P = 0.01) and total MMP-9 (P = 0.01) (Figure 2C).
DISCUSSION
Various studies have reported high levels of MMP-2 and MMP-9[6,20], and lipocalin-2[6,21,22] in human gastrointestinal cancers, detected mainly by immune and zymography assays. In accordance with these, this study shows that the absolute quantity of MMP9 mRNA is significantly enhanced in gastric cancer compared to normal mucosa, but interpretation of the differential mRNA expression among gastric cancer and normal mucosa of MMP2 is conflictive, and appears to depend on the normalization factor employed. Our findings would suggest that the commonly used housekeeping genes 18SRNA and HPRT-1 are constitutively expressed at different levels in normal mucosa, gastritis and gastric cancer samples, although further studies with larger numbers of samples are required to confirm these findings. In addition, this study found that minor differences of the highly expressed gene MMP2 between normal and cancer tissues could be obscured when using the equally highly expressed gene 18SRNA as a normalization factor. Kubben and collaborators first reported that MMP-9 in complex with lipocalin-2 is increased in human gastric cancers compared to adjacent control tissue following detection by zymograms and immunoblotting[23]. Our qGZ analysis reveals that MMP-9 homodimers, MMP-9/lipocalin-2 complexes, MMP-2 and total MMP-9 are significantly enhanced in gastric cancer compared to normal gastric mucosa. The formation of MMP-9/lipocalin-2 complexes has potential clinical value, as this action has been shown to protect MMP-9 from degradation in vitro[24]. This may lead to the maintenance of an extracellular pool of latent MMP-9[23]. Enhanced levels of these complexes are associated with poor prognosis in comparison to enhanced levels of MMP-9 alone. This study does not investigate the existence of possible ternary complexes of MMP-9/lipocalin-2/TIMP-1, which have been reported previously and show low gelatinase activity[25]. Enhanced expression of MMP-2 and MMP-9 in human gastritis mucosal biopsy homogenates which are Helicobacter pylori (H. pylori)-positive has been reported using semiquantitative gelatin zymography[19]; MMP-9 in serum samples from patients with gastritis H. pylori-positive[26] and MMP-9 in infiltrative human gastric mucosal lymphocytes of H. pylori-associated gastritis are detected by flow cytometry[27]. Interestingly, it has been shown by immunochemistry that Mmp-9 is increased in the murine H. felis-associated gastritis model and it has been associated with infection response and recruitment of immune cells[28]. However, using qRT-PCR and qGZ technology we did not find significant differences in levels of MMP2 and MMP9 in gastritis biopsies compared to normal mucosa. There are several reasons which may account for this: (1) employment of different technology, qRT-PCR and qGZ, vs flow cytometry immunochemistry and semiquantitative gelatin zymography; (2) existence of multiple levels of regulation of MMP expression and activity; and (3) our model was human superficial gastritis in which, at least initially, inflammation is confined to the portion of mucosa occupied by foveolae and the basal membrane is intact[29]. In this regard it would be interesting to determine MMP expression and activity in gastritis characterized by strong tissue remodeling and degradation of the basal membrane, such as erosive gastritis[29]. We would anticipate enhanced activity of MMPs in erosive gastritis compared to superficial gastritis.
In summary, we have shown that MMP9 mRNA is significantly enhanced in gastric cancer compared to normal mucosa, while interpretation of the differential of MMP2 transcripts among cancer and normal gastric mucosa is conflictive. The commonly used housekeeping genes 18SRNA and HPRT-1 appear to be constitutively expressed at different levels in normal mucosa, gastritis and gastric cancer tissues, although further studies are required. qGZ analysis reveals that MMP-9 homodimers, MMP-9/lipocalin-2 complexes, MMP-2 and total MMP-9 are significantly enhanced in gastric cancer compared to normal gastric mucosa. The potential clinical value of these findings should be fully explored in larger groups of gastritis and cancer patients.
COMMENTS
Background
Gastric cancer in Mexico is the most frequent gastrointestinal malignant neoplasm and mortality due to this disease has been reported to be steady during the past three decades. Several precursor conditions, such as chronic gastritis, have been associated with the development of gastric cancer. Proteolysis represents an important mechanism for achieving precise cellular control of biological processes, through the highly specific hydrolysis of peptide bonds. Proteases such as matrix metalloproteinases 2 (MMP-2) and MMP-9 degrade basement membrane, cleave chemokines, shed cellular ectodomains and have been implicated in different gastrointestinal diseases, such as gastritis and cancer.
Research frontiers
MMP-2 and MMP-9 are fundamental enzymes in extracellular matrix (ECM) homeostasis. In this study the authors demonstrate differences in MMP9 mRNA between gastric cancer and to normal mucosa; interpretation of differential expression of MMP2 among cancer and normal gastric mucosa is conflictive. No differences for both genes were detected between gastritis and normal mucosa. Analysis of metalloproteinase activity has shown that MMP-9 homodimers, MMP-9/lipocalin-2 complexes, MMP-2 and total MMP-9 are significantly enhanced in gastric cancer compared to normal gastric mucosa but no differences were found among gastritis and normal condition.
Innovations and breakthroughs
Besides the ability of MMPs to degrade extracellular matrix components, they participate in regulating immune response, inflammation, invasion, angiogenesis, survival and epithelial mesenchymal transition; hence proteolytic parameters may be suitable as prognosis tools in gastrointestinal diseases. In the Mexican population, this is the first study to report MMP2 and MMP9 expression in two related gastric diseases: gastritis and cancer.
Applications
Knowledge about proteolytic profiles between normal gastric mucosa, precursor conditions and malignant mucosa is central to elucidation of regulatory pathways involved in cancer development and progression. Combination of proteolytic gene expression profile and clinicopathological factors may provide insight into biology of these diseases.
Terminology
The MMPs are a family of zinc-dependent endopeptidases that consists of at least 24 members. These enzymes are able to degrade most components of ECM. The ECM is all secreted molecules that are outside cells. This network supports adhesion of cells and transmits signals through cell-surface adhesion receptors. The group of gelatinases comprises MMP-2 and MMP-9 both is noteworthy because they are involved in a great number of physiological and pathological processes including cancer.
Peer review
The authors investigated differential expression of MMP2 and MMP9 in gastric tumor and gastritis tissues using a population from Mexico, a high risk population of gastric cancer. They observed a significantly increased expression of MMP9 in tumor tissues. Results of this study were supported by previous studies. Strengths of this study are (1) biologically plausible mechanism, and (2) two different approaches for assessing expression of enzymes.
Acknowledgments
We wish to thank Cuevas B, Saldaña G, Morales J, Ortiz C, de la Peña P, Ochoa M, Puente L, Abner P, Oropeza R, Coronel-Brizio P, Andrade E, Valverde A, Dunn J and Pineda E for helpful assistance.
Footnotes
Supported by The National Council on Science and Technology (CONACYT: 85675 and 79628), Institute of Public Health (POA: 2008-2010) and Research Office of Veracruzana University and Public Education Secretariat (SEP-PROMEP-UV: PTC-319)
Peer reviewer: Jong Park, PhD, MPH, MS, Associate Professor, Division of Cancer Prevention and Control, H. Lee Moffitt Cancer Center, College of Medicine, University of South Florida, 12902 Magnolia Dr. MRC209, Tampa, FL 33612, United States
S- Editor Wang JL L- Editor O'Neill M E- Editor Lin YP
References
- 1.Göõz M, Göõz P, Smolka AJ. Epithelial and bacterial metalloproteinases and their inhibitors in H. pylori infection of human gastric cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G823–G832. doi: 10.1152/ajpgi.2001.281.3.G823. [DOI] [PubMed] [Google Scholar]
- 2.de Mingo M, Morán A, Sánchez-Pernaute A, Iniesta P, Díez-Valladares L, Pérez-Aguirre E, de Juan C, García-Aranda C, Díaz-López A, García-Botella A, et al. Expression of MMP-9 and TIMP-1 as prognostic markers in gastric carcinoma. Hepatogastroenterology. 2007;54:315–319. [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. J Biol Chem. 2001;276:16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 5.Peake NJ, Foster HE, Khawaja K, Cawston TE, Rowan AD. Assessment of the clinical significance of gelatinase activity in patients with juvenile idiopathic arthritis using quantitative protein substrate zymography. Ann Rheum Dis. 2006;65:501–507. doi: 10.1136/ard.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB, Verspaget HW. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torii A, Kodera Y, Uesaka K, Hirai T, Yasui K, Morimoto T, Yamamura Y, Kato T, Hayakawa T, Fujimoto N, et al. Plasma concentration of matrix metalloproteinase 9 in gastric cancer. Br J Surg. 1997;84:133–136. [PubMed] [Google Scholar]
- 8.Dragutinović VV, Radovanović NS, Izrael-Zivković LT, Vrvić MM. Detection of gelatinase B activity in serum of gastric cancer patients. World J Gastroenterol. 2006;12:105–109. doi: 10.3748/wjg.v12.i1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR--a perspective. J Mol Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- 10.Young DA, Billingham O, Sampieri CL, Edwards DR, Clark IM. Differential effects of histone deacetylase inhibitors on phorbol ester- and TGF-beta1 induced murine tissue inhibitor of metalloproteinases-1 gene expression. FEBS J. 2005;272:1912–1926. doi: 10.1111/j.1742-4658.2005.04622.x. [DOI] [PubMed] [Google Scholar]
- 11.Shukla CJ, Pennington CJ, Riddick AC, Sethia KK, Ball RY, Edwards DR. Laser-capture microdissection in prostate cancer research: establishment and validation of a powerful tool for the assessment of tumour-stroma interactions. BJU Int. 2008;101:765–774. doi: 10.1111/j.1464-410X.2007.07372.x. [DOI] [PubMed] [Google Scholar]
- 12.Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, Tibshirani R, Levy R. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945–951. doi: 10.1182/blood.v98.4.945. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros LJ, Hai S, Thomazy VA, Estalilla OC, Romaguera J, Luthra R. Real-time RT-PCR assay for quantifying cyclin D1 mRNA in B-cell non-Hodgkin's lymphomas. Mod Pathol. 2002;15:556–564. doi: 10.1038/modpathol.3880562. [DOI] [PubMed] [Google Scholar]
- 14.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 15.Jacques C, Baris O, Prunier-Mirebeau D, Savagner F, Rodien P, Rohmer V, Franc B, Guyetant S, Malthiery Y, Reynier P. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J Clin Endocrinol Metab. 2005;90:2314–2320. doi: 10.1210/jc.2004-1337. [DOI] [PubMed] [Google Scholar]
- 16.Stadtländer CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 17.Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- 18.Makowski GS, Ramsby ML. Zymographic analysis of latent and activated forms of matrix metalloproteinase-2 and -9 in synovial fluid: correlation to polymorphonuclear leukocyte infiltration and in response to infection. Clin Chim Acta. 2003;329:77–81. doi: 10.1016/s0009-8981(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 19.Kubben FJ, Sier CF, Schram MT, Witte AM, Veenendaal RA, van Duijn W, Verheijen JH, Hanemaaijer R, Lamers CB, Verspaget HW. Eradication of Helicobacter pylori infection favourably affects altered gastric mucosal MMP-9 levels. Helicobacter. 2007;12:498–504. doi: 10.1111/j.1523-5378.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 20.Mrena J, Wiksten JP, Nordling S, Kokkola A, Ristimäki A, Haglund C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol. 2006;59:618–623. doi: 10.1136/jcp.2005.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furutani M, Arii S, Mizumoto M, Kato M, Imamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122:209–214. doi: 10.1016/s0304-3835(97)00391-1. [DOI] [PubMed] [Google Scholar]
- 23.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Fernández CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5395. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 25.Kolkenbrock H, Hecker-Kia A, Orgel D, Kinawi A, Ulbrich N. Progelatinase B forms from human neutrophils. complex formation of monomer/lipocalin with TIMP-1. Biol Chem. 1996;377:529–533. doi: 10.1515/bchm3.1996.377.7-8.529. [DOI] [PubMed] [Google Scholar]
- 26.Rautelin HI, Oksanen AM, Veijola LI, Sipponen PI, Tervahartiala TI, Sorsa TA, Lauhio A. Enhanced systemic matrix metalloproteinase response in Helicobacter pylori gastritis. Ann Med. 2009;41:208–215. doi: 10.1080/07853890802482452. [DOI] [PubMed] [Google Scholar]
- 27.Koyama S. Significance of cell-surface expression of matrix metalloproteinases and their inhibitors on gastric epithelium and infiltrating mucosal lymphocytes in progression of Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 2004;39:1046–1053. doi: 10.1080/00365520410003245. [DOI] [PubMed] [Google Scholar]
- 28.Bergin PJ, Raghavan S, Svensson H, Starckx S, Van Aelst I, Gjertsson I, Opdenakker G, Quiding-Järbrink M. Gastric gelatinase B/matrix metalloproteinase-9 is rapidly increased in Helicobacter felis-induced gastritis. FEMS Immunol Med Microbiol. 2008;52:88–98. doi: 10.1111/j.1574-695X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 29.Owen DA. Gastritis and carditis. Mod Pathol. 2003;16:325–341. doi: 10.1097/01.MP.0000062995.72390.14. [DOI] [PubMed] [Google Scholar]