Abstract
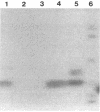
The gene, cblA, encoding the species-specific, clavulanate-susceptible, endogenous cephalosporinase was cloned from Bacteroides uniformis WAL-7088. The nucleotide sequence was determined, and the cblA structural gene was found to be 891 nucleotides, with a 48% G+C composition, which is similar to that of the B. uniformis genome. The cblA open reading frame encoded an Ambler class A beta-lactamase polypeptide precursor of 296 amino acid residues with a predicted molecular weight of 33,450. A beta-lactamase-deficient B. uniformis mutant with increased beta-lactam susceptibility was constructed by insertional inactivation of the chromosomal gene. This mutant was complemented by plasmids bearing the cblA gene, and the resulting strains were resistant to cephaloridine and had a beta-lactamase that comigrated with the parental beta-lactamase on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (30,500 Da) and in isoelectric focusing gels (pI 4.6), confirming a role for this beta-lactamase in resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Characterization of beta-lactamases. Antimicrob Agents Chemother. 1989 Mar;33(3):259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P. Sensitivity of four species of bacteroides to antibiotics. Br Med J. 1955 Dec 24;2(4955):1529–1531. doi: 10.1136/bmj.2.4955.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Klein J. R., Henrich B., Plapp R. Molecular analysis and nucleotide sequence of the envCD operon of Escherichia coli. Mol Gen Genet. 1991 Nov;230(1-2):230–240. doi: 10.1007/BF00290673. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung H. B., Kvalnes-Krick K. L., Meyer S. L., deRiel J. K., Schramm V. L. Structure and regulation of the AMP nucleosidase gene (amn) from Escherichia coli. Biochemistry. 1989 Oct 31;28(22):8726–8733. doi: 10.1021/bi00448a008. [DOI] [PubMed] [Google Scholar]
- Nikolich M. P., Shoemaker N. B., Salyers A. A. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob Agents Chemother. 1992 May;36(5):1005–1012. doi: 10.1128/aac.36.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Hedberg M. Resistance to beta-lactam antibiotics in anaerobic bacteria. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 2):S231–S234. doi: 10.1093/clinids/12.supplement_2.s231. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson-Liljequist B., Dornbusch K., Nord C. E. Characterization of three different beta-lactamases from the Bacteroides fragilis group. Antimicrob Agents Chemother. 1980 Aug;18(2):220–225. doi: 10.1128/aac.18.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A. C., Smith C. J. Genetic and biochemical analysis of a novel Ambler class A beta-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993 May;37(5):1028–1036. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Gluzman Y., Tally F. P. Escherichia coli chromosomal mutations that permit direct cloning of the Bacteroides fragilis metallo-beta-lactamase gene, ccrA. Mol Microbiol. 1991 May;5(5):1211–1219. doi: 10.1111/j.1365-2958.1991.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Rogers M. B., Parker A. C., Smith C. J. Cloning and characterization of the endogenous cephalosporinase gene, cepA, from Bacteroides fragilis reveals a new subgroup of Ambler class A beta-lactamases. Antimicrob Agents Chemother. 1993 Nov;37(11):2391–2400. doi: 10.1128/aac.37.11.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Miyata K., Inoue M., Mitsuhashi S. Characterization of cephalosporinases from Bacteroides fragilis, Bacteroides thetaiotaomicron and Bacteroides vulgatus. J Antibiot (Tokyo) 1983 Jan;36(1):76–85. doi: 10.7164/antibiotics.36.76. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J. Characterization of Bacteroides ovatus plasmid pBI136 and structure of its clindamycin resistance region. J Bacteriol. 1985 Mar;161(3):1069–1073. doi: 10.1128/jb.161.3.1069-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Owen C., Kirby L. Activation of a cryptic streptomycin-resistance gene in the Bacteroides erm transposon, Tn4551. Mol Microbiol. 1992 Aug;6(16):2287–2297. doi: 10.1111/j.1365-2958.1992.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Parker A. C. Identification of a circular intermediate in the transfer and transposition of Tn4555, a mobilizable transposon from Bacteroides spp. J Bacteriol. 1993 May;175(9):2682–2691. doi: 10.1128/jb.175.9.2682-2691.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Rogers M. B., McKee M. L. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992 Mar;27(2):141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- Tajima M., Sawa K., Watanabe K., Ueno K. The beta-lactamases of genus Bacteroides. J Antibiot (Tokyo) 1983 Apr;36(4):423–428. doi: 10.7164/antibiotics.36.423. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Malamy M. H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus beta-lactamase II. J Bacteriol. 1990 May;172(5):2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Wexler H. M., Halebian S. Alterations to the penicillin-binding proteins in the Bacteroides fragilis group: a mechanism for non-beta-lactamase mediated cefoxitin resistance. J Antimicrob Chemother. 1990 Jul;26(1):7–20. doi: 10.1093/jac/26.1.7. [DOI] [PubMed] [Google Scholar]