Abstract
There is increased interest in the effects of secretory products from aged cells on promoting both benign and malignant cell growth. We identified a human fibroblast line, AG04382, from an aged donor that naturally demonstrated senescence-associated features and whose conditioned media significantly induced proliferation of benign prostatic hyperplasia (BPH1) cells. Candidate cytokines mediating this effect were identified with protein arrays and validated by ELISA. We found that the AG04382 fibroblast line secreted high levels of CXCL5, CCL5, and CCL2, but relative to the other lines, its conditioned media was unique in its increased expression of CCL5. Blocking studies using specific antibodies against CXCL5, CCL5, and CCL2 in the conditioned media of AG04382 showed that only CCL5 contributed significantly to BPH1 proliferation. Stimulation of BPH1 cells with rhuCCL5 resulted in increased proliferation and migration, as well as significant changes in the expression of genes that influence angiogenesis. These data suggest that CCL5 is a candidate chemokine secreted by aged cells that promotes prostate growth and regulates angiogenesis.
Keywords: BPH, chemokines, senescence, migration
Introduction
There is a close correlation between host age and the prevalence of both benign (prostatic hyperplasia) and malignant (cancer) prostate disease (Balducci and Ershler 2005; Untergasser et al. 2005; Nelen 2007). Accordingly, much attention has focused on the role of “senescent” stromal cells in the aged prostate (Choi et al. 2000; Begley et al. 2005; Bavik et al. 2006; Dean and Nelson 2008). Although senescence is strictly defined as replicative arrest, it is increasingly appreciated that senescent cells remain highly metabolically active (DiPaolo et al. 1995; Choi et al. 2000; Reed et al. 2001; Castro et al. 2003; Begley et al. 2005; Begley et al. 2007; Liu and Hornsby 2007; Begley et al. 2008; Sprenger et al. 2008). Indeed, as stromal cells age, their gene expression and secretory profiles change and dynamically influence the behavior of nearby epithelial cells (Campisi 2005; Bavik et al. 2006). Stromal-epithelial interactions in the tissue microenvironment are proposed to regulate both the initiation and progression of prostate growth (Ao et al. 2007). For example, fibroblast derived secretory products, such as CXCL-12, CXCL- 13, IL6, IL8, and fibroblast growth factor (FGF) 7, induce the proliferation of prostate epithelium (Begley et al. 2005; Campisi 2005; Egeblad et al. 2005; Bavik et al. 2006). Conversely, epithelial cells express factors that further promote stromal cell replication and secretion (Giri and Ittmann 2000; Giri and Ittmann 2001). Many of these paracrine interactions are mediated by cytokines, which then potentiate cross-talk between the stroma, the epithelium, and the matrix resulting in the complicated histology noted in BPH (Begley et al. 2008).
Identification of specific mediators that are produced by aged cells will promote understanding of how aging initiates and/or potentiates the growth of certain tissues. Defining these interactions is valuable whether the cells are frankly senescent or derived from an aged host and exhibiting markers of senescence. In this study, we identified a fibroblast line from an elderly donor that had phenotypic features of senescence. Conditioned media from these cells enhanced the growth of the benign prostatic hyperplasia epithelial cell line, BPH1. Further analyses for potential paracrine modulators demonstrated that the chemokine, CCL5, in the conditioned media of the aged fibroblasts was responsible, in part, for inducing proliferation of the BPH1 cells. In addition, CCL5 stimulation resulted in significant changes in a limited number of BPH1 genes that regulate tissue growth and angiogenesis.
Methods
Cells
Primary fibroblast cultures AG13153 (30yrs), AG11747 (22yrs), AG04152 (82yrs), and AG04382 (81yrs) were obtained from the Aging Cell Repository, NIA at the Coriell Institute (Camden, NJ), and were grown in DMEM (Mediatech, Inc., Manassas, VA) with 5% FBS, 2mM L- glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin, and 2.5 ug/ml fungizone/amphotericin B (Invitrogen, Carlsbad, CA). Only early passage (passage number at <50% of expected population doublings) cells were utilized for the experiments reported below. To collect conditioned media, fibroblasts were grown to confluence and then changed to serum free DMEM for 8 hours. Media were then changed to a minimal amount of serum free media that were then collected after 48 hours of incubation. Media were spun down to eliminate any floating cells and stored at −80°C.
BPH1 cells (a kind gift from Dr. Simon Hayward, Vanderbilt University) represent a non-tumorigenic line established from epithelial cells of benign prostatic hyperplasia and then immortalized with SV40-T. BPH1 cells were used for most of the experiments because of their derivation from hyperplastic prostate tissue and their stable phenotype in vitro (Hayward et al 1995). BPH1 cells were grown in DMEM (Mediatech, Inc) with 5% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin, and 2.5 ug/ml fungizone/amphotericin B (Invitrogen).
Prostate epithelial cells were isolated from a primary prostate cell line that were initially primarily stromal in content (Lonza, Walkersville, MD). Cells were confirmed to be epithelial by morphology and cytokeratin 18 staining (Santa Cruz Biotechnology, Santa Cruz, CA).
Senescence-associated beta-galactosidase (SA-beta-gal) Staining
Fibroblasts were grown to subconfluence on 35 mm cell culture dishes, washed with PBS, and fixed with 2% formaldehyde/2% glutaraldehyde in PBS for 10 min. After 2 rinses in PBS, cells were incubated with beta-galactosidase substrate staining solution (150 mM NaCL, 2 mM MgCl2, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 40 mM citric acid, and 12 mM sodium phosphate, pH 6.0, containing 1 mg/ml X-gal {5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside}) (Invitrogen) for 24 h at 37°C. Senescent cells were identified by blue/green-staining (Dimri et al. 1995).
Proliferation Assays
Proliferation was measured in a Packard Fusion 3.5 Universal microplate analyzer using the CyQuant assay (Invitrogen) according to themanufacturer’s protocol. Briefly, cells wereplated into 96-well plates at 3,000 cells per well and allowedto attach overnight. The cells were then fed with conditioned media from fibroblasts with 1%FBS or control media (DMEM with 1%FBS) and incubated for an additional 24, 48, and 72 hours. To measure the specific effect of CCL5 on BPH1 and PEC proliferation, 2,000 cells were plated in 96 well plates and allowed to attach overnight. Media were changed to serum free for 8 hours and then exposed to control media alone or with recombinant human CCL5 (R&D Systems, Minneapolis, MN) at 10, 20, and 100ng/ml. Proliferation was measured at 24 and 48 hours as above. All proliferation assays were performed at least twice in duplicate. DMEM with 10% FBS served as a positive stimulus.
Protein Arrays
The relative amounts of cytokines secreted by the different fibroblast lines were analyzed with the RayBio® Human Cytokine Antibody arrays (RayBiotech, Inc, Norcross, GA). The array assesses 42 cytokines with 3 positive and 3 negative controls. Equivalent amounts of total protein from conditioned media of fibroblast lines AG13153, AG11747, AG04152, and AG04382 were added in duplicate wells according to the manufacturer’s instructions. Relative values were quantified by densitometry.
CCL5 ELISA
Conditioned media were collected as described above. Media representing equivalent amounts of total protein from each fibroblast line were assessed for CCL5 using the Human RANTES ELISA kit (RayBiotech) as per manufacturer’s protocol. Data were analyzed from conditioned media obtained on 2 different collections.
Blocking Antibodies
The specific effects of cytokines highly expressed in the conditioned media of fibroblasts AG04382 were determined using blocking antibodies to CCL5, CXCL5, and CCL2 (R&D Systems) in the proliferation assay. Briefly, 2,000 cells were plated in 96 well plates and allowed to attach overnight. Media were changed to serum free for 8 hours. Cells were then exposed to conditioned media from AG04382 alone or conditioned media from AG04382 with blocking antibodies to CCL5, CXCL5, CCL2 (R&D Systems) at 8 and 16μg/ml. GM6001 was used as a negative control at 25μM (Sigma, St. Louis, MO) and proliferation was measured at 24 and 48 hours as above in duplicate experiments performed on different dates.
Migration Assays
BPH1 cells were plated at confluence in the upper wells of a 24 well Transwell Fluoroblock Assay (8μm pore size, BD Biosciences Falcon, Franklin Lakes, NJ) and allowed to attach for 3 hours in DMEM with 5% FBS. After attachment, media were changed twice to achieve DMEM with 1%FBS. The bottom wells were then filled with either control media (DMEM with 1% FBS) alone or control media with CCL5 20ng/ml. DMEM with 10% FBS was a positive stimulus. Cells were allowed to migrate through the pores for 18 hours. Migrated cells were stained with DAPI (1μM) and counted in 4 fields from at least 2 wells. Data were analyzed from assays performed on 2 separate dates.
SA Biosciences Arrays
RT PCR arrays were analyzed to determine the effect of CCL5 stimulation on the expression of BPH1 genes. The pathways chosen were 1) Extracellular matrix and adhesion molecules and 2) Angiogenesis promoters and inhibitors (SA Biosciences, Frederick, MD), based on our interest in genes that would affect the extracellular microenvironment and blood vessel growth. Each array contains 84 genes and 5 housekeeping genes including beta-2-microglobulin, hypoxanthine phosphoribosyl transferase, ribosomal protein L13a, actin/beta, and GAPDH. In addition each array has 3 PCR positive controls, 3 reverse transcription controls and a control for detecting genomic DNA contamination. RNA was extracted by Rneasy plus minikit (Qiagen, Valencia, CA) from BPH1 cells treated with control or CCL5 (20ng/ml) for 24 hrs. cDNA was made and RT PCR were performed as per the manufacture’s protocol from SA Biosciences. Two separate experiments were analyzed on different dates for each Array. Fold differences were calculated using PCR array data analysis web portal (SA Biosciences). Genes that were either up regulated or down regulated by >40% in both experiments were considered significant based on prior studies describing the effects of age on protein secretion and cellular functions (Swift et al. 1999; Sadoun and Reed 2003).
Statistical Analysis
To determine differences between experimental and control groups statistical significance (p <0.05 with Bonferroni corrections for multiple comparisons) was determined by the two-tailed student’s paired t-test with unequal variance.
Results
Conditioned media from aged fibroblasts induce the proliferation of BPH cells
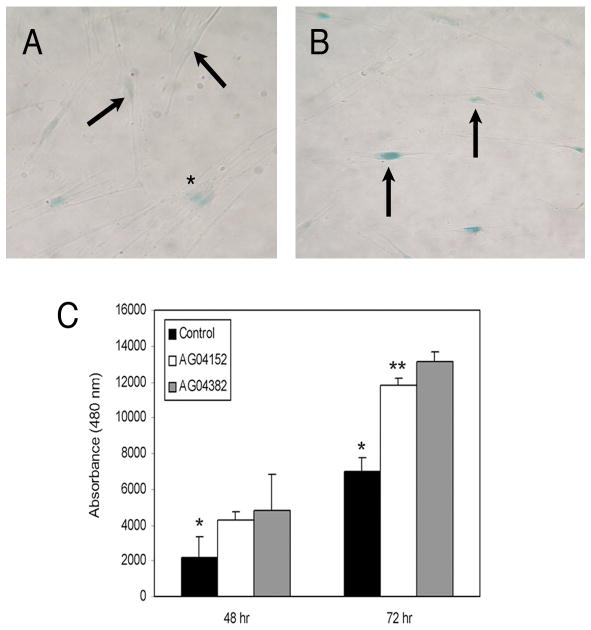
We began by examination of 4 human fibroblast lines that we previously characterized (Reed et al. 1994; Reed et al. 2001): AG13153 (30yrs), AG11747 (22yrs), AG04152 (82yrs), and AG04382 (81yrs). Fibroblasts were used because of their careful derivation, stable phenotype, their longstanding use in studies of aging, and their availability (at early passage) from donors of a wide range of ages. Each line was examined at early passage for features associated with senescence including in situ staining for SA-beta-galactosidase (Dimri et al. 1995), increased secretion of matrix metalloproteinase (MMP) 1/2 activity, and decreased proliferative activity (Reed et al. 2001). Relative to the other fibroblasts, cells from line AG04382 naturally demonstrated more staining for senescence associated beta galactosidase (Figure 1B), slower proliferation, increased MMP1/2 activity and an increased cytoplasmic to nuclear ratio (data not shown). Both AG04382 and media from AG04152, a cell line that did not highly express features of senescence (Figure 1A), induced significant increases in proliferation relative to control media (DMEM with 1%) at 48 and 72 hours. However, conditioned media from AG04382 had a significantly greater effect than that of AG04152 on the proliferation of BPH1 cells at 72 hours (Figure 1C).
Figure 1.
Aged fibroblasts that demonstrate features of senescence produce conditioned media that significantly increases proliferation of BPH1 cells. Panel B is a representative image of dermal fibroblasts from aged donor AG04382 showing increased expression of senescence associated β-galactosidase (SA-β-Gal) (arrows) relative to cells from aged donor AG04152 (Panel A). Magnification is 400X. Panel C shows that relative to control media (DMEM with 1% FBS), conditioned media from both AG04152 and AG04382 stimulate the proliferation of BPH1 cells at 48 hours and 72 hours (*=p<0.03 at both 48 and 72 hours), but the effect of AG04382 is greater than that of conditioned media from AG04152 at 72 hours (**=p<0.002). Data are mean +/− standard deviation.
The cytokine profiles of conditioned media from aged fibroblasts differ from that of non-senescent fibroblasts
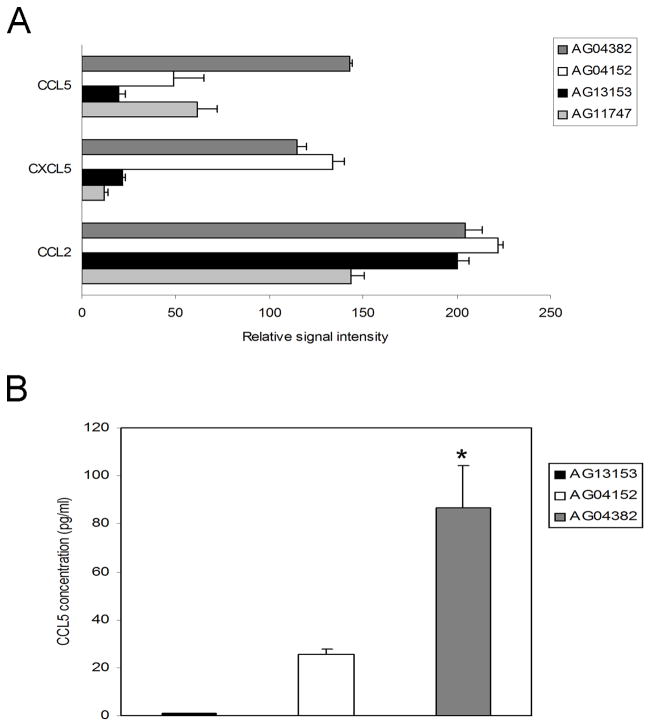
The secretory profile of the 4 fibroblast lines were analyzed, focusing on cytokines which have been reported to be increasingly expressed by aged prostate stromal cells (Giri and Ittmann 2000; Giri and Ittmann 2001; Castro et al. 2003; Castro et al. 2004; Konig et al. 2004; Begley et al. 2005; Ao et al. 2007; Begley et al. 2007). Using RayBio human cytokine antibody arrays, we noted significant differences in relative secretion of CXCL5, CCL5, and CCL2 among the cell lines (Figure 2A). Unlike CXCL5 and CCL2, CCL5 expression was higher in media from AG04382 relative to the other fibroblasts. A CCL5 ELISA confirmed increased expression of CCL5 in the conditioned media of line AG04382 compared to the other fibroblast lines (Figure 2B).
Figure 2.
Fibroblasts that demonstrate features of senescence secrete greater amounts of CCL5. Panel A shows cytokines that differed in the conditioned media of the 4 fibroblast lines as measured by a protein array. Expression of cytokines CCL2/MCP-1, CXCL5/ENA78, and CCL5/RANTES varied among aged fibroblast donors AG04382 and AG04152 and younger donors AG13153 and AG11747. Note that CCL5 is highly expressed in conditioned media from AG04382 relative to the other cell lines. The increase in CCL5 in the conditioned media from AG04382, compared to the media from other lines, was confirmed by specific ELISA as shown in Panel B. Data is mean +/− standard deviation. *=p<0.02. AG04382 relative to AG04152.
Blockade of CCL5 in the conditioned media of senescent fibroblasts inhibits the proliferation of BPH cells
We next sought to determine the effect of blocking CCL5 in the conditioned media of AG04382 on BPH1 proliferation. BPH1 cells were used because they are an SV40-T immortalized primary human prostate epithelial cell line derived from hyperplastic prostatic tissue and maintain a consistent phenotype in cell culture. Neutralizing antibodies directed against CCL5, CXCL5, or CCL2 were added to the conditioned media from AG04382 and then incubated with BPH1 cells in a proliferation assay. GM6001 was added as a complete inhibitor of proliferation. Blocking antibodies to CCL5 significantly reduced proliferation relative to cells incubated in conditioned media alone or with conditioned media containing blocking antibodies to CCL2 or CXCL5 (Figure 3). These results suggest that CCL5 is a specific chemokine in the media from AG04382 that promotes the growth of BPH1 cells.
Figure 3.
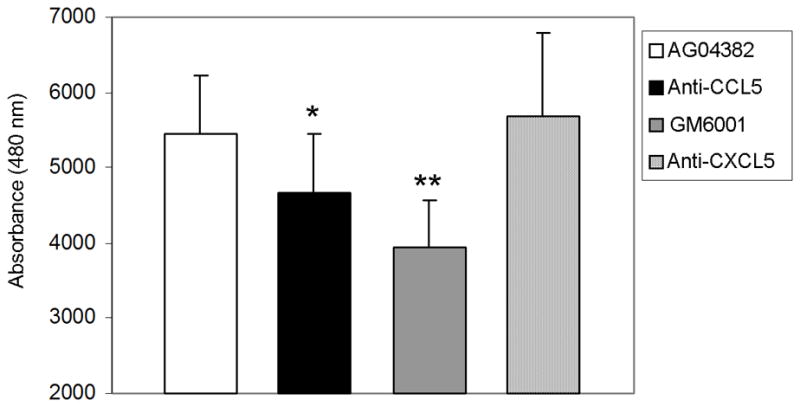
Blockade of CCL5 in the conditioned media of AG04382 inhibits the proliferation of BPH1 cells. Figure shows the effect of a blocking antibody to CCL5 on the proliferation of BPH1 cells. Both 8 and 16 μg/ml of blocking antibody added to conditioned media from AG04382 significantly inhibits proliferation of BPH1 cells. Blocking antibodies to CXCL5 did not have an effect on BPH1 proliferation. GM6001 is shown as a negative control. Data are mean +/− standard deviation. * and **=p<0.03 and <0.01, anti-CCL5 and GM6001 compared to AG04382, respectively.
Addition of CCL5 induces increased migration and proliferation
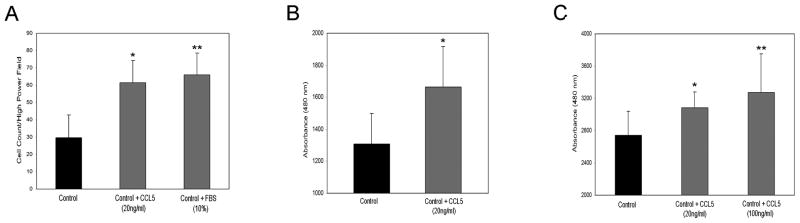
It is established that CCL5 induces proliferation, migration, and invasiveness of prostate and other tumor cells (Stormes et al. 2005; Vaday et al. 2006). Similarly, BPH1 cells stimulated with bioactive recombinant human CCL5 (20ng/ml in control media) significantly increased migration across an 8μm pore Transwell membrane compared to control media (DMEM with 1% FBS) alone. DMEM with 10% FBS was used as a positive control (Figure 4A).
Figure 4.
Addition of recombinant human CCL5 increases migration and proliferation of BPH1 cells. Panel A shows the increase, relative to control media, in the migration of BPH1 cells across an 8μm transwell membrane in the presence of CCL5 (20ng/ml in control media). DMEM with 10% FBS served as a positive stimulus. * and **=p<0.01 and <0.01, CCL5 and 10% FBS compared to control media, respectively. Panel B shows a significant increase in BPH1 proliferation after 24 hours of treatment with 20 ng/ml of CCL5 compared to control media. *=p<0.02. Stimulation with rhuCCL5 at both 20 ng/ml and 100 ng/ml also increased the proliferation of primary prostate epithelial cells (Panel C). * and **=p<0.05 and <0.05, CCL5 20ng/ml and CCL5 100ng/ml compared to control media, respectively. All data are mean +/− standard deviation.
The addition of rhuCCL5 (20ng/ml) to control media also resulted in significantly increased proliferation of BPH1 cells relative to BPH1 cells grown in control media (DMEM with 1%FBS) for 24 hours (Figure 4B). A similar increase in proliferation was noted when a primary prostate epithelial cell line was stimulated for 24 hours with both 20ng and 100ng/ml of rhuCCL5 (Figure 4C).
Stimulation of BPH1 cells with CCL5 influences the gene profile
We then examined the effect of CCL5 stimulation on the expression of BPH1 genes representing regulators of cell adhesion, matrix turnover, and angiogenesis. Two separate 96 gene arrays (one for adhesion molecules and one for regulators of angiogenesis) were examined in duplicate experiments on separate dates. CCL5 (20ng/ml) had little influence on matrix and adhesion molecules with significant, but not large, increases noted in integrin alpha 7, NCAM, and collagen XV alpha 1 and small decreases in laminin alpha 2.
CCL5 did not alter BPH1 gene expression of key regulators of matrix and angiogenesis, such as MMP9, vascular endothelial cell growth factor (VEGF) A, or thrombospondin (TSP) 1. CCL5 stimulation enhanced expression of Chromogranin A, a secretory protein released by neuroendocrine cells, and tumstatin, a cleavage fragment from collagen type IV alpha 3 that inhibits formation of new blood vessels. Of greater relevance to prostate cells and tissues, CCL5 induced significant and sizeable increases in BPH1 transcripts for the pro-angiogenic peptides angiogenin and pleiotrophin and decreases in the angiogenesis inhibitor interleukin 12β (Figure 5).
Figure 5.
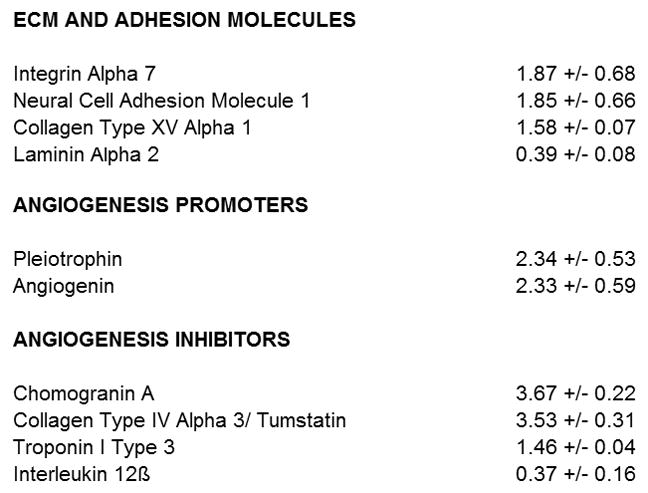
Stimulation of BPH1 cells with rhuCCL5 modulates a limited number of genes representing adhesion molecules and regulators of angiogenesis. Figure shows all the genes from 2 separate 96 gene RT PCR pathway arrays (Matrix and Adhesion Molecules and Angiogenesis Promoters and Inhibitors, SA Biosciences) that demonstrate at least a >40% increase or decrease in BPH1 gene expression after stimulation with rhuCCL5 (20ng/ml). Relative to the other pathways, CCL5 induced large changes in transcripts for genes that regulate angiogenesis. Of note, pro-angiogenic molecules that are highly expressed in the prostate are increased in response to CCL5.
Discussion
The secretory profile of aged cells differs markedly from those of their non-aged counterparts (Reed et al. 2001; Rumpold et al. 2002; Begley et al. 2008). This stimulatory activity is enhanced when a subset of the cells exhibit senescence (Bavik et al. 2006). The subsequent secretion products are believed to contribute to age related diseases, such as benign proliferative hyperplasia (BPH) of the prostate, a significant source of morbidity for older men (Untergasser et al. 2005). In BPH, nodules comprised of stromal cells, epithelial cells, and matrix components increase in prevalence and size in the transitional and periurethral zones of the prostate (Choi et al. 2000; Begley et al. 2008). Nodules grow, in part, in response to mediators expressed by aged prostate cells that have both direct and indirect effects on promotion of cell growth and matrix deposition. For example, over expression of CXCL12 by aged fibroblasts enhances proliferation of human prostate epithelial cells (Begley et al. 2005) and IL-1 secretion by prostate epithelial cells in hyperplastic nodules results in increased stromal cell production of FGF7 (Giri and Ittmann 2000; Castro et al. 2004). Indeed, many studies of the aged prostate have focused on a broad range of cytokines that are traditionally thought of as inflammatory markers, but are also potent paracrine modulators of stromal-epithelial interactions (Giri and Ittmann 2000; Giri and Ittmann 2001; Castro et al. 2004; Konig et al. 2004; Begley et al. 2005; Ao et al. 2007; Begley et al. 2007). Whether inflammation is the starting point or a response to mechanical changes due to prostate enlargement is a matter of ongoing debate (Mishra et al. 2007; Nickel 2008). Certainly, many BPH nodules demonstrate rapidly replicating stromal and epithelial cells, accompanied by marked deposition of extracellular matrix proteins, in the absence of significant inflammation (True et al. 2008).
In this study, we identified the chemokine CCL5 as a specific product of aged fibroblasts that induces the proliferation of BPH1 cells as well as primary prostate epithelial cells. BPH1 cells were utilized because of their derivation from a prostatic hyperplasia and their consistent phenotype in culture (Hayward et al. 1995). CCL5, also known as RANTES (Regulated upon activation, normal t-cell regulated and secreted), is an 8kD pro-angiogenic cytokine that is secreted by many cell types, including stromal cells. CCL5 is a member of a large family of molecules whose expression is activated by inflammatory signals as well as cellular senescence (Singh et al. 2007; Begley et al. 2008). Receptors for CCL5 include CCR1, CCR3, and CCR5 (Elsner et al. 2000; Capoulade-Metay et al. 2006; Vaday et al. 2006; Aldinucci et al. 2008). CCL5 promotes proliferation and is chemotactic for T-cells, white blood cells, and endothelial cells, thereby playing a key role in recruiting compounds to inflammatory sites (Bakhiet et al. 2001; Corti et al. 2001; Adler et al. 2003; Crola Da Silva et al. 2008). CCL5 has myriad interactions with other bioactive molecules. For example, CCL5 is highly associated with regulation of MMPs, a family of enzymes that control matrix turnover. CCL5 promotes the expression of MMP9 by THP-1 monocytic cells and is correlated with decreased gene expression of MMP2, MMP3, MMP10 and MMP17 (Stormes et al. 2005).
CCL5 stimulates prostate cancer invasion and its expression in senescent prostate stroma is associated with tumor progression (Vaday et al. 2006). CCL5 is highly expressed in malignant tissues (Borczuk et al. 2008), but much less is known about the potential role of CCL5 in stromal-epithelial interactions that contribute to benign processes, such as BPH. In the normal human prostate, the tissue vasculature remains stable due to the predominance of angiogenesis inhibitors in the tissue matrix. During BPH progression, this balance shifts to favor angiogenesis. Key pro-angiogenic mediators are increased, e.g. VEGF and FGF2 and MMPs, at the same time endothelial inhibitors, e.g. Thrombospondin 1, are down regulated (Doll et al. 2001). CCL5 increases vascularity in the chick membrane assay of angiogenesis in vivo (Azenshtein et al. 2002) and promotes angiogenesis in stimulated tissues (Westerweel et al. 2008), but its relationship to vessel growth in the prostate remains to be defined.
Based on the studies above, we focused on potential CCL5 effects on gene expression of molecules that regulate angiogenesis, cell adhesion and matrix turnover, all factors that contribute to prostate pathology in aging. Surprisingly, CCL5 did not significantly alter the expression of BPH1 genes for traditional growth factors, such as VEGF, or potent regulators of matrix turnover, such as the MMPs. Moreover, CCL5 had little influence on matrix and adhesion proteins with small increases noted only for genes representing integrin alpha 7 and NCAM, as well as collagen XV. CCL5 stimulation also resulted in decrease in BPH1 gene expression of laminin alpha 2. The implications of these relatively minor changes are unclear as integrin alpha 7 is a tumor suppressor gene in prostate cancer, whereas expression of collagen XV is increased in prostate cancer (Gaston et al. 2005; Ren et al. 2007)
Stimulation with CCL5 also resulted in increases in gene expression of potential angiogenesis inhibitors, such as chromogranin A and tumstatin (Collagen type IV, alpha 3). Chromogranin A is expressed at very low levels in most tissues except for neuroendocrine tumors (Belloni et al. 2007). Tumstatin is a member of the large family of collagen cleavage products that contribute to endothelial cell function (Hamano and Kalluri 2005). Other molecules in this category include endostatin and angiostatin (Nyberg et al. 2005). Although not readily detected in prostate tissue, these fragments of extracellular matrix proteins are potential focal modulators of vessel growth in situ.
Of greater relevance to the prostate, CCL5 significantly enhanced BPH1 gene expression of the ubiquitous molecules, angiogenin and pleiotrophin, both potent inducers of angiogenesis. Angiogenin is a potent polypeptide that is known to directly promote the growth of new vessels in the prostate (Katona et al. 2005; Yoshioka et al. 2006). Pleiotrophin is a widely expressed heparin binding cytokine that initiates angiogenesis through multiple pathways including augmenting the expression of the growth factors FGF and VEGF (Perez-Pinera et al. 2007; Perez-Pinera et al. 2008). Although pleiotrophin has been previously reported not to be present in BPH (Vacherot et al. 1999), recent descriptions of its multifunctional nature (Magnusson et al. 2007; Perez-Pinera et al. 2008) indicate it is likely to be at least transiently operative during prostate nodule growth. In addition, CCL5 inhibited the expression of the cytokine interleukin 12β, a potential paracrine inhibitor of angiogenesis.
In summary, we have found that aged fibroblasts with features of the senescent phenotype specifically increase secretion of the chemokine, CCL5. The latter induces the proliferation of BPH1 cells. Moreover, stimulation of BPH1 cells with CCL5 enhances the expression of genes that are modulators of angiogenesis. We propose that CCL5 is a mediator of blood vessel development during prostate growth in the aged host.
Acknowledgments
Funding: NIH U54 CA126540 (SP) and R01 AG 15837 (MJR)
Literature Cited
- Adler EP, Lemken CA, Katchen NS, Kurt RA. A dual role for tumor-derived chemokine RANTES (CCL5) Immunol Lett. 2003;90(2–3):187–94. doi: 10.1016/j.imlet.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A, Colombatti A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122(4):769–76. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–53. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62(4):1093–102. [PubMed] [Google Scholar]
- Bakhiet M, Tjernlund A, Mousa A, Gad A, Stromblad S, Kuziel WA, Seiger A, Andersson J. RANTES promotes growth and survival of human first-trimester forebrain astrocytes. Nat Cell Biol. 2001;3(2):150–7. doi: 10.1038/35055057. [DOI] [PubMed] [Google Scholar]
- Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5(8):655–62. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66(2):794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005;4(6):291–8. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Begley LA, MacDonald JW, Day ML, Macoska JA. CXCL12 activates a robust transcriptional response in human prostate epithelial cells. J Biol Chem. 2007;282(37):26767–74. doi: 10.1074/jbc.M700440200. [DOI] [PubMed] [Google Scholar]
- Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43(2):194–9. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni D, Scabini S, Foglieni C, Veschini L, Giazzon A, Colombo B, Fulgenzi A, Helle KB, Ferrero ME, Corti A, Ferrero E. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. Faseb J. 2007;21(12):3052–62. doi: 10.1096/fj.06-6829com. [DOI] [PubMed] [Google Scholar]
- Borczuk AC, Papanikolaou N, Toonkel RL, Sole M, Gorenstein LA, Ginsburg ME, Sonett JR, Friedman RA, Powell CA. Lung adenocarcinoma invasion in TGFbetaRII-deficient cells is mediated by CCL5/RANTES. Oncogene. 2008;27(4):557–64. doi: 10.1038/sj.onc.1210662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Capoulade-Metay C, Ayouba A, Kfutwah A, Lole K, Petres S, Dudoit Y, Deterre P, Menu E, Barre-Sinoussi F, Debre P, Theodorou I. A natural CCL5/RANTES variant antagonist for CCR1 and CCR3. Immunogenetics. 2006;58(7):533–41. doi: 10.1007/s00251-006-0133-2. [DOI] [PubMed] [Google Scholar]
- Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55(1):30–8. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- Castro P, Xia C, Gomez L, Lamb DJ, Ittmann M. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate. 2004;60(2):153–9. doi: 10.1002/pros.20051. [DOI] [PubMed] [Google Scholar]
- Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56(1):160–6. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- Corti S, Salani S, Del Bo R, Sironi M, Strazzer S, D’Angelo MG, Comi GP, Bresolin N, Scarlato G. Chemotactic factors enhance myogenic cell migration across an endothelial monolayer. Exp Cell Res. 2001;268(1):36–44. doi: 10.1006/excr.2001.5267. [DOI] [PubMed] [Google Scholar]
- Crola Da Silva C, Lamerant-Fayel N, Paprocka M, Mitterrand M, Gosset D, Dus D, Kieda C. Selective human endothelial cell activation by chemokines as a guide to cell homing. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98(2):245–9. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo BR, Pignolo RJ, Cristofalo VJ. Identification of proteins differentially expressed in quiescent and proliferatively senescent fibroblast cultures. Exp Cell Res. 1995;220(1):178–85. doi: 10.1006/excr.1995.1304. [DOI] [PubMed] [Google Scholar]
- Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49(4):293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Littlepage LE, Werb Z. The fibroblastic coconspirator in cancer progression. Cold Spring Harb Symp Quant Biol. 2005;70:383–8. doi: 10.1101/sqb.2005.70.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner J, Mack M, Bruhl H, Dulkys Y, Kimmig D, Simmons G, Clapham PR, Schlondorff D, Kapp A, Wells TN, Proudfoot AE. Differential activation of CC chemokine receptors by AOP-RANTES. J Biol Chem. 2000;275(11):7787–94. doi: 10.1074/jbc.275.11.7787. [DOI] [PubMed] [Google Scholar]
- Gaston SM, Soares MA, Siddiqui MM, Vu D, Lee JM, Goldner DL, Brice MJ, Shih JC, Upton MP, Perides G, Baptista J, Lavin PT, Bloch BN, Genega EM, Rubin MA, Lenkinski RE. Tissue-print and print-phoresis as platform technologies for the molecular analysis of human surgical specimens: mapping tumor invasion of the prostate capsule. Nat Med. 2005;11(1):95–101. doi: 10.1038/nm1169. [DOI] [PubMed] [Google Scholar]
- Giri D, Ittmann M. Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2000;157(1):249–55. doi: 10.1016/s0002-9440(10)64535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri D, Ittmann M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2001;159(1):139–47. doi: 10.1016/S0002-9440(10)61681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y, Kalluri R. Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333(2):292–8. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31(1):14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Katona TM, Neubauer BL, Iversen PW, Zhang S, Baldridge LA, Cheng L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin Cancer Res. 2005;11(23):8358–63. doi: 10.1158/1078-0432.CCR-05-0962. [DOI] [PubMed] [Google Scholar]
- Konig JE, Senge T, Allhoff EP, Konig W. Analysis of the inflammatory network in benign prostate hyperplasia and prostate cancer. Prostate. 2004;58(2):121–9. doi: 10.1002/pros.10317. [DOI] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67(7):3117–26. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Magnusson PU, Dimberg A, Mellberg S, Lukinius A, Claesson-Welsh L. FGFR-1 regulates angiogenesis through cytokines interleukin-4 and pleiotrophin. Blood. 2007;110(13):4214–22. doi: 10.1182/blood-2007-01-067314. [DOI] [PubMed] [Google Scholar]
- Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, Motiwala HG, Laniado ME. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100(2):327–31. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- Nelen V. Epidemiology of prostate cancer. Recent Results Cancer Res. 2007;175:1–8. doi: 10.1007/978-3-540-40901-4_1. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Inflammation and benign prostatic hyperplasia. Urol Clin North Am. 2008;35(1):109–15. vii. doi: 10.1016/j.ucl.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65(10):3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Berenson JR, Deuel TF. Pleiotrophin, a multifunctional angiogenic factor: mechanisms and pathways in normal and pathological angiogenesis. Curr Opin Hematol. 2008;15(3):210–4. doi: 10.1097/MOH.0b013e3282fdc69e. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Chang Y, Deuel TF. Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment, and activation of stromal fibroblasts. Cell Cycle. 2007;6(23):2877–83. doi: 10.4161/cc.6.23.5090. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev. 2001;122(11):1203–20. doi: 10.1016/s0047-6374(01)00260-3. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158(1):169–79. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- Ren B, Yu YP, Tseng GC, Wu C, Chen K, Rao UN, Nelson J, Michalopoulos GK, Luo JH. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst. 2007;99(11):868–80. doi: 10.1093/jnci/djk199. [DOI] [PubMed] [Google Scholar]
- Rumpold H, Mascher K, Untergasser G, Plas E, Hermann M, Berger P. Trans-differentiation of prostatic stromal cells leads to decreased glycoprotein hormone alpha production. J Clin Endocrinol Metab. 2002;87(11):5297–303. doi: 10.1210/jc.2002-020596. [DOI] [PubMed] [Google Scholar]
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51(9):1119–30. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26(3–4):453–67. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger CC, Plymate SR, Reed MJ. Extracellular influences on tumour angiogenesis in the aged host. Br J Cancer. 2008;98(2):250–255. doi: 10.1038/sj.bjc.6604144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormes KA, Lemken CA, Lepre JV, Marinucci MN, Kurt RA. Inhibition of metastasis by inhibition of tumor-derived CCL5. Breast Cancer Res Treat. 2005;89(2):209–12. doi: 10.1007/s10549-004-5328-3. [DOI] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. lab Invest. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- True LD, Hawley S, Norwood TH, Braun KR, Evanko SP, Chan CK, Lebaron RC, Wight TN. The accumulation of versican in the nodules of benign prostatic hyperplasia. Prostate. 2008 doi: 10.1002/pros.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005;40(3):121–8. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vacherot F, Caruelle D, Chopin D, Gil-Diez S, Barritault D, Caruelle JP, Courty J. Involvement of heparin affin regulatory peptide in human prostate cancer. Prostate. 1999;38(2):126–36. doi: 10.1002/(sici)1097-0045(19990201)38:2<126::aid-pros6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66(2):124–34. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- Westerweel PE, Rabelink TJ, Rookmaaker MB, Grone HJ, Verhaar MC. RANTES is required for ischaemia-induced angiogenesis, which may hamper RANTES-targeted anti-atherosclerotic therapy. Thromb Haemost. 2008;99(4):794–5. doi: 10.1160/TH07-10-0628. [DOI] [PubMed] [Google Scholar]
- Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci U S A. 2006;103(39):14519–24. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]