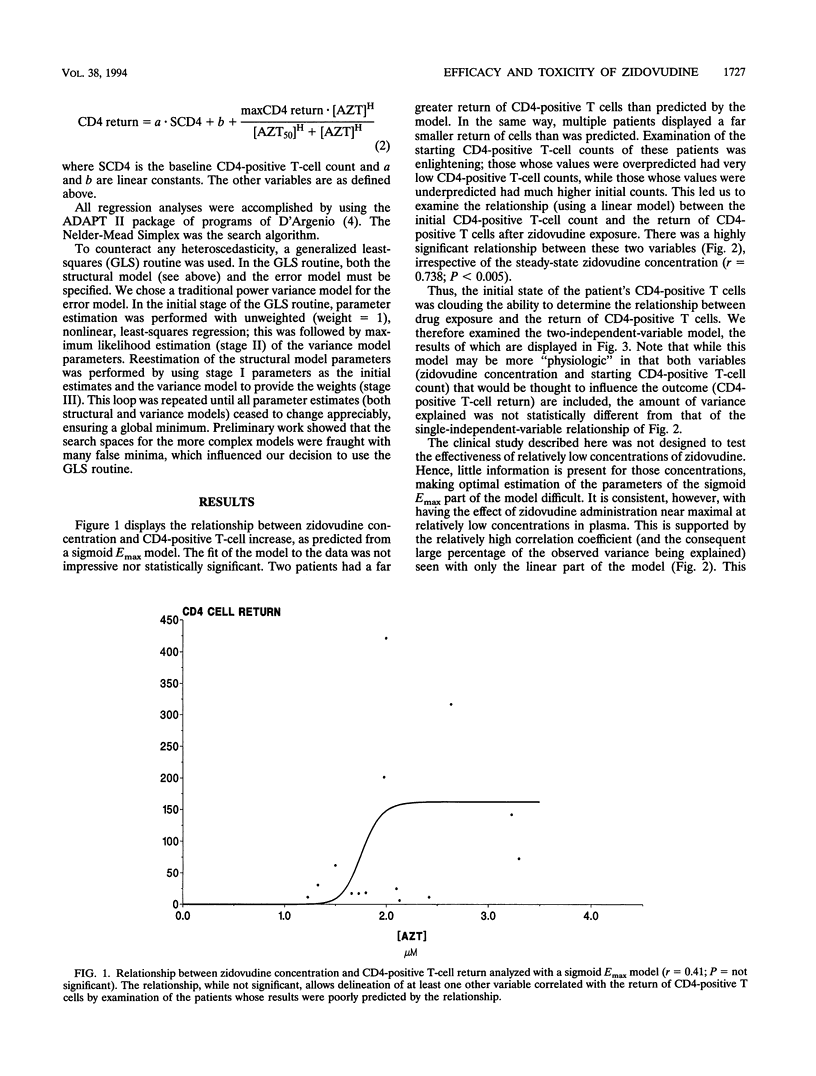
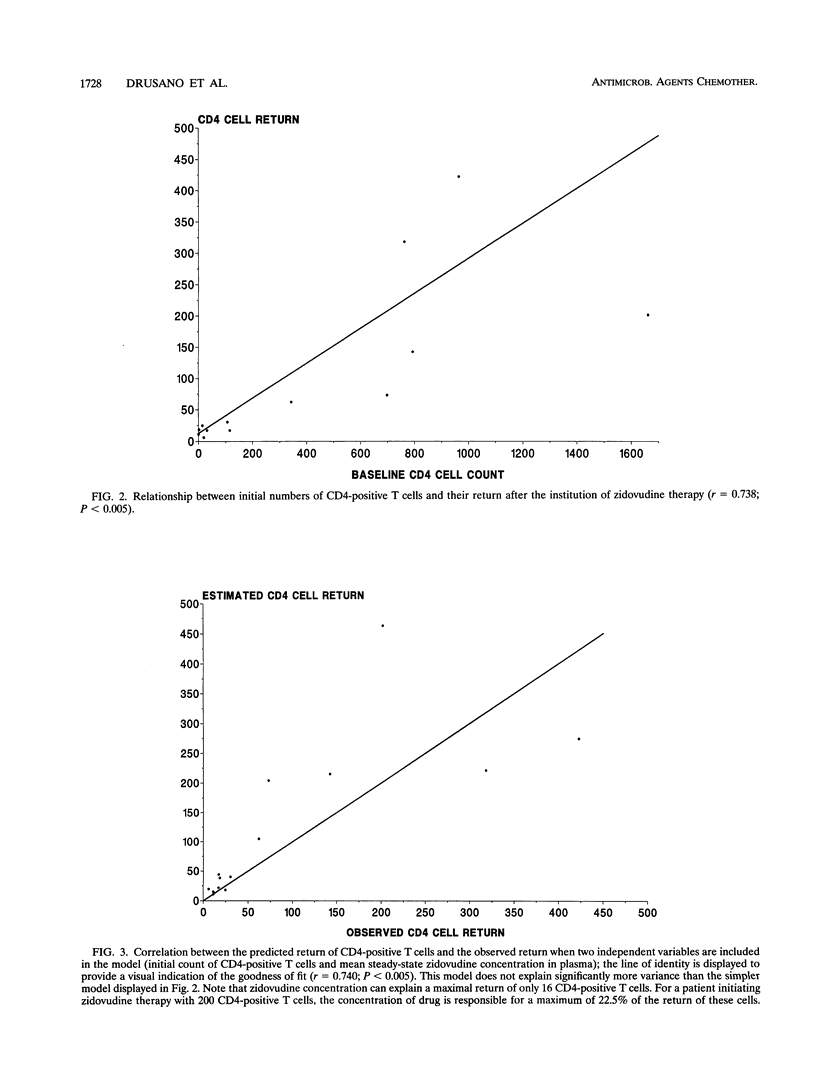
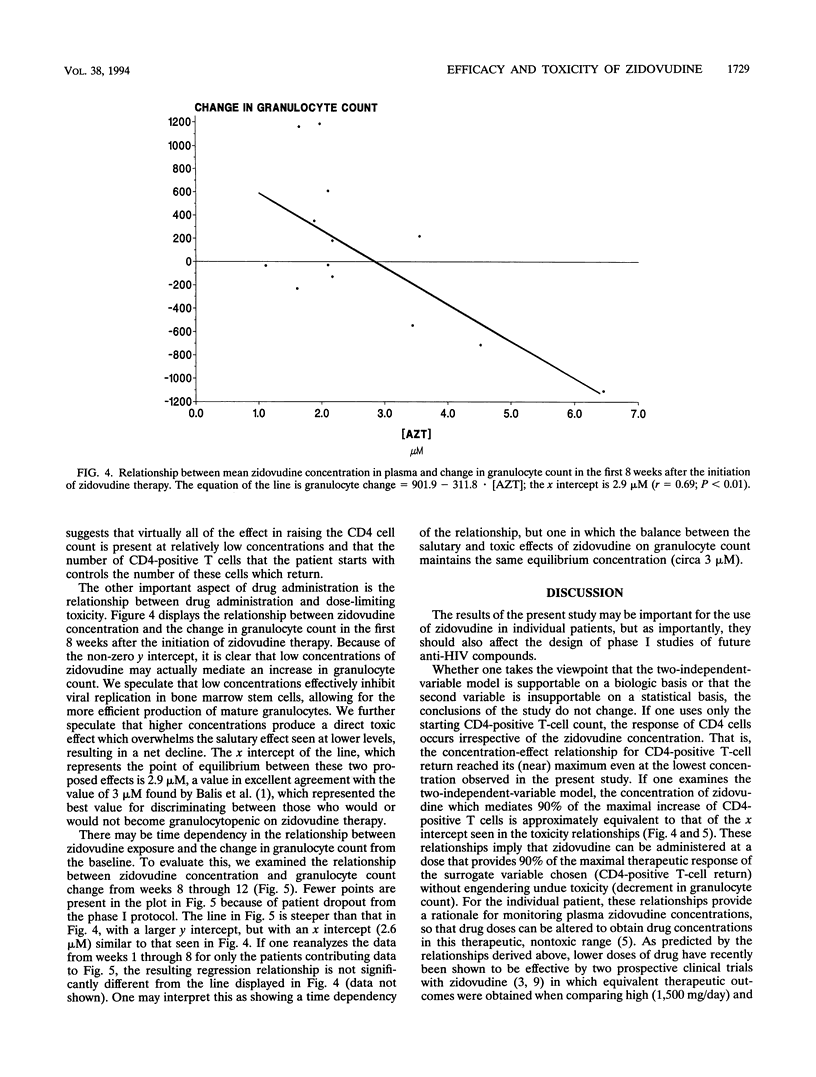
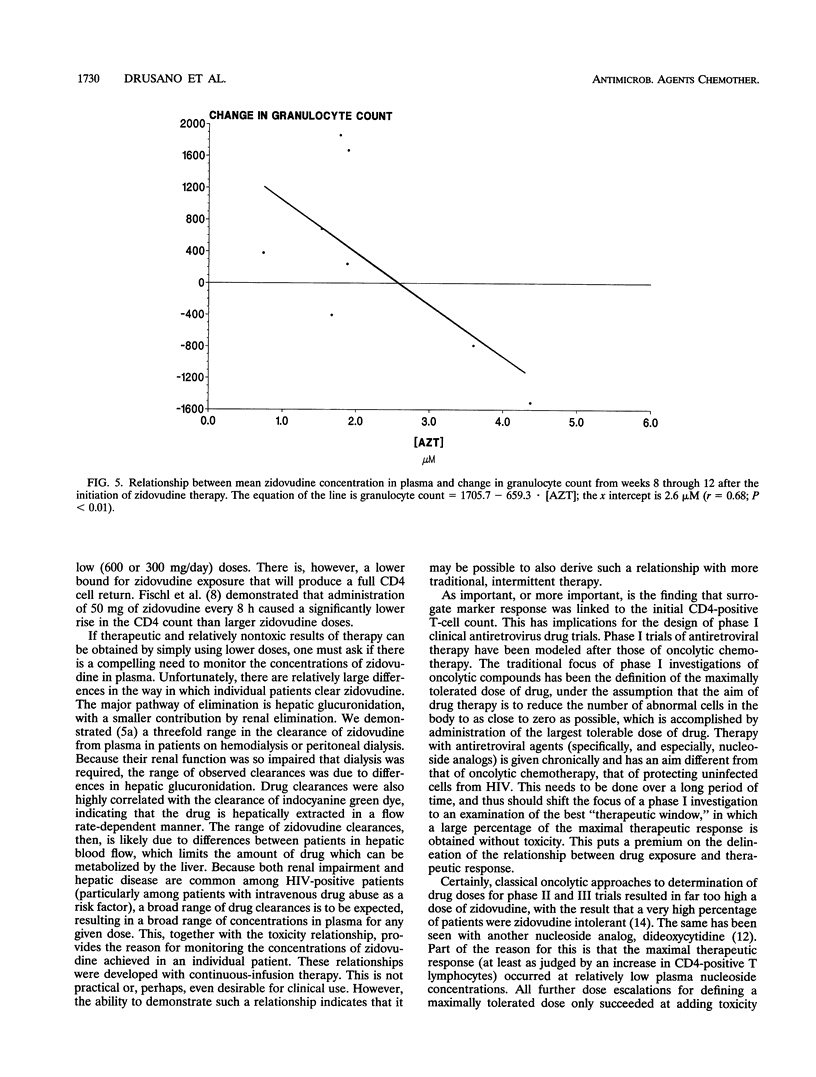
Abstract
We examined the relationship between the concentrations of zidovudine in plasma given by continuous intravenous infusion to human immunodeficiency virus-positive pediatric patients and a surrogate marker of outcome (measured by the increase in the number of CD4-positive T cells) as well as drug-mediated toxicity (change in granulocyte count). The return of CD4-positive T cells was most strongly related to the number of these cells present at the start of therapy. Drug concentration data added little explanatory power to this relationship, indicating that the effect of zidovudine was near maximal throughout the range of concentrations examined. The change in granulocyte count was significantly correlated with zidovudine concentration both from weeks 1 through 8 and from weeks 8 through 12. These findings imply that it may be wise to stratify phase I antiretrovirus drug trials for the entry level of CD4-positive T cells if pharmacodynamic relationships with this marker as the dependent variable are to be sought. Continued efforts need to be made to derive quantitative relationships between drug exposure and measures of both efficacy and toxicity so that the maximal amount of information is derived from small phase I studies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balis F. M., Pizzo P. A., Murphy R. F., Eddy J., Jarosinski P. F., Falloon J., Broder S., Poplack D. G. The pharmacokinetics of zidovudine administered by continuous infusion in children. Ann Intern Med. 1989 Feb 15;110(4):279–285. doi: 10.7326/0003-4819-110-4-279. [DOI] [PubMed] [Google Scholar]
- Choi S., Lagakos S. W., Schooley R. T., Volberding P. A. CD4+ lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993 May 1;118(9):674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- Collier A. C., Bozzette S., Coombs R. W., Causey D. M., Schoenfeld D. A., Spector S. A., Pettinelli C. B., Davies G., Richman D. D., Leedom J. M. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N Engl J Med. 1990 Oct 11;323(15):1015–1021. doi: 10.1056/NEJM199010113231502. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Yuen G. J., Lambert J. S., Seidlin M., Dolin R., Valentine F. T. Relationship between dideoxyinosine exposure, CD4 counts, and p24 antigen levels in human immunodeficiency virus infection. A phase I trial. Ann Intern Med. 1992 Apr 1;116(7):562–566. doi: 10.7326/0003-4819-116-7-562. [DOI] [PubMed] [Google Scholar]
- Egorin M. J., Van Echo D. A., Olman E. A., Whitacre M. Y., Forrest A., Aisner J. Prospective validation of a pharmacologically based dosing scheme for the cis-diamminedichloroplatinum(II) analogue diamminecyclobutanedicarboxylatoplatinum. Cancer Res. 1985 Dec;45(12 Pt 1):6502–6506. [PubMed] [Google Scholar]
- Fischl M. A., Parker C. B., Pettinelli C., Wulfsohn M., Hirsch M. S., Collier A. C., Antoniskis D., Ho M., Richman D. D., Fuchs E. A randomized controlled trial of a reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1990 Oct 11;323(15):1009–1014. doi: 10.1056/NEJM199010113231501. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Kahn J. O., Lagakos S. W., Richman D. D., Cross A., Pettinelli C., Liou S. H., Brown M., Volberding P. A., Crumpacker C. S., Beall G. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. The NIAID AIDS Clinical Trials Group. N Engl J Med. 1992 Aug 27;327(9):581–587. doi: 10.1056/NEJM199208273270901. [DOI] [PubMed] [Google Scholar]
- Meng T. C., Fischl M. A., Boota A. M., Spector S. A., Bennett D., Bassiakos Y., Lai S. H., Wright B., Richman D. D. Combination therapy with zidovudine and dideoxycytidine in patients with advanced human immunodeficiency virus infection. A phase I/II study. Ann Intern Med. 1992 Jan 1;116(1):13–20. doi: 10.7326/0003-4819-116-1-13. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Skowron G. Safety and tolerance of dideoxycytidine as a single agent. Results of early-phase studies in patients with acquired immunodeficiency syndrome (AIDS) or advanced AIDS-related complex. Study Group of the AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. Am J Med. 1990 May 21;88(5B):11S–15S. doi: 10.1016/0002-9343(90)90415-a. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Eddy J., Falloon J., Balis F. M., Murphy R. F., Moss H., Wolters P., Brouwers P., Jarosinski P., Rubin M. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988 Oct 6;319(14):889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Jusko W. J. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed. 1983 Jun;16(3):203–216. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Volberding P. A., Lagakos S. W., Koch M. A., Pettinelli C., Myers M. W., Booth D. K., Balfour H. H., Jr, Reichman R. C., Bartlett J. A., Hirsch M. S. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med. 1990 Apr 5;322(14):941–949. doi: 10.1056/NEJM199004053221401. [DOI] [PubMed] [Google Scholar]


