Abstract
The transition-metal-catalyzed functionalization of C-H bonds is a powerful method for generating carbon-carbon bonds. Although significant advances to this field have been reported during the last decade, many challenges remain. First, most of the methods are substrate-specific and thus cannot be generalized. Second, conversions of unactivated (i.e. not benzylic or alpha to heteroatom) sp3 C–H bonds to C–C bonds are rare, with most examples limited to t-butyl groups—a conversion that is inherently simple because there are no β-hydrogens that can be eliminated. Finally, the palladium, rhodium, and ruthenium catalysts routinely used for the conversion of C–H bonds to C–C bonds are expensive. Catalytically active metals that are cheaper and less exotic (e.g. copper, iron, and manganese) are rarely used.
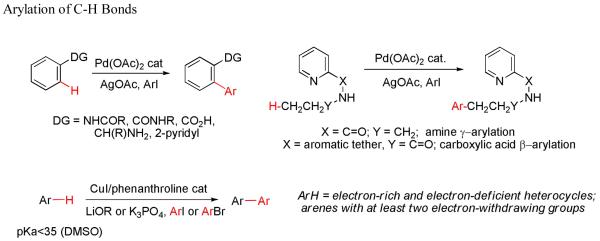
This Account describes our attempts to provide solutions to these three problems. We have developed a general method for directing-group-containing arene arylation by aryl iodides. Using palladium acetate as the catalyst, we arylated anilides, benzamides, benzoic acids, benzylamines, and 2-substituted pyridine derivatives under nearly identical conditions. We have also developed a method for the palladium-catalyzed auxiliary-assisted arylation of unactivated sp3 C–H bonds. This procedure allows for the β-arylation of carboxylic acid derivatives and the γ-arylation of amine derivatives. Furthermore, copper catalysis can be used to mediate the arylation of acidic arene C–H bonds (i.e. those with pKa values <35 in DMSO). Using a copper iodide catalyst in combination with a base and a phenanthroline ligand, we successfully arylated electron-rich and electron-deficient heterocycles and electron-poor arenes possessing at least two electron-withdrawing groups. The reaction exhibits unusual regioselectivity: arylation occurs at the most hindered position. This copper-catalyzed method supplements the well-known C–H activation/borylation methodology, in which functionalization usually occurs at the least hindered position.
We also describe preliminary investigations to determine the mechanisms of these transformations. We anticipate that other transition metals, including iron, nickel, cobalt, and silver, will also be able to facilitate deprotonation/arylation reaction sequences.
1. Introduction
It can be argued that the goal of any methodology project is to increase the efficiency of the desired chemical transformation. This goal may be achieved either by optimization of the current synthetic methods or by introducing new transformations that allow synthesis of the desired substances to be carried out in fewer steps or in higher yield. Increased efficiency results in labor and energy savings, and is usually environmentally beneficial by decreasing the amount of disposable reaction waste such as byproducts and solvents. In this context, employing C-H bond as a functional group for direct functionalization is advantageous.1 Most of the starting materials that are obtained in large scale from petrochemical sources contain only carbon-carbon and carbon-hydrogen bonds. Thus, the C-H or C-C bonds in these compounds need to be converted into other functional groups. However, selective one-step conversion of C-H bonds to most functional groups usually is not possible and several steps are required to obtain the desired products. Consequently, methods for selective functionalization of C-H bonds may result in shorter synthetic schemes. While the advantages of such procedures were recognized some time ago, formidable difficulties have prevented development of user-friendly, viable, and general methods in this field until the last decade. Majority of the transition-metal-catalyzed C-H bond functionalization examples involve regioselective arylation of sp2 C-H bonds in directing-group-containing arenes2 or electron-rich heterocycles such as azoles or indoles.3 However, these methods often are not general and are usually applicable to only a few substrate classes. Different reaction conditions and catalysts are required even for closely related structures. Formation of carbon-carbon bonds from unactivated (not benzylic or alpha to heteroatom) sp3 C-H bonds is rare,4 and most of the examples described in literature involve functionalization of t-butyl groups that is a particularly easy case due to entropic considerations and lack of β-hydride elimination from metalated intermediates. Additionally, catalysts that are employed for arylation usually are complexes of rhodium, palladium, platinum, and ruthenium. Less expensive metals such as copper or iron only rarely have been used for functionalization of C-H bonds.5
Thus, three major problems can be formulated for C-H bond arylation reactions. First, reactions are not general and that may be a reason why such reactions have only rarely been used in synthetic regime or for industrial applications. Second, unactivated (alkane) sp3 C-H bond conversion to C-C bonds is rare. Third, expensive late transition metals with relatively high catalyst loadings are generally employed. Our efforts described in this Account are directed towards addressing these issues.
2. Discussion
2.1. Directing-Group-Containing Arene Arylation
2.1.1. Anilides
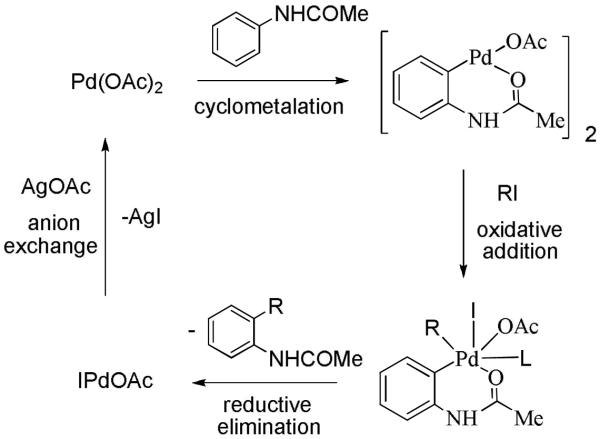
Our interest in this area was spurred by Tremonts’ seminal paper describing palladium acetate-promoted anilide alkylation by alkyl iodides.6 Tremont suggested that the reaction proceeds by either a Pd(II)-Pd(IV) catalytic cycle or a sigma-bond metathesis mechanism.6a The former is more likely due to well-established mechanism for the Catellani reaction where intermediacy of Pd(IV) is not disputed.7 The Pd(II)-Pd(IV) catalytic cycle is presented in Scheme 1. Cyclometallation of the directing-group-containing arene is followed by oxidative addition of aryl or alkyl iodide to Pd(II) species affording a high-energy Pd(IV) intermediate. Fast reductive elimination and anion exchange affords the product and regenerates the catalyst. Inspection of the scheme allows to come to several conclusions. Since Catellani has shown that both alkyl and aryl iodides participate in Pd(II)-Pd(IV) catalytic cycles,7 it should be possible to employ aryl iodides in the reactions. Arylations can be rendered catalytic because ArI is compatible with silver salts used for iodide removal from palladium coordination sphere. It has been shown that Pd(IV) complexes possessing hard ligands are isolable in some cases;8a hence, if arene contains a hard directing group and can be cyclopalladated, we should be able to arylate it. Palladacycles have been studied extensively;8b however, alternative mechanistic pathways for C-H activation by iridium complexes have also been proposed.8c
Scheme 1.
Catalytic Cycle
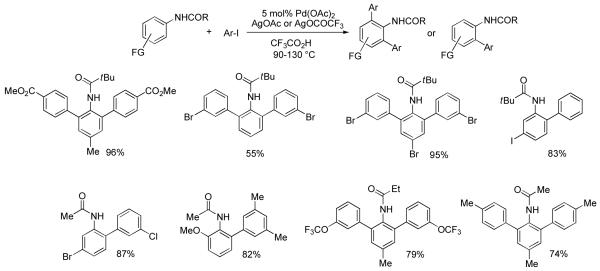
The initial targets were anilides since they are known to be reactive under Tremont conditions. We were pleased to discover that a combination of aryl iodide, silver acetate, and catalytic palladium acetate efficiently arylates pivalanilides (Scheme 2).9a The reactions proceed in trifluoroacetic acid at elevated temperatures affording the ortho-arylated products in good to excellent yields. Interestingly, bromide is tolerated both on the aryl iodide and pivalanilide coupling partners. As a result, scaffolds amenable for further functionalization by using Pd(0)-Pd(II) coupling processes can be obtained. While the pivaloylated anilines afford the cleanest results, it is difficult to remove the acyl group from the arylation product. Use of removable directing groups such as acetyl, propionyl, or trifluoroacetyl would allow a short synthesis of 2-aminobiphenyl- or terphenyl derivatives. After a short optimization, we showed that also acetanilides and propionanilides can be efficiently arylated under conditions similar to the ones for pivalanilide arylation (Scheme 2).9b After deprotection, ortho-amino biphenyl or terphenyl derivatives are obtained in excellent yields. Access to these compounds requires multiple step syntheses from starting materials that may not be readily available. Direct arylation methodology allows for the short and convenient synthesis of such materials. Sanford has reported arylation of anilides by iodonium salts.10 Boronic acids have been employed in arylation of anilides.11
Scheme 2.
Anilide Arylation
2.1.2. Benzamides
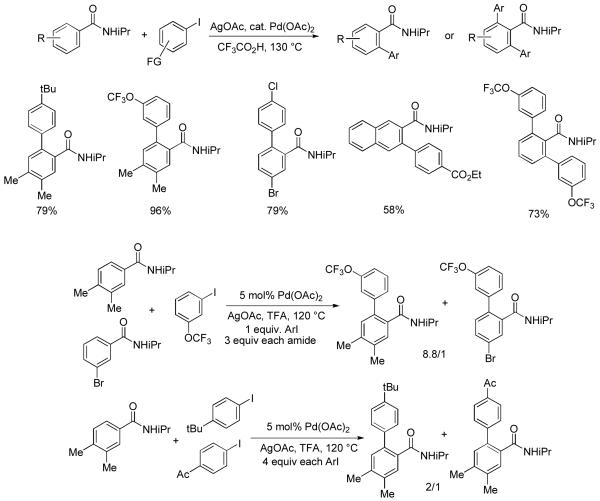
According to Scheme 1, if an arene contains a hard directing group and can be palladated, we should be able to arylate it provided the mechanistic considerations are valid. Miura has demonstrated that benzamide derivatives can be arylated under Pd(0)-Pd(II) catalytic cycle conditions.12 We reasoned that the arylation of benzamide derivatives should be possible also under Pd(II)-Pd(IV) couple conditions. Gratifyingly, conditions that were developed for anilide arylation worked well for benzamide arylation (Scheme 3).13 The best results were obtained with n-propylamide and isopropylamide derivatives. Benzamide derivatives can be arylated by both electron-rich and electron-poor aryl iodides and good functional group tolerance is observed. A competition experiment was carried out by reacting a mixture of an electron-rich and electron-poor benzamide with aryl iodide. The electron-rich benzamide was shown to react preferentially (8.8/1). The faster reaction of electron-rich benzamide can be explained by its preferential coordination to Pd(II) before the metalation step as shown by Sanford.14 More interestingly, electron-rich aryl iodides react faster than electron-poor ones (Scheme 3). The aryl iodide enters the catalytic cycle at the stage of oxidative addition to Pd(II), which is most likely the rate-determining step since arylation rate depends on ArI and reductive elimination from Pd(IV) is expected to be fast.8a Oxidative addition step must be faster for electron-rich aryl iodides which is different from the usual Pd(0)-Pd(II) coupling cycle where electron-deficient aryl halides are more reactive.15 In general, 2- or 3-substituted benzamides are monoarylated, presumably due to steric interference of the substituent. It is well-known that the palladation of sterically hindered positions is unfavorable.8b,d
Scheme 3.
Benzamide Arylation
2.1.3. Benzoic Acid Arylation
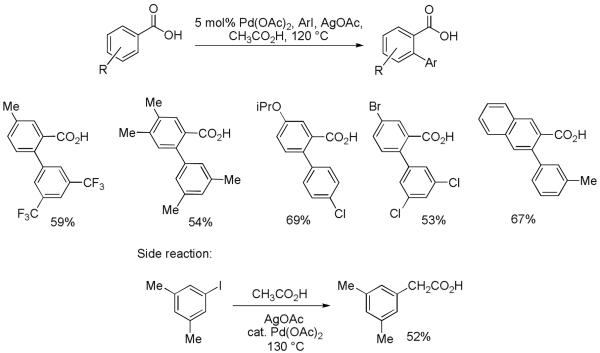
Ortho-arylation of benzoic acids is often preferable to ortho-arylation of benzamides if conversion of amide moiety to other functional group is desired. However, only a few reports have dealt with the ortho-functionalization of free benzoic acids.4e,16a,b Direct functionalization of C-H bonds in carboxylic acids is challenging. The reactions can be complicated by decarboxylation of the product and/or the starting material. If successful, direct arylation of aromatic carboxylic acids would allow one-step synthesis of 2-arylbenzoic acids. Amides of such acids are potent MTP and ApoB inhibitors.16c We were pleased to discover that benzoic acid arylation under Pd(II)-Pd(IV) catalytic cycle conditions proceeds under conditions that are similar to the conditions for benzamide and anilide arylation; however, the optimal solvent for the arylation reaction is acetic acid. One can employ from electron-rich to moderately electron-deficient benzoic acids and from electron-rich to electron-deficient aryl iodides (Scheme 4).17 Reactions generally proceed in lower yields than amide or anilide arylations due to competing solvent arylation (Scheme 4). Thus, temperature needs to be controlled carefully for achieving optimal results. Use of trifluoroacetic acid solvent often results in product or starting material decarboxylation.
Scheme 4.
Benzoic Acid Arylation
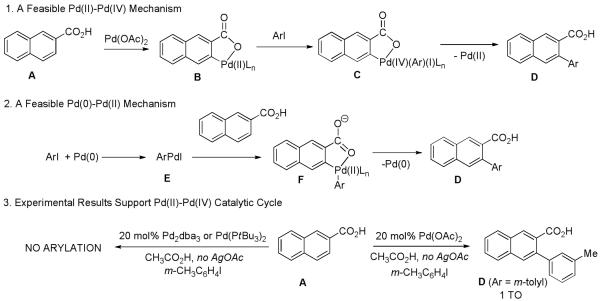
Several mechanistic pathways are possible for benzoic acid arylation (Scheme 5). The reaction may proceed via a Pd(II)-Pd(IV) catalytic cycle (Scheme 5-1). Cyclometallation of benzoic acid A followed by oxidative addition of aryl iodide would afford a high-energy Pd(IV) intermediate C. Reductive elimination would produce the arylated carboxylic acid D. Alternatively, a Pd(0)-Pd(II) catalytic cycle may be operative. The catalytically active Pd(0) is produced by an in situ reduction of Pd(II). Oxidative addition of aryl iodide would afford an arylpalladium iodide E. Cyclometallation followed by reductive elimination would produce the arylated carboxylic acid D and regenerate the Pd(0) catalyst. β-Naphthoic acid was reacted with 3-iodotoluene in the presence of a 20 mol% palladium source. If palladium acetate was used, one turnover to the arylated product D was observed. Reactions in the presence of Pd(0) complexes did not afford tolylnaphthoic acid D. Instead, formation of 3,3′-dimethylbiphenyl was observed. This result suggests a Pd(II)-Pd(IV) catalytic cycle. However, a sigma-bond metathesis mechanism suggested by Tremont can not be rigorously excluded.
Scheme 5.
Mechanistic Considerations
2.1.4. Benzylamine Arylation
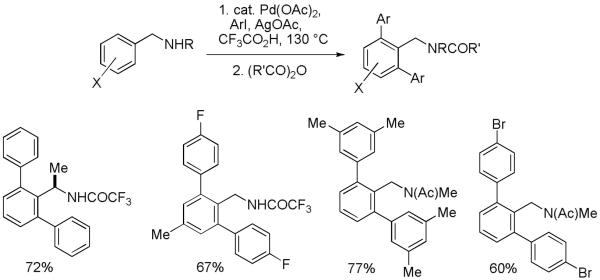
Palladium-catalyzed ortho-arylation is often complementary to the existing lithiation/boronation/cross-coupling methodologies. However, there are cases where ortho-lithiation strategies are not likely to be successful. Examples include functionalization of compounds containing aryl-bromine or iodine bonds or substances possessing multiple acidic protons such as benzylamines. Furthermore, it is known that even unsubstituted benzylamines can be palladated.18 According to mechanistic considerations of Scheme 1, benzylamine arylation should be feasible. Thepalladation of benzylamines in strong acid might be retarded due to protonation of the directing amino group. Employing a limited amount of trifluoroacetic acid solvent allowed to achieve efficient benzylamine arylation. Specifically, the benzylamine arylation reactions are the fastest if about five equivalents of trifluoroacetic acid solvent are used. A number of benzylamines and N-methylbenzylamines were shown to be reactive under these conditions (Scheme 6).19 The products are acylated after the reaction to facilitate isolation.
Scheme 6.
Benzylamine Arylation
2.1.5. Arylation of 2-Substituted Pyridines
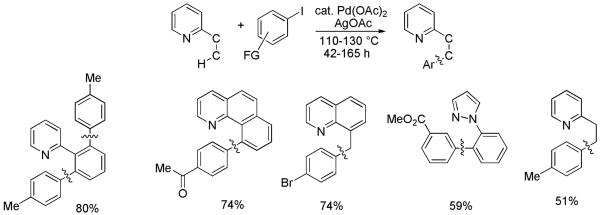
Ruthenium-catalyzed 2-substituted pyridine C-H bond arylation by aryl halides was reported by Oi and Inoue in 2001.20a We were interested in developing a complementary palladium-catalyzed method that would expand the scope of our arylation protocol (Scheme 7).20b Acetic acid solvent is usually employed, and extended reaction times, typically several days, are required. Interestingly, the most reactive substrate is 8-methylquinoline showing that arylation of benzylic sp3 C-H bonds is more facile than the arylation of sp2 C-H bonds. The most interesting arylation example is the p-tolylation of 2-ethylpyridine. This is one of the first examples of palladium-catalyzed C-C bond formation from an unactivated sp3 C-H bond that is not a part of a t-butyl group. Unfortunately, the arylation of other 2-alkylsubstituted pyridines results in product mixtures. Furthermore, requirement for the pyridine directing group limits the generality of the reaction. The generality issue is addressed in Section 2.2. Aryliodonium salts have also been used for 2-substituted pyridine functionalization under Pd(II)-Pd(IV) catalytic cycle.10
Scheme 7.
Pyridine and Pyrazole Derivative Arylation
2.2 Auxiliary-Directed Arylation of sp2 and sp3 C-H Bonds
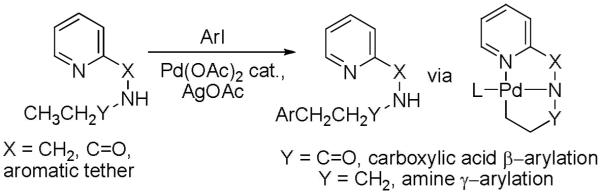
The development of a general method for the arylation of directing-group containing arenes is described in Section 2.1. If the directing group is pyridine, even sp3 C-H bonds can be arylated. The generality of the method could be improved by employing pyridine as a part of a removable auxiliary ligand. Several issues were considered while contemplating attachment of the auxiliary ligand. The reaction mechanism involves a C-H activation step and, most likely, a step where Pd(II) is converted to Pd(IV). Additional pyridine ligand should stabilize a high-energy Pd(IV) species and presumably increase its the rate of formation.8a It is likely that the Pd(II) to Pd(IV) transformation is the rate determining step in the catalytic cycle. The C-H activation step will also most likely be facilitated by an additional coordination site. β-Hydride elimination should be retarded by saturating the coordination sites on palladium. The logical auxiliary structures are pyridines linked to the group to be arylated by an amide linkage. After the arylation step the auxiliary could be removed by acid or base hydrolysis. Both the arylation of carboxylic acid and amine derivatives should be possible, depending on how the auxiliary is attached (equation 1). The arylation regiochemistry is dictated by 5-membered palladated chelate formation.
 |
(1) |
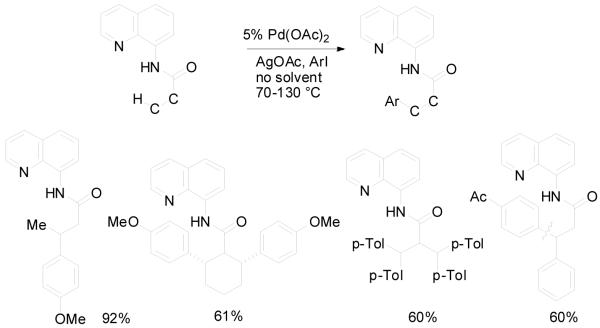
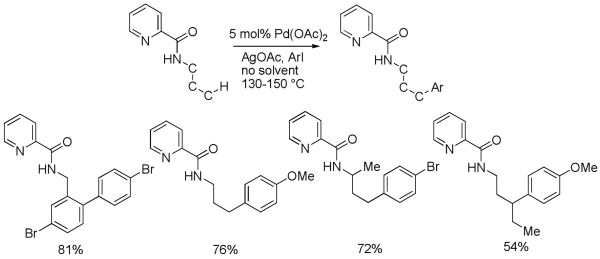
Auxiliary-assisted sp3 C-H bond arylation results are presented in Schemes 8 and 9.21 As expected, carboxylic acid derivatives are arylated in β-positions (Scheme 8). Aliphatic C-H bonds are arylated extremely easily in this case. The butyramide of 8-aminoquinoline undergoes p-methoxylation in the methylene group in less than 5 minutes at 110 °C. Cyclohexanecarboxylic acid amide is diarylated. The reaction proceeds at 70 °C and is diastereoselective (8:1) for the formation of the all-cis product. For a Pd-catalyzed functionalization of secondary aliphatic C-H bonds these are exceptionally mild conditions. Interestingly, secondary C-H bonds react faster than primary bonds, allowing the tetraarylation of isobutyric amide. This method was recently used for the synthesis of modified amino acid derivatives.22 The transposition of the amide group allows to arylate benzyl and alkylamine derivatives by employing picolinic acid as a removable directing group (Scheme 9). This is an unprecedented remote functionalization reaction that allows selective arylation of the γ-position of an aliphatic amine. The reaction conditions are harsher than for aminoquinoline derivatives, showing that the role of the auxiliary ligand is quite important.
Scheme 8.
Carboxylic Acid Derivative Arylation
Scheme 9.
Amine Derivative Arylation
Remote functionalizations resulting in the formation of C-C bonds are rare.23 Most of these methods involve radical reactions. While it is relatively easy to functionalize the enolizable α-position of the carboxylic acid derivatives, the selective functionalization of β-positions is substantially more difficult.24 In a somewhat related case, Sen has described the Pt-catalyzed oxidation of the terminal methyl group in propionic acid; however, in the next homologue, butyric acid, the γ-position is reactive.24a Du Bois has described Rh-catalyzed β- and γ-amination of alcohol and amine derivatives.25
Selective γ-arylation of aliphatic amine chains is unique. The reactions shown above are also the first examples of palladium-catalyzed unactivated sp3 C-H bond transformation into C-C bonds with no adjacent tertiary centers.4
2.3. Arylation by Aryl Chlorides: A Pd(0)-Pd(II) Catalytic Cycle
2.3.1 Benzoic Acid Arylation
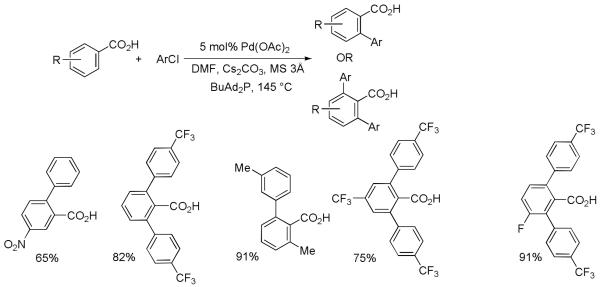
While the Pd(II)-Pd(IV) catalytic cycle conditions allow for the arylation of a wide variety of directing-group-containing arenes, use of comparatively expensive aryl iodides is required. Aryl chlorides are now routinely used for C-C bond creation under Pd(0)-Pd(II) catalytic cycle conditions.26 As a consequence, use of cheaper ArCl in C-H bond functionalization should also be possible if appropriate ligands are used and catalytic cycle for the arylation is changed from Pd(II)-Pd(IV) to Pd(0)-Pd(II) couple. Ohta has published a method for palladium-catalyzed indole arylation by activated pyrazinyl chlorides in 1980’s.27 Fagnou and Ackermann have previously reported C-H bond arylation by unactivated ArCl.28 We were interested in developing the method for benzoic acid arylation by aryl chlorides. During the optimization, substantial conversions to product were observed with many electron rich, bulky phosphine ligands. The best conversions were observed by employing n-butyl-di-1-adamantylphosphine ligand in combination with cesium carbonate base and palladium acetate precatalyst.17 Benzoic acids of any electronic properties are reactive (Scheme 10). However, the combination of an electron-rich aryl chloride and an electron-rich benzoic acid is occasionally problematic due to decarboxylation of either product or reactant benzoic acid. Halogens other than fluorine are not compatible with the reaction conditions in contrast with the ArI/AgOAc method.
Scheme 10.
Benzoic Acid Arylation by Aryl Chlorides
2.3.2 Electron-Rich Heterocycles
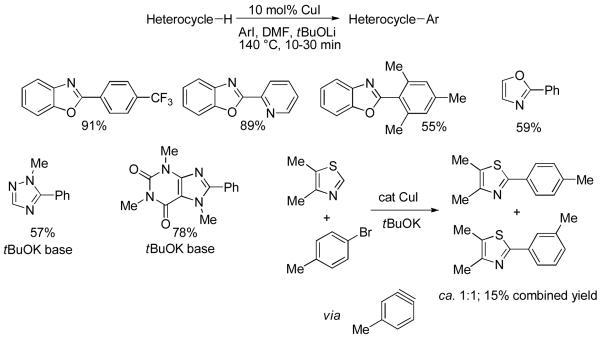
While aryl bromides are widely used for direct arylation of electron-rich heterocycles,1,3 use of aryl chlorides in such reaction has been rare.3e,27,29 A simple optimization of conditions that were successful for benzoic acid arylation afforded a method for electron-rich heterocycle arylation.30 The ligand of choice that afforded the best combination of arylation scope, price, and operational simplicity is n-butyl-di-1-adamantylphosphine. Thiophene, benzothiophene, isoxazole and thiazole derivatives, benzoxazole, benzothiazole, imidazole, alkyltriazoles, and caffeine can all be efficiently arylated (Scheme 11).
Scheme 11.
Heterocycle Arylation by Aryl Chlorides
While the method is very general, additional optimization may be required to maximize reaction yields, since the heterocycles that can be arylated are very structurally different. For example, the optimized procedure for the phenylation of isobutylthiazole involves decreased amount of phosphine ligand, added sodium acetate reagent, and DMA instead of NMP solvent.31
2.4 Copper Catalysis
The palladium catalysis allows for the arylation of a wide variety of directing-group-containing arenes and unactivated sp3 C-H bonds. Aryl chlorides can be used in some of the arylations. However, it would be advantageous to employ cheaper catalysts for such transformations. Copper is underutilized as a catalyst for C-H bond functionalization even though it was the first transition metal shown to promote carbon-hydrogen bond arylation.32 Our attention was drawn to the observation that copper salts can affect the regioselectivity of palladium-catalyzed electron-rich heterocycle arylation,3a and that both electron-poor arenes, such as trinitrobenzene, and electron-rich heterocycles, such as thiophene, can be arylated under harsh conditions by employing stoichiometric copper (I) oxide reagent.32 These observations can be explained by invoking an organocopper intermediate. Arylated benzene or heterocycle would be formed by the reaction of the arylcopper with aryl iodide. If the presumed arylcopper intermediate could be generated efficiently, a copper-catalyzed method for the carbon-hydrogen bond arylation would be achieved. A logical approach to organocopper species generation involves employing a relatively strong base. This approach was successful as described in the following sections.
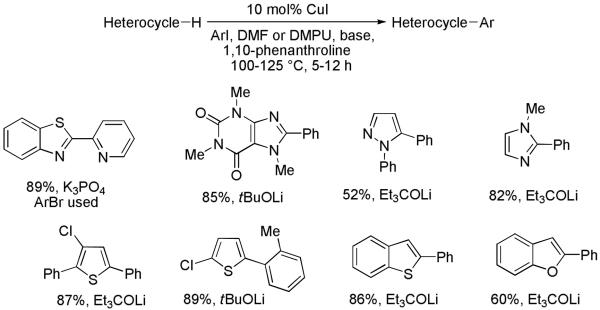
2.4.1 Electron-rich Heterocycle Arylation
Two sets of condition have been developed for electron-rich heterocycle arylation. More acidic heterocycles such as benzoxazole or benzothiazole may be arylated by employing tBuOLi base, aryl iodide, and DMF solvent. These reactions proceed at 140 °C in minutes. However, for less acidic imidazole, 1,2,4-triazole, and caffeine derivatives a stronger tBuOK base is required, and the reaction proceeds by a benzyne-type mechanism.33a Formation of regioisomer mixtures was observed if substituted aryl halides were used in combination with tBuOK base (Scheme 12).
Scheme 12.
Copper-Catalyzed Arylation of Acidic Heterocycles
Several other issues had to be considered for developing a more widely applicable procedure. For slower reactions, formation of tert-butyl aryl ether by the reaction of tert-butoxide base with aryl iodide was observed, resulting in decreased conversion to the arylation products. Copper catalyst is relatively unstable at the temperature required for the arylation, and only fast reactions were successful. Therefore, a second set of conditions was developed. Employing phenanthroline ligand should allow for a more efficient heterocycle arylation by stabilizing the copper catalyst and facilitating the halide displacement step. Replacing tBuOK with a weaker lithium alkoxide or K3PO4 base should shut down the benzyne mechanism ensuring arylation regioselectivity. Employing hindered Et3COLi base instead of tBuOLi should slow down the nucleophilic substitution of aryl iodide while not influencing the arylation rate. We were pleased to discover that addition of a phenanthroline ligand34 allows tBuOLi or Et3COLi base to be used for less acidic heterocycle arylation avoiding the problems associated with the benzyne mechanism.33c The modified reaction conditions allow for the arylation of heterocycles that were not reactive under our previous conditions (Scheme 13). It is possible to employ K3PO4 base in the arylation of the most acidic heterocycles possessing DMSO pKa’s below 27. Thiophenes, caffeine, alkylimidazoles, alkyltriazoles, benzothiophene, and benzofuran can be arylated by employing either tBuOLi or Et3COLi base. The limitations of the method are as follows. Furans and N-substituted indoles are unreactive, while heterocycles possessing acidic N-H bonds are arylated on the nitrogen. The following DMSO pKa’s of heterocycle C-H bonds have been reported: N-alkylindoles, about 37; furan, 35; N-methylimidazole, 33.35 It can be concluded that copper-catalyzed electron-rich heterocycle arylation is successful for compounds possessing pKa’s below 35.
Scheme 13.
Electron-Rich Heterocycle Arylation
2.4.2 Electron-Deficient Heterocycles and Benzene Derivatives
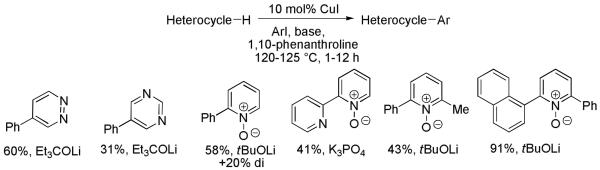
If the mechanistic considerations are valid, arylation of any arenes with C-H bond DMSO pKa’s below 35 should be possible. The reaction conditions developed for electron-rich heterocycles were applied for electron-deficient heterocycle and benzene arylation. Pyridine oxides, pyrimidine, and pyridazine can be efficiently functionalized (Scheme 14).33c The most acidic position of the heterocycle is selectively arylated.
Scheme 14.
Electron-Deficient Heterocycle Arylation
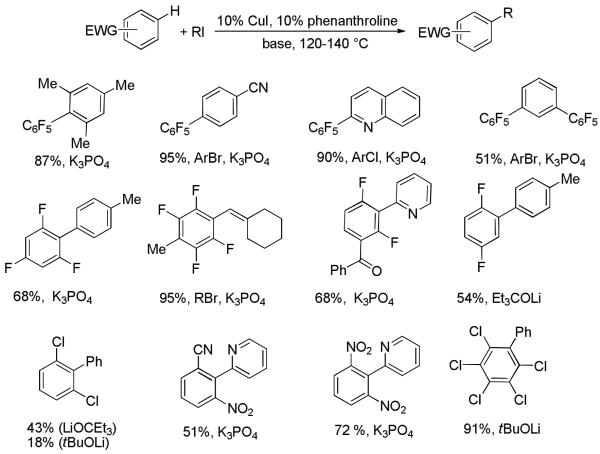
Arylation of electron-deficient benzene derivatives proceeds smoothly (Scheme 15).33b,c All polyfluorobenzenes are arylated efficiently. The reactivity parallels the acidity of C-H bonds, with the most acidic C-H bonds, those flanked by two electron-withdrawing groups, arylated most efficiently. Potassium phosphate can be used as a base if an arene contains more than two fluorine substituents or two fluorine substituents and an additional electron-withdrawing group. Penta-, tetra-, 1,3,5-tri-, and 1,3-dichlorobenzenes can be arylated in excellent to reasonable yield. 1,3-Dinitrobenzene and 3-nitrobenzonitrile are also reactive affording the arylation products in moderate yields. Arenes possessing only one electron-withdrawing group, such as nitro-, chloro-, fluoro-, and cyanobenzene, are unreactive.
Scheme 15.
Electron-Deficient Benzene Arylation
2.4.3 Mechanistic Considerations
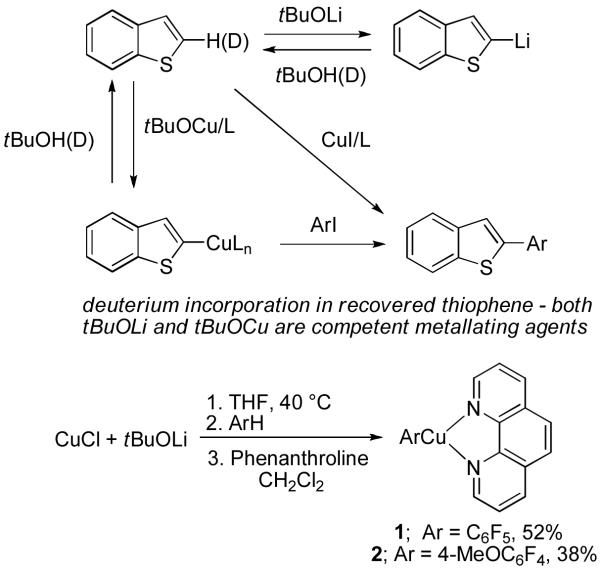
The arylation reaction can be divided into three parts: metalation, transmetalation with copper halide, and reaction of arylcopper with a haloarene. Both tBuOLi and tBuOCu are competent metalating agents under the reaction conditions (Scheme 16). Intermediacy of an arylcopper species was proven by in situ NMR studies. The reaction of copper iodide, potassium phosphate, pentafluorobenzene, and phenanthroline in DMF under the conditions of the catalytic process affords pentafluorophenylcopper-phenanthroline complex 1 as determined by 19F NMR of the crude reaction mixture. Furthermore, 4-methoxy-2,3,5,6-tetrafluorophenylcopper-phenanthroline complex 2 was prepared by reacting tBuOCu with 2,3,5,6-tetrafluoroanisole followed by addition of phenanthroline ligand (Scheme 16). The structures of 1 and 2 have been verified by X-ray crystallographic analysis.33c Complex 1 reacts with aryl iodides affording the coupling products. Consequently, the reaction mechanism parallels that of copper-catalyzed heteroatom arylation.36
Scheme 16.
Mechanistic Considerations
3. Summary and Outlook
This Account details our progress in developing direct, transition-metal-catalyzed carbon-hydrogen bond arylation procedures. By building on early observations of Tremont and Liebeskind,6 we were able to develop a very general method that allows conversion of directing-group-containing arene C-H bonds into carbon-carbon bonds. Anilides, benzamides, benzoic acids, benzylamines, and 2-substituted pyridine derivatives can be arylated by aryl iodides under very similar reaction conditions thus attesting to the generality of the method. Others have shown that arylation of imines and heterocyclic substrates are possible under nearly identical conditions.37 Furthermore, extension of the method to the arylation of unactivated sp3 C-H bonds has been achieved by incorporating a removable, pyridine-based auxiliary. Highly regioselective arylation of β-positions of carboxylic acid derivatives and γ-positions of amine derivatives is thus possible. For several classes of substrates, such as benzoic acids and electron-rich heterocycles, aryl chloride coupling partners can be employed under Pd(0)-Pd(II) catalytic cycle conditions. For the arylation of acidic (DMSO pKa below 35) arene C-H bonds, copper catalysis can be employed. Electron-rich and electron-deficient heterocycles as well as electron-poor arenes possessing at least two electron-withdrawing groups can be arylated by using copper catalyst in combination with a base and phenanthroline ligand. The most acidic position, which usually is the most hindered one, is arylated. The copper-catalyzed method complements the C-H activation/borylation methodology where functionalization usually occurs at the least hindered position.38 It is interesting to consider differences between the palladium- and copper-catalyzed arylations. Mechanistically, palladium complexes are intimately involved both in the C-H activation and the subsequent C-C bond formation steps. This feature allows a greater scope of substrates to be functionalized by the virtue of many substrate classes that can be palladated. On the other hand, mechanistic details are quite complicated and synthetic procedures are often developed by a trial-and-error method. The copper-catalyzed C-H bond arylation, in contrast, involves a simple acid-base reaction followed by a Cu-catalyzed C-C bond formation. Only relatively acidic (pKa below 35) substrates can be functionalized; however, simple mechanistic picture allows for an easy and predictable development of synthetic procedures. Since transition metal is involved only in the C-C bond formation, copper-catalyzed arylation is conceptually similar to Kumada, Negishi, and Castro cross-couplings. It is likely that other transition metals such as iron, nickel, cobalt, or silver can be employed in deprotonation/arylation reaction sequences.
ACKNOWLEDGMENT
We thank the Welch foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), A. P. Sloan Foundation, Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Biographical Information
Olafs Daugulis received an undergraduate degree in Chemical Engineering from Riga Technical University. After earning a PhD from University of Wisconsin under the guidance of Prof. Edwin Vedejs in 1999, he spent three years as a postdoctoral associate with Prof. Maurice Brookhart at University of North Carolina at Chapel Hill. He joined the chemistry faculty at the University of Houston in 2003. He is interested in the application of organometallic chemistry to organic chemistry problems.
Hien-Quang Do received his B.S. in chemistry from the University of Natural Sciences at Ho Chi Minh City in Vietnam. A few years after obtaining a Masters degree from the same University in 2003, he came to the University of Houston where he currently is a third-year graduate student in Prof. Daugulis’ group. He developed the method for copper-catalyzed arylation of acidic sp2 C-H bonds and is currently working on expanding the arylation scope.
Dmitry Shabashov obtained his B.S. degree in Chemical Engineering from Riga Technical University in 2001 followed by a MS degree in 2004. He is currently a fourth-year graduate student at the University of Houston in Prof. Daugulis’ group. His research involves auxiliary-directed arylation of sp3 carbon-hydrogen bonds.
REFERENCES
- 1.Recent reviews: Seregin IV, Gevorgyan V. Direct Transition Metal-Catalyzed Functionalization of Heteroaromatic Compounds. Chem. Soc. Rev. 2007;36:1173–1193. doi: 10.1039/b606984n. Alberico D, Scott ME, Lautens M. Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007;107:174–238. doi: 10.1021/cr0509760. Ackermann L. Catalytic Arylations with Challenging Substrates: from Air-Stable HASPO Preligands to Indole Syntheses and C-H Bond Functionalizations. Synlett. 2007:507–526. Campeau L-C, Fagnou K. Applications of and Alternatives to π-Electron-Deficient Azine Organometallics in Metal Catalyzed Cross-Coupling Reactions. Chem. Soc. Rev. 2007;36:1058–1068. doi: 10.1039/b616082d. Lewis JC, Bergman RG, Ellman JA. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc. Chem. Res. 2008;41:1013–1025. doi: 10.1021/ar800042p.
- 2.(a) Kakiuchi F, Kan S, Igi K, Chatani N, Murai S. A Ruthenium-Catalyzed Reaction of Aromatic Ketones with Arylboronates: A New Method for the Arylation of Aromatic Compounds via C-H Bond Cleavage. J. Am. Chem. Soc. 2003;125:1698–1699. doi: 10.1021/ja029273f. [DOI] [PubMed] [Google Scholar]; (b) Oi S, Fukita S, Inoue Y. Rhodium-Catalyzed Direct ortho-Arylation of 2-Arylpyridines with Arylstannanes via C-H Activation. Chem. Commun. 1998:2439–2440. [Google Scholar]; (c) Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. The Catalytic Intermolecular ortho-Arylation of Phenols. Angew. Chem., Int. Ed. 2003;42:112–114. doi: 10.1002/anie.200390037. [DOI] [PubMed] [Google Scholar]; (d) Caron L, Campeau L-C, Fagnou K. Palladium-Catalyzed Direct Arylation of Nitro-Substituted Aromatics with Aryl Halides. Org. Lett. 2008;10:4533–4536. doi: 10.1021/ol801839w. [DOI] [PubMed] [Google Scholar]; (e) Cho SH, Hwang SJ, Chang S. Palladium-Catalyzed C-H Functionalization of Pyridine N-Oxides: Highly Selective Alkenylation and Direct Arylation with Unactivated Arenes. J. Am. Chem. Soc. 2008;130:9254–9256. doi: 10.1021/ja8026295. [DOI] [PubMed] [Google Scholar]; (f) Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. Palladium-Catalyzed Alkylation of Aryl C-H Bonds with sp3 Organotin Reagents Using Benzoquinone as a Crucial Promoter. J. Am. Chem. Soc. 2006;128:78–79. doi: 10.1021/ja0570943. [DOI] [PubMed] [Google Scholar]
- 3.(a) Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Palladium-Catalyzed Arylation of Azole Compounds with Aryl Halides in the Presence of Alkali Metal Carbonates and the Use of Copper Iodide in the Reaction. Bull. Chem. Soc. Jpn. 1998;71:467–473. [Google Scholar]; (b) Park C-H, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Palladium-Catalyzed Arylation and Heteroarylation of Indolizines. Org. Lett. 2004;6:1159–1162. doi: 10.1021/ol049866q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bellina F, Cauteruccio S, Mannina L, Rossi R, Viel S. Regioselective Synthesis of 1,5-Diaryl-1H-Imidazoles by Palladium-Catalyzed Direct Arylation of 1-Aryl-1H-Imidazoles. J. Org. Chem. 2005;70:3997–4005. doi: 10.1021/jo050274a. [DOI] [PubMed] [Google Scholar]; (d) Flegeau EF, Popkin ME, Greaney MF. Direct Arylation of Oxazoles at C2. A Concise Approach to Consecutively Linked Oxazoles. Org. Lett. 2008;10:2717–2720. doi: 10.1021/ol800869g. [DOI] [PubMed] [Google Scholar]; (e) Iwasaki M, Yorimitsu H, Oshima K. Microwave-Assisted Palladium-Catalyzed Direct Arylation of 1,4-Disubstituted 1,2,3-Triazoles with Aryl Chlorides. Chem. -Asian J. 2007;2:1430–1435. doi: 10.1002/asia.200700206. [DOI] [PubMed] [Google Scholar]
- 4.(a) Chaumontet M, Piccardi R, Baudoin O. Synthesis of 3,4-Dihydroisoquinolines by a C(sp3)-H Activation/Electrocyclization Strategy: Total Synthesis of Coralydine. Angew. Chem. Int. Ed. 2009;48:179–182. doi: 10.1002/anie.200804444. [DOI] [PubMed] [Google Scholar]; (b) Lafrance M, Gorelsky SI, Fagnou K. High-Yielding Palladium-Catalyzed Intramolecular Alkane Arylation: Reaction Development and Mechanistic Studies. J. Am. Chem. Soc. 2007;129:14570–14571. doi: 10.1021/ja076588s. [DOI] [PubMed] [Google Scholar]; (c) Barder TE, Walker SD, Martinelli JR, Buchwald SL. Catalysts for Suzuki-Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]; (d) Dyker G. Palladium-Catalyzed C-H Activation of tert-Butyl Groups: A Simple Synthesis of 1,2-Dihydrocyclobutabenzene Derivatives. Angew. Chem. Int. Ed. 1994;33:103–105. [Google Scholar]; (e) Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. Palladium-Catalyzed Methylation and Arylation of sp2 and sp3 C-H Bonds in Simple Carboxylic Acids. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (f) Wang D-H, Wasa M, Giri R, Yu J-Q. Pd(II)-Catalyzed Cross-Coupling of sp3 C-H Bonds with sp2 and sp3 Boronic Acids Using Air as the Oxidant. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 5.(a) Brasche G, Buchwald SL. C-H Functionalization/C-N Bond Formation: Copper-Catalyzed Synthesis of Benzimidazoles from Amidines. Angew. Chem., Int. Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; (b) Norinder J, Matsumoto A, Yoshikai N, Nakamura E. Iron-Catalyzed Direct Arylation through Directed C-H Bond Activation. J. Am. Chem. Soc. 2008;130:5858–5859. doi: 10.1021/ja800818b. [DOI] [PubMed] [Google Scholar]; (c) Chen X, Hao X-S, Goodhue CE, Yu J-Q. Cu(II)-Catalyzed Functionalizations of Aryl C-H Bonds Using O2 as an Oxidant. J. Am. Chem. Soc. 2006;128:6790–6791. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; (d) Uemura T, Imoto S, Chatani N. Amination of the ortho C-H Bonds by the Cu(OAc)2-Mediated Reaction of 2-Phenylpyridines with Anilines. Chem. Lett. 2006;35:842–843. [Google Scholar]; (e) Phipps RJ, Grimster NP, Gaunt MJ. Cu(Ii)-Catalyzed Direct And Site-Selective Arylation of Indoles Under Mild Conditions. J. Am. Chem. Soc. 2008;130:8172–8174. doi: 10.1021/ja801767s. [DOI] [PubMed] [Google Scholar]; (f) Wen J, Zhang J, Chen S-Y, Li J, Yu X-Q. Iron-Mediated Direct Arylation of Unactivated Arenes. Angew. Chem., Int. Ed. 2008;47:8897–8900. doi: 10.1002/anie.200802526. [DOI] [PubMed] [Google Scholar]; (g) Ueda S, Nagasawa H. Synthesis of 2-Arylbenzoxazoles by Copper-Catalyzed Intramolecular Oxidative C-O Coupling of Benzanilides. Angew. Chem., Int. Ed. 2008;47:6411–6413. doi: 10.1002/anie.200801240. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tremont SJ, Rahman HU. Ortho-Alkylation of Acetanilides Using Alkyl Halides and Palladium Acetate. J. Am. Chem. Soc. 1984;106:5759–5760. [Google Scholar]; (b) McCallum JS, Gasdaska JR, Liebeskind LS, Tremont SJ. Palladium-Mediated 2,6-Dialkylation of N-Benzylidene Imines: Preparation of 2,6-Dialkylbenzaldehydes. Tetrahedron Lett. 1989;30:4085–4088. [Google Scholar]
- 7.Catellani M, Motti E, Della Ca’ N, Ferraccioli R. Recent Developments in Catalytic Aryl Coupling Reactions. Eur. J. Org. Chem. 2007:4153–4165. [Google Scholar]
- 8.(a) Canty AJ, Patel J, Rodemann T, Ryan JH, Skelton BW, White AH. Reactivity of Diaryliodine(III) Triflates toward Palladium(II) and Platinum(II): Reactions of C(sp2)-I Bonds to Form Arylmetal(IV) Complexes; Access to Dialkyl(aryl)metal(IV), 1,4-Benzenediyl-Bridged Platinum(IV), and Triphenylplatinum(IV) Species; and Structural Studies of Platinum(IV) Complexes. Organometallics. 2004;23:3466–3473. [Google Scholar]; (b) Dupont J, Consorti CS, Spencer J. The Potential of Palladacycles: More than Just Precatalysts. Chem. Rev. 2005;105:2527–2572. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]; (c) Zhang X, Kanzelberger M, Emge TJ, Goldman AS. Selective Addition to Iridium of Aryl C-H Bonds Ortho to Coordinating Groups. Not Chelation-Assisted. J. Am. Chem. Soc. 2004;126:13192–13193. doi: 10.1021/ja046476q. [DOI] [PubMed] [Google Scholar]; (d) Desai LV, Malik HA, Sanford MS. Oxone as an Inexpensive, Safe, and Environmentally Benign Oxidant for C-H Bond Oxygenation. Org. Lett. 2006;8:1141–1144. doi: 10.1021/ol0530272. [DOI] [PubMed] [Google Scholar]
- 9.(a) Daugulis O, Zaitsev VG. Anilide ortho-Arylation by Using C-H Activation Methodology. Angew. Chem., Int. Ed. 2005;44:4046–4048. doi: 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. Palladium-Catalyzed Anilide ortho-Arylation and Subsequent One-Pot Cyclization to Phenanthridines. J. Org. Chem. 2007;72:7720–7725. doi: 10.1021/jo701387m. [DOI] [PubMed] [Google Scholar]
- 10.Kalyani D, Deprez NR, Desai LV, Sanford MS. Oxidative C-H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J. Am. Chem. Soc. 2005;127:7330–7331. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]
- 11.Shi Z, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Suzuki-Miyaura Coupling Reaction by Pd(II)-Catalyzed Aromatic C-H Bond Activation Directed by an N-Alkyl Acetamino Group. Angew. Chem., Int. Ed. 2007;46:5554–5558. doi: 10.1002/anie.200700590. [DOI] [PubMed] [Google Scholar]
- 12.Kametani Y, Satoh T, Miura M, Nomura M. Regioselective Arylation of Benzanilides with Aryl Triflates or Bromides under Palladium Catalysis. Tetrahedron Lett. 2000;41:2655–2658. [Google Scholar]
- 13.(a) Shabashov D, Daugulis O. ortho-Arylation of Benzamides. Org. Lett. 2006;8:4947–4949. doi: 10.1021/ol0619866. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Maldonado JRM, Daugulis O. Carbon-Hydrogen Bond Functionalization Approach for the Synthesis of Fluorenones and ortho-Arylated Benzonitriles. J. Org. Chem. 2008;73:7818–7821. doi: 10.1021/jo801300y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai LV, Stowers KJ, Sanford MS. Insights into Directing Group Ability in Palladium-Catalyzed C-H Bond Functionalization. J. Am. Chem. Soc. 2008;130:13285–13293. doi: 10.1021/ja8045519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauvarque J-F, Pflüger F, Troupel M. Kinetics of Oxidative Addition of Zerovalent Palladium to Aromatic Iodides. J. Organomet. Chem. 1981;208:419–427. [Google Scholar]
- 16.(a) Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. Oxidative Cross-Coupling of N-(2′-Phenylphenyl)benzenesulfonamides or Benzoic and Naphthoic Acids with Alkenes Using a Palladium-Copper Catalyst System under Air. J. Org. Chem. 1998;63:5211–5215. [Google Scholar]; (b) Myers AG, Tanaka D, Mannion MR. Development of a Decarboxylative Palladation Reaction and Its Use in a Heck-type Olefination of Arene Carboxylates. J. Am. Chem. Soc. 2002;124:11250–11251. doi: 10.1021/ja027523m. [DOI] [PubMed] [Google Scholar]; (c) Li J, Bertinato P, Cheng H, Cole BM, Bronk BS, Jaynes BH, Hickman A, Haven ML, Kolosko NL, Barry CJ, Manion TB. Discovery of potent and orally active MTP inhibitors as potential anti-obesity agents. Bioorg. Med. Chem. Letters. 2006;16:3039–3042. doi: 10.1016/j.bmcl.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Chiong HA, Pham Q-N, Daugulis O. Two Methods for Direct ortho-Arylation of Benzoic Acids. J. Am. Chem. Soc. 2007;129:9879–9884. doi: 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]
- 18.Vicente J, Saura-Llamas I, Palin MG, Jones PG, Ramírez de Arellano MC. Orthometalation of Primary Amines. 4 Orthopalladation of Primary Benzylamines and (2-Phenylethyl)amine. Organometallics. 1997;16:826–833. [Google Scholar]
- 19.Lazareva A, Daugulis O. Direct Palladium-Catalyzed ortho-Arylation of Benzylamines. Org. Lett. 2006;8:5211–5213. doi: 10.1021/ol061919b. [DOI] [PubMed] [Google Scholar]
- 20.(a) Oi S, Fukita S, Hirata N, Watanuki N, Miyano S, Inoue Y. Ruthenium Complex-Catalyzed Direct Ortho Arylation and Alkenylation of 2-Arylpyridines with Organic Halides. Org. Lett. 2001;3:2579–2581. doi: 10.1021/ol016257z. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. Catalytic Coupling of C-H and C-I Bonds Using Pyridine As a Directing Group. Org. Lett. 2005;7:3657–3659. doi: 10.1021/ol051255q. [DOI] [PubMed] [Google Scholar]
- 21.Zaitsev VG, Shabashov D, Daugulis O. Highly Regioselective Arylation of sp3 C-H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 22.Reddy BVS, Reddy LR, Corey EJ. Novel Acetoxylation and C-C Coupling Reactions at Unactivated Positions in α-Amino Acid Derivatives. Org. Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 23.Breslow R, Rothbard J, Herman F, Rodriguez ML. Remote Functionalization Reactions as Conformational Probes for Flexible Alkyl Chains. J. Am. Chem. Soc. 1978;100:1213–1218. [Google Scholar]
- 24.(a) Kao L-C, Sen A. Platinum(II) Catalyzed Selective Remote Oxidation of Unactivated C-H Bonds in Aliphatic Carboxylic Acids. J. Chem. Soc., Chem. Commun. 1991:1242–1243. [Google Scholar]; (b) Dangel BD, Johnson JA, Sames D. Selective Functionalization of Amino Acids in Water: A Synthetic Method via Catalytic C-H Bond Activation. J. Am. Chem. Soc. 2001;123:8149–8150. doi: 10.1021/ja016280f. [DOI] [PubMed] [Google Scholar]
- 25.(a) Espino CG, Fiori KW, Kim M, Du Bois J. Expanding the Scope of C-H Amination through Catalyst Design. J. Am. Chem. Soc. 2004;126:15378–15379. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]; (b) Espino CG, Wehn PM, Chow J, Du Bois J. Synthesis of 1,3-Difunctionalized Amine Derivatives through Selective C-H Bond Oxidation. J. Am. Chem. Soc. 2001;123:6935–6936. [Google Scholar]
- 26.Fu GC. The Development of Versatile Methods for Palladium-Catalyzed Coupling Reactions of Aryl Electrophiles through the Use of P(t-Bu)3 and PCy3 as Ligands. Acc. Chem. Res. 2008;41:1555–1564. doi: 10.1021/ar800148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akita Y, Inoue A, Yamamoto K, Ohta A, Kurihara T, Shimizu M. Palladium-Catalyzed Coupling Reaction of Chloropyrazines with Indole. Heterocycles. 1985;23:2327–2333. [Google Scholar]
- 28.(a) Ackermann L. Phosphine Oxides as Preligands in Ruthenium-Catalyzed Arylations via C-H Bond Functionalization Using Aryl Chlorides. Org. Lett. 2005;7:3123–3125. doi: 10.1021/ol051216e. [DOI] [PubMed] [Google Scholar]; (b) Campeau L-C, Thansandote P, Fagnou K. High-Yielding Intramolecular Direct Arylation Reactions with Aryl Chlorides. Org. Lett. 2005;7:1857–1860. doi: 10.1021/ol050501v. [DOI] [PubMed] [Google Scholar]
- 29.Rieth RD, Mankad NP, Calimano E, Sadighi JP. Palladium-Catalyzed Cross-Coupling of Pyrrole Anions with Aryl Chlorides, Bromides, and Iodides. Org. Lett. 2004;6:3981–3983. doi: 10.1021/ol048367m. [DOI] [PubMed] [Google Scholar]
- 30.Chiong HA, Daugulis O. Palladium-Catalyzed Arylation of Electron-Rich Heterocycles with Aryl Chlorides. Org. Lett. 2007;9:1449–1451. doi: 10.1021/ol0702324. [DOI] [PubMed] [Google Scholar]
- 31.Lazareva A, Chiong HA, Daugulis O. Palladium(II) Acetate-Butyldi-1-adamantylphosphine Catalyzed Arylation of Electron-Rich Heterocycles. Preparation of 5-Phenyl-2-Isobutylthiazole. Org. Synth. 2009;86:105–113. [Google Scholar]
- 32.(a) Steinkopf W, Leitsmann R, Hofmann KH. Studien in der Thiophenreihe. LVII. Über α-Polythienyle. Liebigs Ann. Chem. 1941;546:180–199. [Google Scholar]; (b) Björklund C, Nilsson M. Preparation of 2,6-Dinitrobiphenyls from m-Dinitrobenzene and Iodoarenes with Copper(I) Oxide in Quinoline. Acta Chem. Scand. 1968;22:2338–2346. [Google Scholar]; (c) Ljusberg H, Wahren R. Copper-Promoted Arylation of Pentafluorobenzene. Acta Chem. Scand. 1973;27:2717–2721. [Google Scholar]; (d) Nilsson M. Reaction of 2-Thienylcopper with Iodobenzene. Tetrahedron Lett. 1966;7:679–682. [Google Scholar]
- 33.(a) Do H-Q, Daugulis O. Copper-Catalyzed Arylation of Heterocycle C-H Bonds. J. Am. Chem. Soc. 2007;129:12404–12405. doi: 10.1021/ja075802+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do H-Q, Daugulis O. Copper-Catalyzed Arylation and Alkenylation of Polyfluoroarene C-H Bonds. J. Am. Chem. Soc. 2008;130:1128–1129. doi: 10.1021/ja077862l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Do H-Q, Khan RMK, Daugulis O. A General Method for Copper-Catalyzed Arylation of Arene C-H Bonds. J. Am. Chem. Soc. 2008;130:15185–15192. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(a) Altman RA, Shafir A, Choi A, Lichtor PA, Buchwald SL. An Improved Cu-Based Catalyst System for the Reactions of Alcohols with Aryl Halides. J. Org. Chem. 2008;73:284–286. doi: 10.1021/jo702024p. [DOI] [PubMed] [Google Scholar]; (b) Gujadhur RK, Bates CG, Venkataraman D. Formation of Aryl-Nitrogen, Aryl-Oxygen, and Aryl-Carbon Bonds Using Well-Defined Copper(I)-Based Catalysts. Org. Lett. 2001;3:4315–4317. doi: 10.1021/ol0170105. [DOI] [PubMed] [Google Scholar]
- 35.Shen K, Fu Y, Li J-N, Liu L, Guo Q-X. What are the pKa Values of C-H Bonds in Aromatic Heterocyclic Compounds in DMSO? Tetrahedron. 2007;63:1568–1576. [Google Scholar]
- 36.Tye JW, Weng Z, Johns AM, Incarvito CD, Hartwig JF. Copper Complexes of Anionic Nitrogen Ligands in the Amidation and Imidation of Aryl Halides. J. Am. Chem. Soc. 2008;130:9971–9983. doi: 10.1021/ja076668w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(a) Yang F, Wu Y, Zhu Z, Zhang J, Li Y. Direct ortho-Arylation of 2-Arylbenzoxazoles via C-H Activation. Tetrahedron. 2008;64:6782–6787. [Google Scholar]; (b) Thirunavukkarasu VS, Parthasarathy K, Cheng C-H. Synthesis of Fluorenones from Aromatic Aldoxime Ethers and Aryl Halides by Palladium-Catalyzed Dual C-H Activation and Heck Cyclization. Angew. Chem., Int. Ed. 2008;47:9462–9465. doi: 10.1002/anie.200804153. [DOI] [PubMed] [Google Scholar]
- 38.(a) Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002;124:390–391. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; (b) Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr., Smith MR., III Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds. Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]