Abstract
Ischemia/reperfusion injury (IRI) of the liver is an important cause of hepatic dysfunction. Ischemic preconditioning (IP) is associated with adenosine-mediated tissue protection from subsequent IRI. Extracellular nucleotides (e.g. ATP) represent the main source for extracellular adenosine. Therefore, we hypothesized that phosphohydrolysis of ATP/ADP via the ectonucleoside-triphosphate-diphosphohydrolase-1 (CD39, conversion of ATP/ADP to AMP) mediates IP-dependent liver protection. We found that hepatic IP was associated with a significant induction of CD39 transcript, heightened protein expression and improved outcomes after IRI. Targeted gene-deletion or pharmacological inhibition of CD39 abolished hepato-protection by IP as measured by serum markers of liver injury, or histology. Therapeutic studies to mimic IP with i.p. apyrase (a soluble NTPDase) in the absence of IP attenuated hepatic injury after IRI. In additional in vivo studies, siRNA treatment was used to achieve repression of the transcription factor Sp1, known to be implicated in CD39 transcriptional regulation. In fact, Sp1 siRNA treatment was associated with attenuated CD39 induction, and increased hepatic injury in vivo. Our data suggest a Sp1-dependent regulatory pathway for CD39 during hepatic IP. These studies reveal a novel role of CD39 in hepatic protection and suggest soluble apyrase for the treatment of liver ischemia.
Introduction
Ischemia/reperfusion injury (IRI) involves the transient deprivation of blood flow and oxygen followed by the return of blood flow during reperfusion. Liver IRI and subsequent dysfunction or failure occurs in many clinical settings including transplantation surgery, tissue resections or hemorrhagic shock (1, 2). Liver IRI has been reported to cause up to 10% of early organ dysfunction, leading to increased rates of acute and chronic rejection (3). This process has a primarily vascular phase of injury followed by secondary leukocyte recruitment that result in cellular and organ damage (1). Ischemic preconditioning (IP), which is thought to activate endogenous cellular protective mechanisms, represents one of the strongest forms of in vivo protection of the liver (4–8) While the underlying mechanisms of hepatic IP remain unclear, it would be highly desirable to use pharmacological means to recapitulate IP-dependent liver protection (2).
Ectonucleoside triphosphate diphosphohydrolase-1 (CD39) hydrolyzes both extracellular ATP and ADP to AMP. AMP is rapidly degraded to adenosine via the ubiquitously expressed 5’-ecto-nucleotidase (CD73) (9–13). Previous studies suggest that extracellular adenosine is an important pathway for liver protection from ischemia and inflammation (14–18). For example, we previously demonstrated that extracellular adenosine production by CD73 mediates protection during murine hepatic IP (17). Other studies recently demonstrated that the catalysis of extracellular nucleotides by CD39 is required for liver regeneration following partial hepatectomy (19). Based on the fact that extracellular AMP mainly stems from CD39-dependent ATP/ADP-phosphohydrolysis, we hypothesized a central role of CD39 in IP-mediated liver protection. To test this hypothesis, we combined pharmacological and genetic studies to address the role of CD39 in this aspect of hepatic IRI.
Materials and Methods
Mice
All animal experiments were in accordance with German guidelines and approved by the University of Tübingen, Germany. Mice deficient in CD39 (CD39−/−)(20) were compared to littermate controls matched in age, gender and weight (CD39+/+; WT). In some experiments, mice were treated with sodium polyoxotungstate (POM-1, Na6[H2W12O40], 3 mg/kg/h, i.a., 30 min prior to IP or IR) (21, 22), apyrase from potatoes (Sigma, 5U apyrase i.p., 30 min prior to IP or IR), AMP (100 µl/h of 4 mg/kg, i.a.) (21, 22), Sp1 small interfering RNA (Sp1 siRNA, Dharmacon RNA Technologies, Lafayette, CO, 2 mg/kg in transfection reagent, siPORT Amine; Ambion, Austin, TX, i.v., 24 hours prior to IP or IR),(23) or nonsense siRNA (NS siRNA, Silencer Negative Control #1 siRNA, Ambion, 2 mg/kg in transfection reagent, i.v., 24 hours prior to IP or IR).
Technique of portal triad occlusion
Partial hepatic ischemia was performed via portal triad occlusion with the use of a hanging-weight system as described previously (24). Mice underwent 30 min ischemia, followed by 3 h reperfusion or IP (3 cycles of 5 min ischemia/5 min reperfusion) prior to IR (24). Sham mice underwent exposure of the portal triad without IR or IP.
Real-time RT-PCR and Western blot
To measure Sp1 and CD39 transcript levels, the median lobe was excised, followed by isolation of RNA and quantification of mRNA by real-time RT-PCR relative to β-actin (21, 23). For western blot of Sp1, the median lobe was excised and proteins were resolved by SDS-PAGE, transferred to nitrocellulose and probed with anti-Sp1 antibody (Abcam, Cambridge, USA).
Serum markers of liver injury
Lactate dehydrogenase (LDH, Randox, Crumlin, UK), aspartate (AST) and alanine (ALT) aminotransferases (Teco Diagnostics, Anaheim, CA, USA) were measured using commercially available kits.
Histological sections
The median and left liver lobes were placed in OCT Tissue-Tek, frozen, sectioned and stained with H&E. Examination/scoring was carried out by a pathologist blinded to the experimental group using a semi-quantitative grading scale of 0–4 for histopathological assessment of liver necrosis (25): 0=no liver necrosis, 1=single cell necrosis, 2=up to 30% lobular necrosis, 3=up to 60% lobular necrosis, and 4=more than 60% lobular necrosis. Immunohistochemical staining was performed with a polyclonal goat anti-mouse IgG antibody against CD39 (sc-33558 rabbit polyclonal IgG, Santa Cruz, Heidelberg, Germany) or using a negative control rabbit immunoglobulin fraction (DakoCytomation, Glostrup, Denmark).
Adenosine measurements
The left and median liver lobes were removed and immediately snap frozen with clamps pre-cooled to the temperature of liquid nitrogen within a time lag of 3–5 seconds. The frozen tissue was pulverized under liquid nitrogen, protein was precipitated with ice-cold 0.6 N perchloric acid and tissue adenosine or nucleotide levels were determined (22, 26, 27).
Statistical analysis
Data are presented as mean ± SD and analyzed using one-way analysis of variance.
Results
Hepatic CD39 is induced by IP
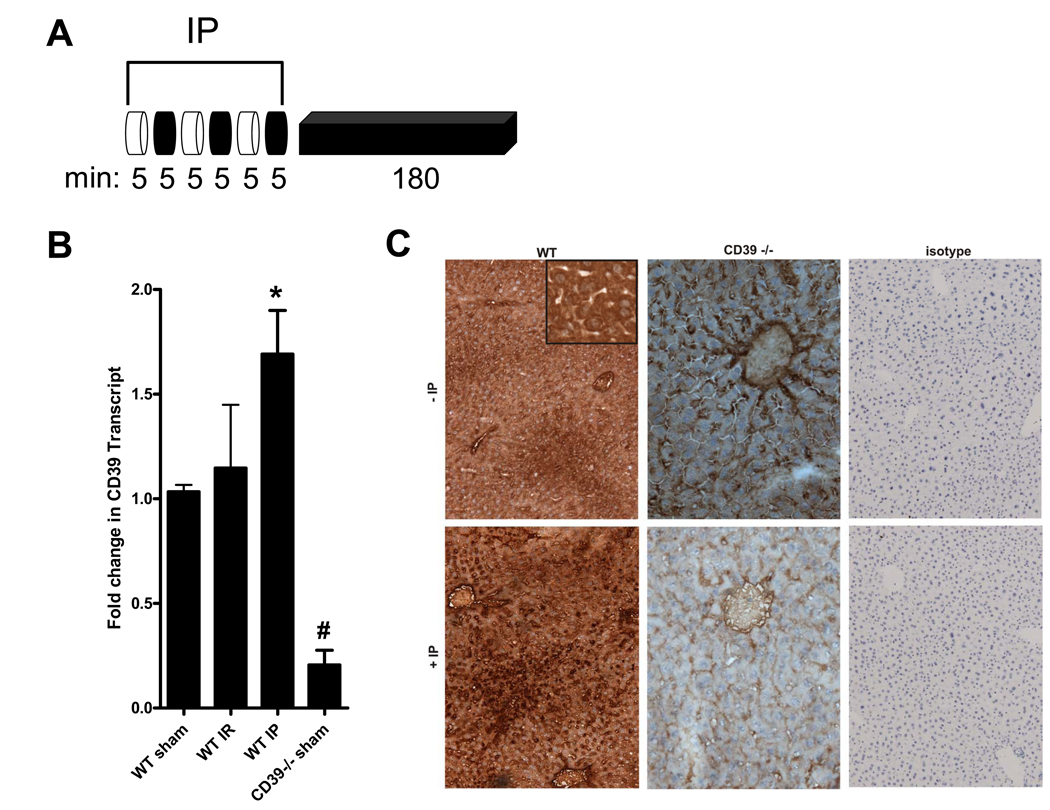
We first investigated liver CD39 expression in mice subjected to three cycles of IP treatment (intermittent portal triad occlusion and reperfusion, 5 min of ischemia/5 min of reperfusion) prior to 180 min reperfusion (Fig. 1A). A significant induction of CD39 mRNA was observed 180 min following hepatic IP (Fig. 1B). Immunohistochemistry confirmed that CD39 protein was increased in hepatocytes (see inset) following IP in WT mice in contrast to CD39−/− mice, which showed only minimal immunostaining for CD39 (Fig. 1C). We did not detect differences in CD39 immunostaining for endothelial cells or pericytes. No nonspecific staining with isotype control antibody was observed. These data support hepatic induction of CD39 following IP treatment.
Figure 1.
CD39 is induced by liver IP. (A) After 3 cycles of IP [5 min ischemia (white), 5 min reperfusion (black)] and 180 min reperfusion the median lobe of the murine liver was excised. (B) Total RNA was isolated from wild-type (WT) or CD39−/− mice and CD39 mRNA was determined by real-time PCR. Data were calculated relative to β-actin and expressed as fold change in transcript relative to housekeeping gene β-actin. Results are expressed as the mean ± SD of 3 mice/group. *p<0.05 significantly higher vs. WT sham. #p<0.05 significantly lower vs. WT sham. (C) The median liver lobe from wild-type (WT) or CD39−/− mice was sectioned and stained using CD39 antibody. WT samples were also stained using isotype control antibody. Note enhanced staining for CD39 in WT mice following IP treatment, particularly in the hepatocytes (inset).
CD39 inhibition attenuates hepatic protection by IP
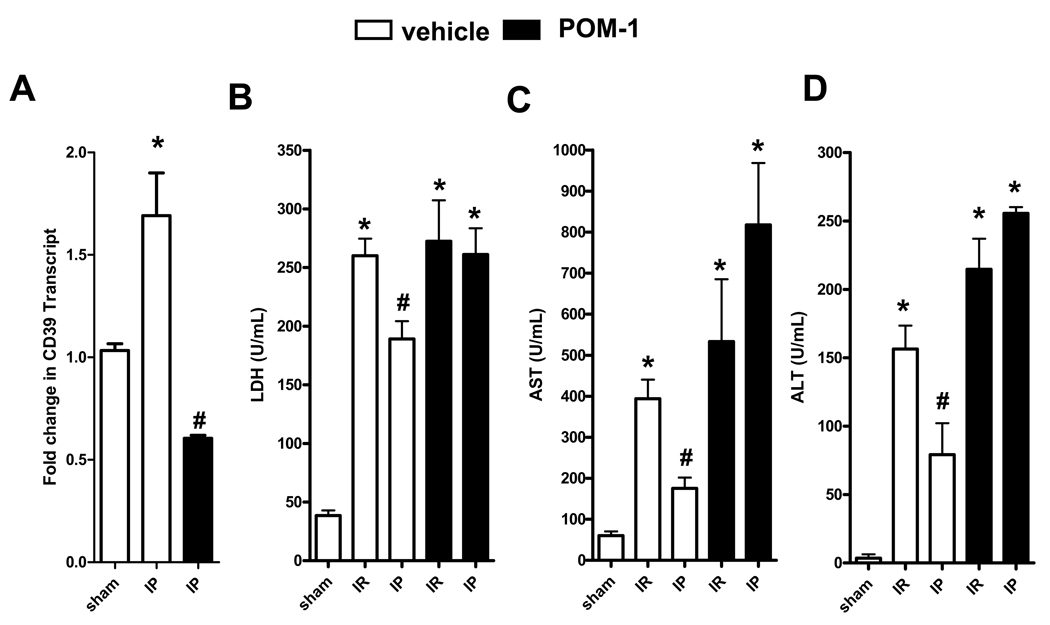
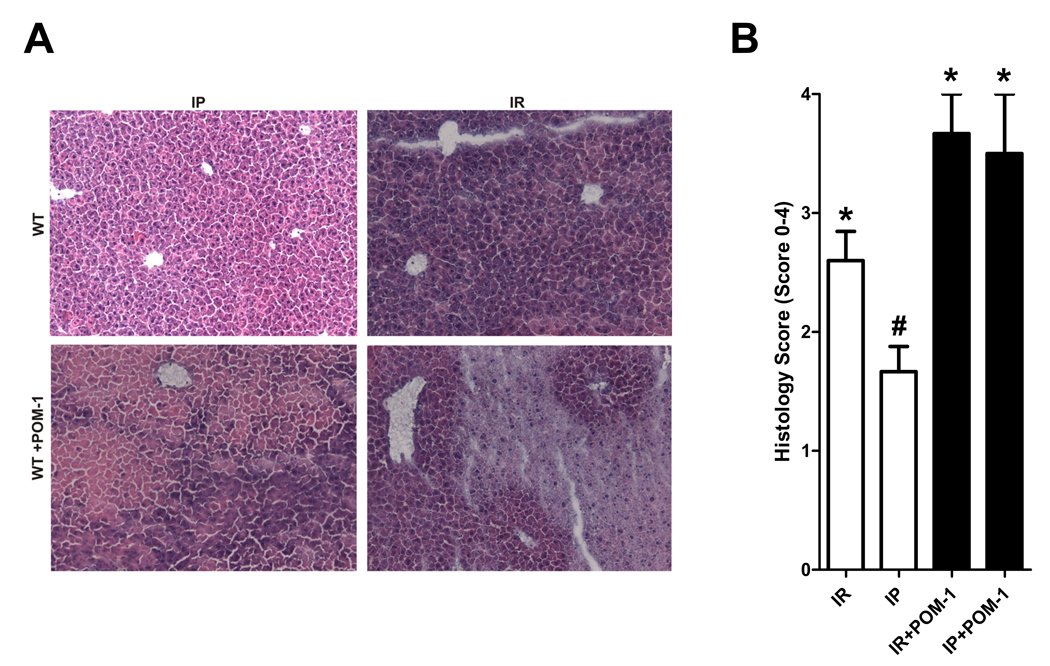
After having shown induction of CD39 with hepatic IP treatment, we next pursued studies to address the functional role of CD39 in hepatoprotection. To determine if inhibition of extracellular phosphohydrolysis attenuates the hepatoprotective effects of IP, we pharmacologically inhibited CD39 using a polyoxometalate (Na6[H2W12O40], POM-1), which was recently described to function as a specific NTPDase inhibitor (21, 22). WT mice were subjected to IR with or without prior IP following treatment with POM-1 (3 mg/kg/h, i.a.) or vehicle (saline). As shown in Figure 2A, CD39 levels following treatment with CD39 inhibitor POM-1 were significantly decreased in WT mice, confirming specificity of POM-1. Following hepatic IR, WT mice demonstrated significantly higher levels of LDH (Fig. 2B), AST (Fig. 2C) and ALT (Fig. 2D) compared to their respective sham controls. Serum LDH, AST and ALT were significantly improved by IP (Fig. 2B–D). Furthermore, POM-1 treatment completely abolished the hepatoprotective effects of IP, demonstrating that blockade of CD39 enzymatic activity provides pharmacological evidence for an essential role of CD39 in hepatic protection by IP, in a comparable manner to the kidney (22) or the heart (21). In addition, histological signs of ischemic injury were substantially increased in WT mice treated with POM-1 (Fig. 3A) as demonstrated by a significant increase in the Suzuki index (Fig. 3B) (25). Taken together, these data provide strong evidence for a role of CD39 in attenuating IR injury during hepatic IP.
Figure 2.
CD39 inhibition abolishes liver protection by IP. Injury was measured after WT mice were pre-treated with the CD39 inhibitor POM-1 (3 mg/kg/h, i.a.) or saline prior to IR (IR) with or without prior IP (IP). Injury was measured for the following parameters: (A) Total RNA was isolated and CD39 mRNA was determined by real-time PCR. Data were calculated relative to β-actin and expressed as fold change in transcript relative to housekeeping gene β-actin. Results are expressed as the mean ± SD of 3 mice/group. *p<0.05 vs. WT sham. (B) LDH, (C) AST, and (D) ALT. Results are expressed as the mean ± SD of 4–8 mice/group. *p<0.05 vs. sham. #p<0.05 vs. IR+vehicle.
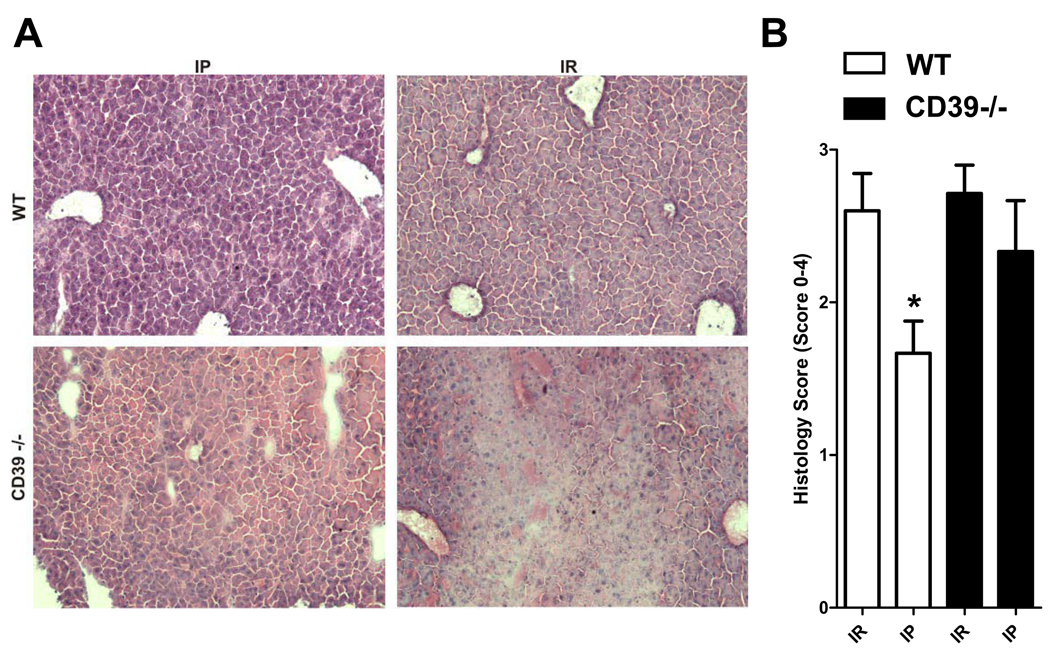
Figure 3.
POM-1 abolishes liver protection by IP. (A) H&E staining of liver sections and (B) quantification of ischemic injury following treatment with the CD39 inhibitor POM-1 (3 mg/kg/h, i.a.) or saline prior to IR (IR) with or without prior IP (IP) (n=3–6 mice/group expressed as mean ± SEM). *p<0.05 vs. respective IR group.
Hepatic protection by IP is abolished in CD39−/− mice
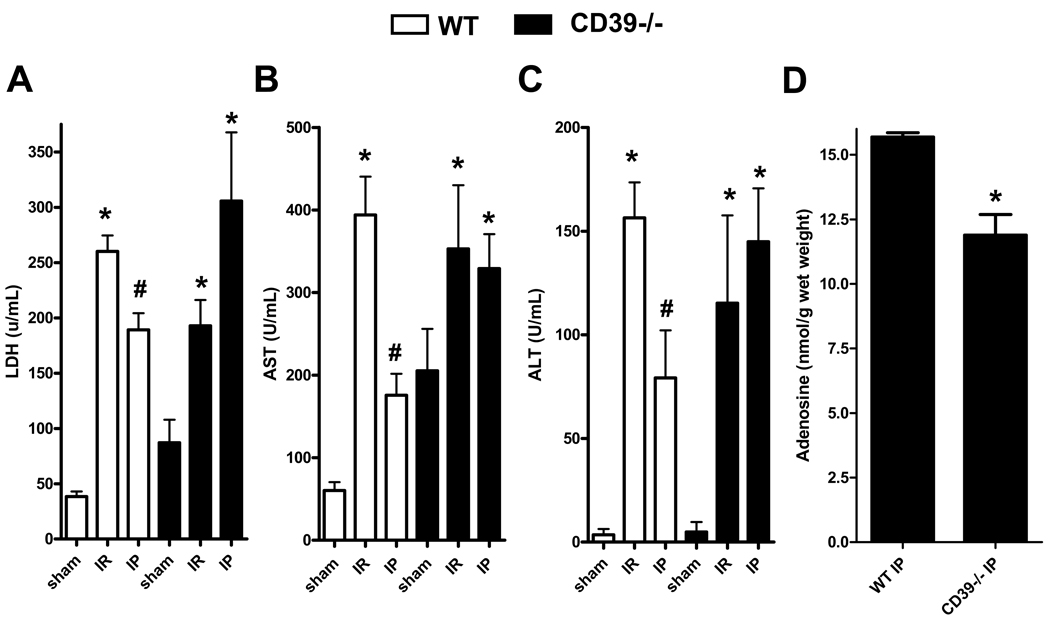
To determine if deficiency of CD39 attenuates the hepatic protective effects of IP, we performed studies in CD39−/− mice (20). Following hepatic IR, WT or CD39−/− mice demonstrated significantly higher levels of LDH (Fig. 4A), AST (Fig. 4B) and ALT (Fig. 4C) compared to their respective sham controls. However, in contrast to the results with WT mice, LDH, AST or ALT serum levels were not improved by IP in CD39−/− mice. To confirm CD39-mediated phosphohydrolysis of ADP we next measured adenosine tissue concentrations and found that hepatic adenosine levels of CD39−/− mice were significantly lower than WT mice (Fig. 4D). Additionally we measured histological signs of ischemic injury and found that they were also not improved by IP in CD39−/− mice (Fig. 5A). Thus, WT mice with IP prior to ischemia showed only mild to moderate histological signs of injury. In contrast, hepatic tissue protection by IP was absent in CD39−/− mice and there was no significant reduction in the Suzuki index (Fig. 5A and 5B respectively) (25). Taken together, these data provide genetic evidence for a critical role of CD39 in protection by IP of the liver.
Figure 4.
Liver protection by IP is abolished in CD39−/− mice. CD39 deficient (CD39−/−) or WT mice were subjected to IR or IP preceding IR (IP). Sham mice underwent the same surgical procedure but without IP or IR. (A) LDH, (B) AST, and (C) ALT. Results are expressed as the mean ± SD of 4–8 mice/group. *p<0.05 vs. respective sham. #p<0.05 vs. respective IR group. (D) Hepatic adenosine concentrations with IP are attenuated in CD39−/− mice. CD39−/− or WT mice were subjected to IP. The liver was snap frozen after the last cycle of IP. Results are expressed as the mean ± SEM of 5 mice/group. *p<0.05 vs. WT+IP.
Figure 5.
Targeted gene-deletion of CD39 abolished hepatic protection by IP. (A) H&E staining of liver sections and (B) quantification of ischemic injury (n=3–7 mice/group expressed as mean ± SEM). *p<0.05 vs. respective IR group.
Hepatic injury following ischemia is attenuated by treatment of WT mice with apyrase
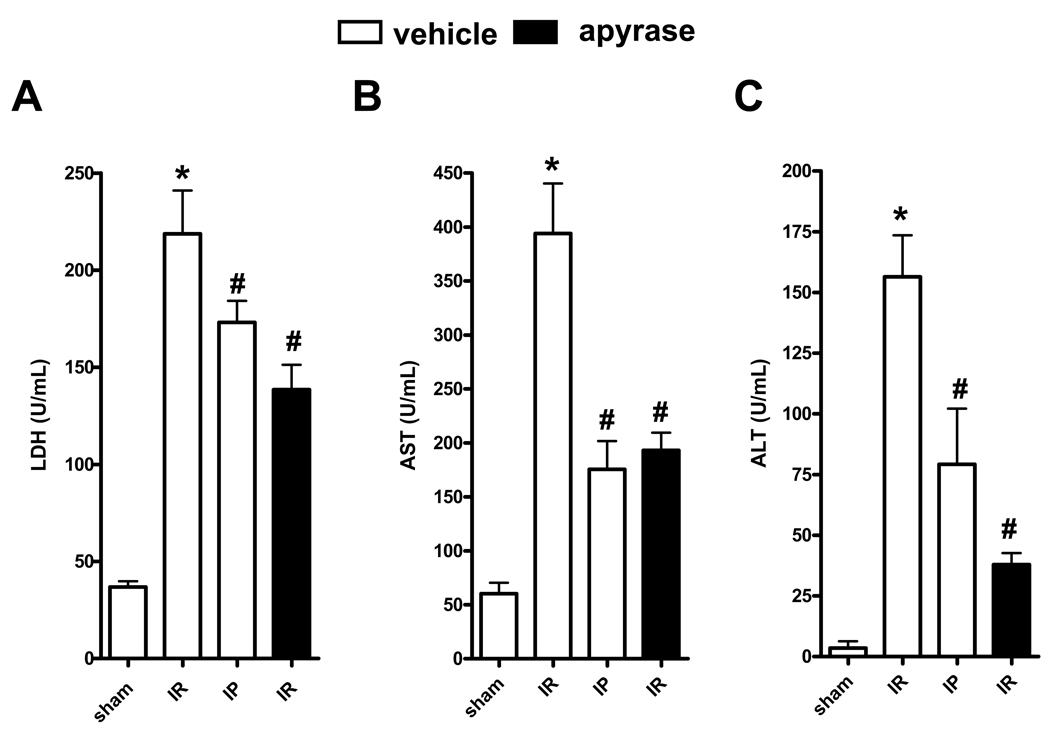
We next investigated treatment of hepatic ischemia with apyrase, an ATP hydrolase which catalyses the removal of the gamma phosphate from ATP and the beta phosphate from ADP. WT mice were treated with saline or apyrase (5 U, i.p.) followed by IR. As shown in Fig. 6A–C, apyrase treatment provided hepato-protection similar to that of IP. These results confirm our pharmacological and genetic studies and further demonstrate the role of CD39 in attenuating hepatic ischemia and reperfusion injury.
Figure 6.
Treatment with apyrase is protective against IR in WT mice. WT mice were treated with saline (vehicle) or apyrase (5 U, i.p.) and subjected to IR with or without prior IP treatment. (A) LDH, (B) AST, and (C) ALT. Results are expressed as the mean ± SEM of 4–8 mice/group. *p<0.05 vs. sham. #p<0.05 vs. IR+vehicle.
Reconstitution of CD39−/−mice with AMP is protective against liver IR injury
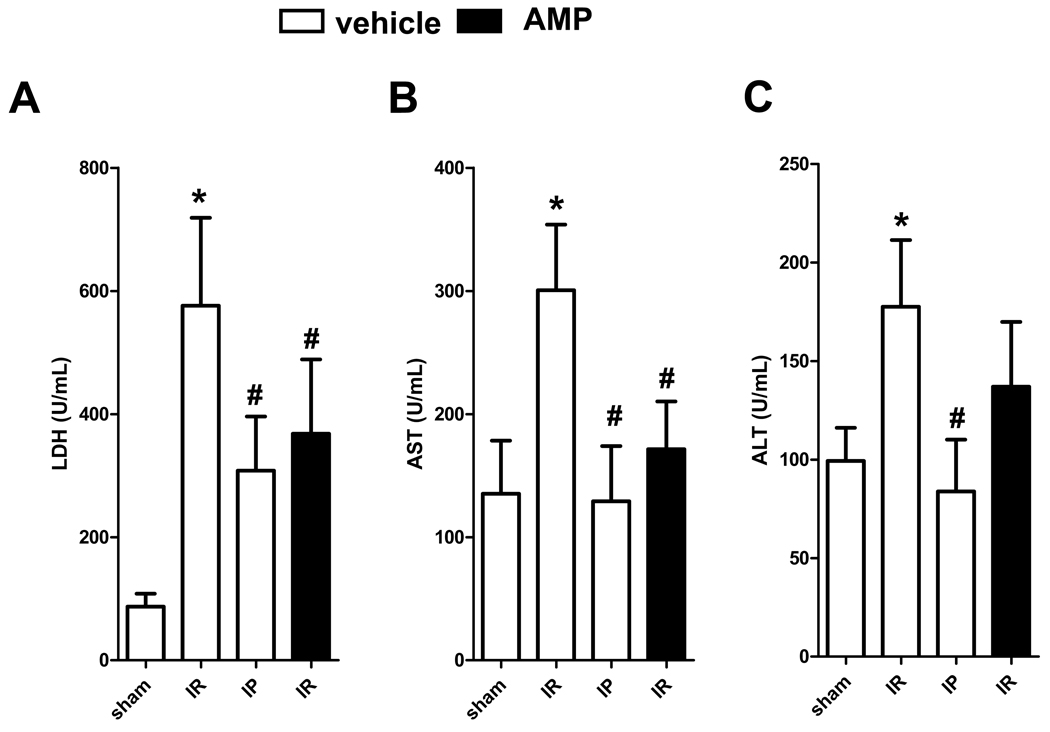
As proof of principle and to demonstrate that the absence of hepatic protection by IP in CD39−/− reflects lack of extracellular AMP, we reconstituted extracellular AMP levels via intra-arterial infusion (100 µl/h of 4 mg/kg, i.a.), a dose we previously determined not to induce hypotension or bradycardia (21, 22). Treatment of CD39−/− mice was associated with decreased LDH, AST and ALT levels similar to that of IP (Figure 7A–C). These results additionally demonstrate that extracellular nucleotide phosphohydrolysis plays a central role in increasing hepatic resistance to ischemia.
Figure 7.
Reconstitution of CD39−/− mice with AMP is protective against IR injury. CD39−/− mice were treated with saline (vehicle) or AMP (100 µl/h of 4 mg/kg, i.a.) and subjected to IR with or without prior IP treatment. (A) LDH, (B) AST, and (C) ALT. Results are expressed as the mean ± SEM of 3–8 mice/group. *p<0.05 vs. sham. #p<0.05 vs. IR+vehicle.
Role of Sp1-regulated CD39 in hepatic protection
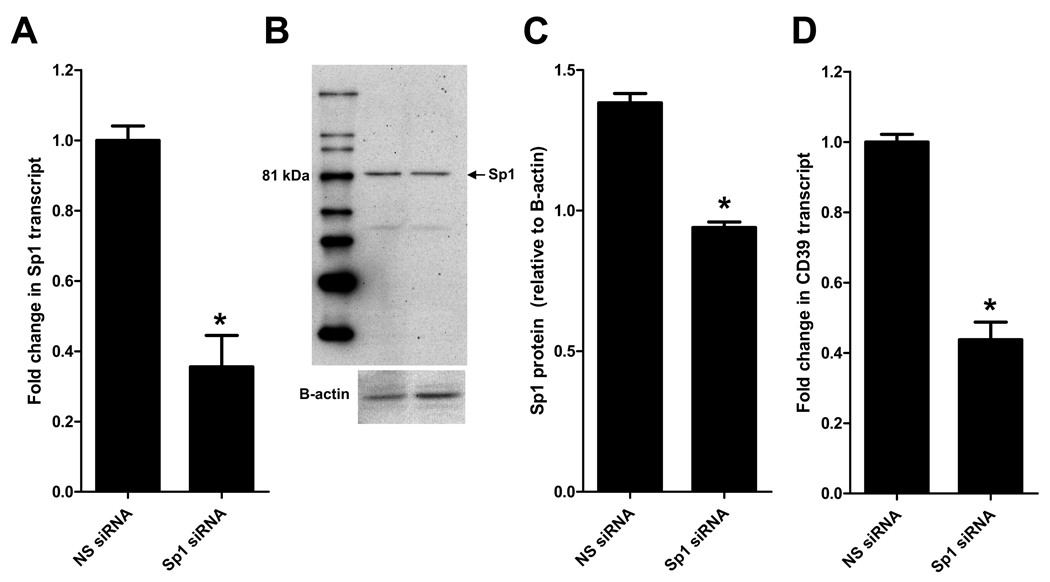
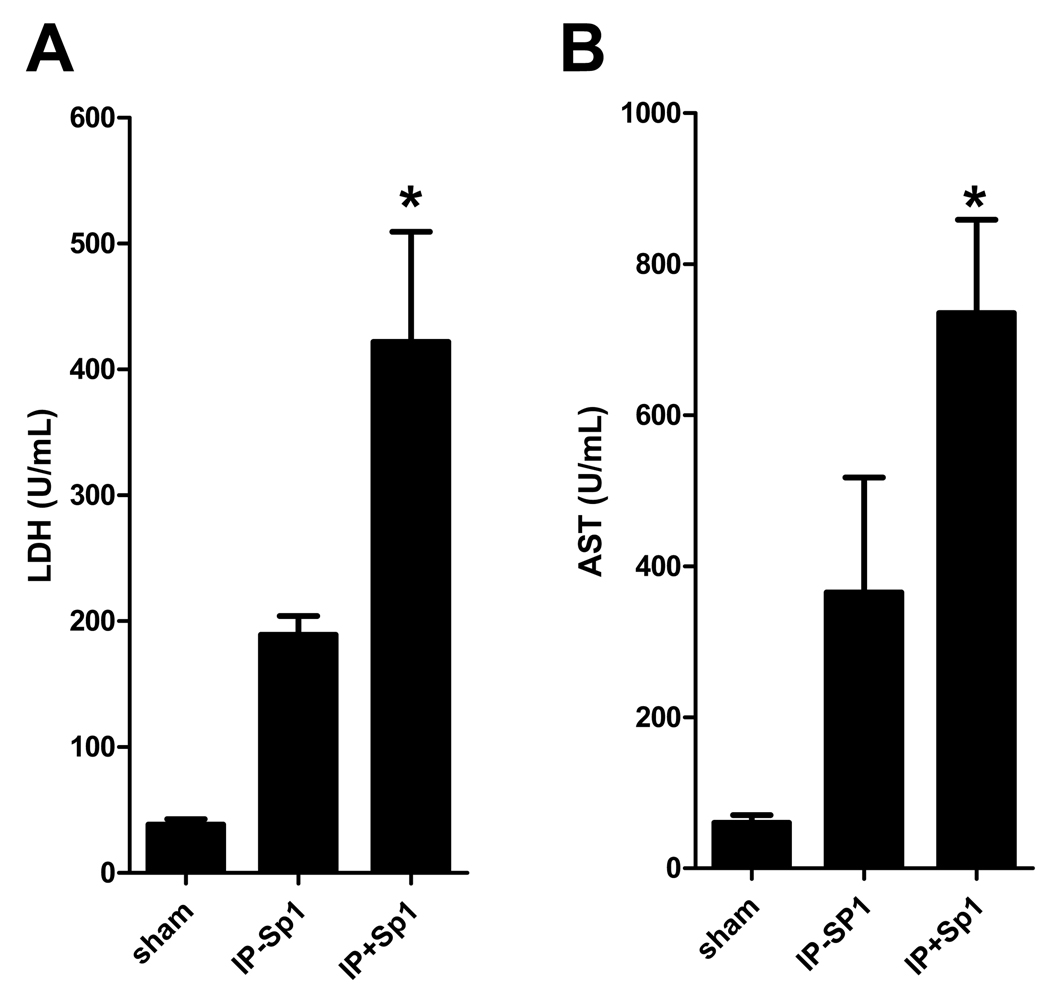
Sp1 is a member of a ubiquitously expressed family of Sp/XKLF transcription factors (28). These transcription factors are best known for maintenance and transcriptional housekeeping responsibilities. However, Sp1 in particular has been strongly implicated in hypoxic gene transcription and has recently been shown to play a protective role in regulation of CD39 during hypoxia and cardiac ischemia (23, 29, 30). To gain mechanistic insight on regulation of CD39 during hepatic IP, we treated WT mice with nonsense (NS siRNA) or Sp1 siRNA in combination with transfection reagent (2 mg/kg i.v.). To confirm efficacy of Sp1siRNA we measured Sp1 transcript and protein levels 24 hours after treatment. These studies revealed a significant repression of Sp1 transcript (Fig. 8A) and protein levels as measured by western blot (Fig. 8B) and densitometry (Fig. 8C) following treatment with Sp1 siRNA compared to NS siRNA. There was a significant decrease in CD39 transcript (Fig. 8D) levels following treatment with Sp1 siRNA compared to NS siRNA control. Furthermore, Sp1 siRNA significantly increased LDH and AST injury parameters in IP treated mice compared to NS siRNA (Fig 9A–B), but interestingly had no effect on ALT. Collectively this data suggests regulation of CD39 by Sp1 during liver IP.
Figure 8.
Sp1 siRNA decreases SP1 and CD39 in the liver. Wild-type (WT) mice were treated with nonsense (NS siRNA) or Sp1 siRNA in combination with transfection reagent (2 mg/kg). (A) Total RNA was isolated and Sp1 transcript levels were determined by real-time PCR. Data were calculated relative to β-actin. Results are expressed as the mean ± SEM of 3 mice/group. *p<0.05 vs. NS siRNA. (B) Proteins were resolved by SDS-PAGE, transferred to nitrocellulose and probed with anti-Sp1 antibody and re-probed for β-actin. (C) Sp1 protein was calculated by densitometry relative to β-actin. (D) Total RNA was isolated and CD39 transcript levels were determined by real-time PCR. Data were calculated relative to β-actin. Results are expressed as the mean ± SEM of 3 mice/group. *p<0.05 vs. NS siRNA.
Figure 9.
Sp1 siRNA decreases SP1 and CD39 in the liver and inhibits injury. Wild-type (WT) mice were treated with nonsense (NS siRNA) or Sp1 siRNA in combination with transfection reagent (2 mg/kg). (A) LDH and (B) AST . Results are expressed as the mean ± SEM of 4 mice/group. *p<0.05 vs. IP-Sp1.
Discussion
The study of liver IRI and subsequent cellular and organ damage remains an important field of investigation. Liver IRI significantly contributes to injury in various clinical settings including transplantation, hepatectomy for cancer, and shock (1, 2). IRI of the liver can activate a cascade of innate-dominated pro-inflammatory immune responses, which then trigger the adaptive immune response that culminates in transplant rejection (31). Hepatic IP significantly protects the liver from ischemic injury (4, 5). Because hepatic IP is thought to activate endogenous cellular protective mechanisms that are associated with adenosine, we used a mouse model of IRI and IP to investigate the protective role of CD39 in hepatic IRI and IP. Initial experiments suggested that preconditioning of the liver selectively induced CD39. Moreover, treatment with soluble apyrase was associated with liver protection from ischemia, emulating the hepatic protective effects of IP. Further experiments demonstrated that genetic deficiency or pharmacological inhibition of CD39 strongly attenuated the protective effects of IP demonstrating an essential role for CD39 in IP-dependent hepato-protection. Finally, the present studies provide insight into transcriptional mechanisms by demonstrating SP1 in hepatic CD39 induction during IP treatment. Taken together, these studies combine pharmacological and genetic evidence for hepatic protection in the context of IP against ischemic injury by CD39.
In the present study, we also explored the molecular mechanisms by which CD39 transcription is regulated during hepatic IP. Our results indicate that Sp1 siRNA (23, 32), but not nonsense controls, resulted in inhibition of CD39 and prevented IP protection. Although we did not examine other transcriptional regulators, Sp1 appears to contribute to CD39-mediated ischemic preconditioning protection. Sp1 has been strongly implicated in gene transcription during hypoxic events (23, 29, 30). Furthermore, our group recently showed Sp1-mediated induction of CD39 in cardioprotection during hypoxia/ischemia (23). Such findings strongly implicate Sp1-regulated transcription as a primary regulator of CD39 during ischemia.
We did not directly measure adenosine production in the present study, however, recent studies suggest that during hepatic ischemia, extracellular levels of adenosine are significantly elevated and play a protective role in liver protection from ischemia (15–18). It was previously shown that adenosine elevation plays a key role in promoting liver protection by IP (33, 34). As such, we recently demonstrated that increases in extracellular adenosine with hepatic IP were significantly attenuated in CD73-deficient mice or in WT mice treated with APCP, an inhibitor of CD73 (17). In conjunction with this study (17) and the present study, it appears that during hepatic IP, CD39 and consequently CD73 are transcriptionally induced, and thus increase adenosine levels, thereby protecting the liver from IRI injury. Adenosine then likely binds to A2A (14, 35) or A2B (36–38) adenosine receptors to mediate protection by hepatic IP (34, 39–43).
Our pharmacologic and genetic results are consistent with a previous study investigating the role of CD39 in the liver. Beldi et al. demonstrated that lack of CD39 activity is associated with decreased hepatic regeneration (19). Furthermore, this work has shown that apyrase treatment boosted levels of replicating hepatocytes post partial hepatectomy. The present study additionally demonstrates that treatment with apyrase protects mice from liver IRI-induced injury. Collectively, these studies suggest that therapeutics in which CD39 enzymatic activity is increased using apyrase, recombinant soluble CD39, or some other type of derivative may result in better clinical outcomes during liver injury.
Although we did not fully determine cell-specificity of CD39 in the ischemic liver, we show that hepatocytes from WT mice stain for CD39 in contrast to CD39−/− mice which show very minimal hepatocyte immunostaining for CD39. We did not detect differences in CD39 immunostaining for endothelial cells and pericytes. Previous studies have shown that CD39 is present on endothelial cells and Kupffer cells utilizing immunohistochemistry (44, 45). Interestingly, quiescent sinusoidal endothelial cells do not express CD39, conversely, under specific conditions of activation such as proliferation after partial hepatectomy, expression of CD39 on sinusoidal endothelial cells is upregulated (19). Unlike CD73 (46–48), CD39 canalicular or biliary expression has not been observed. Hepatic stellate cells have also been shown to express CD39 mRNA both under quiescent and activated conditions (44).
In contrast to the present studies suggesting that extracellular adenosine generation protects the liver from acute ischemic injury, more chronic elevations of adenosine may drive liver fibrosis or alcohol-associated liver disease. For example, a very elegant study demonstrated that wildtype mice fed ethanol on the Lieber-DeCarli diet developed hepatic steatosis, including increased hepatic triglyceride content, while mice lacking ecto-5'-nucleotidase or adenosine A1 or A2B receptors were protected from developing fatty liver (49). Similar protection was also seen in wild-type mice treated with either an adenosine A1 or A2B receptor antagonist. Moreover, in vitro studies supported roles for adenosine A1 receptors in promoting fatty acid synthesis and for A2B receptors in decreasing fatty acid metabolism. These results indicate that adenosine generated by ethanol metabolism plays a critical role in ethanol-induced hepatic steatosis via both A1 and A2B receptors and suggest that targeting adenosine receptors may be effective in the prevention of alcohol-induced fatty liver.
Collectively this study suggests that CD39 is a critical control point for endogenous liver protection during hepatic IP. This study also suggests that therapeutic strategies to increase CD39 enzymatic activity may be beneficial for the treatment of hepatic ischemia. Soluble derivatives conferring the enzymatic properties of CD39 may result in better clinical outcomes during liver IRI and subsequent dysfunction or failure. However, in an obligate surgical population, effects on bleeding or platelet functions would have to be considered (50). Future challenges will involve the translation of this work from mouse to man, and the definition of specific clinical treatment protocols.
Acknowledgements
We gratefully acknowledge Stephanie Zug, Stephanie Laucher and David Köhler for technical assistance.
Grant Support: Supported by University of Tübingen Grants [Fortune Grant F1211269 (MLH; HKE)], European Society of Anaesthesiology (ESA) Grant D3008762 (MLH; HKE), German Research Foundation (DFG) Grants [EL274/2-2, EL274/1 and EL274/4 (HKE)], Foundation for Anesthesia Education and Research (FAER) grants (HKE) and NIH R01 HL092188 (HKE), and P01-HL076540 (SCR).
Abbreviations
- ALT
alanine
- AST
aspartate aminotransferase
- CD73
5’-ectonucleotidase
- CD39
ectonucleoside triphosphate diphosphohydrolase-1
- IP
ischemic preconditioning
- IRI
ischemia/reperfusion injury
- LDH
lactate dehydrogenase
- NS
nonsense
- siRNA
small interfering RNA
- POM-1
sodium polyoxotungstate
Footnotes
Authors Contributions: MLH: Performed experiments, helped with study design, wrote first draft of manuscript. ISC: Performed experiments, helped with data analysis, and interpretation. JS: Performed experiments; SCR: Provided gene-targeted mice, helped with research design and data interpretation, helped with writing of the manuscript. HKE: Responsible for research design, writing of manuscript and intellectual content.
Conflicts of Interest: none
References
- 1.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Experimental and molecular pathology. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 4.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480–1491. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 6.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 7.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 8.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Medicine. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 13.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 15.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 16.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Matsuhashi T, Horikawa Y, Hatakeyama N, Oyake J, Ohba R, Linden J, Watanabe S. Selective A2A adenosine agonist ATL-146e attenuates acute lethal liver injury in mice. J Gastroenterol. 2005;40:526–529. doi: 10.1007/s00535-005-1609-9. [DOI] [PubMed] [Google Scholar]
- 17.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. Extracellular adenosine production by ecto-5'-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. doi: 10.1053/j.gastro.2008.07.064. e1733. [DOI] [PubMed] [Google Scholar]
- 18.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 21.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 22.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart ML, Much C, Kohler D, Schittenhelm J, Gorzolla IC, Stahl GL, Eltzschig HK. Use of a hanging-weight system for liver ischemic preconditioning in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1431–G1440. doi: 10.1152/ajpgi.00083.2008. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5'-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 27.Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;724:231–238. doi: 10.1016/s0378-4347(98)00580-5. [DOI] [PubMed] [Google Scholar]
- 28.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay D, Knebelmann B, Cohen HT, Ananth S, Sukhatme VP. The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Molecular and cellular biology. 1997;17:5629–5639. doi: 10.1128/mcb.17.9.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79:505–514. doi: 10.1097/01.tp.0000153160.82975.86. [DOI] [PubMed] [Google Scholar]
- 32.Hata Y, Duhc E, Zhang K, Robinson GS, Aiello LP. Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J Biol Chem. 1998;273:19294–19303. doi: 10.1074/jbc.273.30.19294. [DOI] [PubMed] [Google Scholar]
- 33.Peralta C, Hotter G, Closa D, Gelpi E, Bulbena O, Rosello-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 34.Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, Rosello-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- 35.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 36.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 38.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama H, Yamamoto Y, Kume M, Yamagami K, Yamamoto H, Kimoto S, Ishikawa Y, Ozaki N, Shimahara Y, Yamaoka Y. Pharmacologic stimulation of adenosine A2 receptor supplants ischemic preconditioning in providing ischemic tolerance in rat livers. Surgery. 1999;126:945–954. doi: 10.1016/s0039-6060(99)70037-1. [DOI] [PubMed] [Google Scholar]
- 41.Arai M, Tejima K, Ikeda H, Tomiya T, Yanase M, Inoue Y, Nagashima K, Nishikawa T, Watanabe N, Omata M, Fujiwara K. Ischemic preconditioning in liver pathophysiology. J Gastroenterol Hepatol. 2007;22 Suppl 1:S65–S67. doi: 10.1111/j.1440-1746.2006.04656.x. [DOI] [PubMed] [Google Scholar]
- 42.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009 doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevigny J, Robson SC, Waelkens E, Csizmadia E, Smith RN, Lemmens R. Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem. 2000;275:5640–5647. doi: 10.1074/jbc.275.8.5640. [DOI] [PubMed] [Google Scholar]
- 46.Matsuura S, Eto S, Kato K, Tashiro Y. Ferritin immunoelectron microscopic localization of 5'-nucleotidase on rat liver cell surface. J Cell Biol. 1984;99:166–173. doi: 10.1083/jcb.99.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frederiks WM, Marx F. A quantitative histochemical study of 5'-nucleotidase activity in rat liver using the lead salt method and polyvinyl alcohol. Histochem J. 1988;20:207–214. doi: 10.1007/BF01747465. [DOI] [PubMed] [Google Scholar]
- 48.Song J, Bosch KS, Tigchelaar W, Van Den Munckhof RJ, Schellens JP, Van Noorden CJ, Frederiks WM. Demonstration of 5'-nucleotidase activity in unfixed cryostat sections of rat liver using a combined light- and electron-microscope procedure. Histochem J. 1995;27:914–922. [PubMed] [Google Scholar]
- 49.Peng Z, Borea PA, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart ML, Kohler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK. Direct treatment of mouse or human blood with soluble 5'-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28:1477–1483. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]