Abstract
Most strains of Pseudomonas aeruginosa are significantly more resistant, even in the absence of R plasmids, to many antimicrobial agents, including beta-lactams, tetracycline, chloramphenicol, and fluoroquinolones, than most other gram-negative rods. This broad-range resistance has so far been assumed to be mainly due to the low permeability of the P. aeruginosa outer membrane. The intrinsic-resistance phenotype becomes further enhanced in "intrinsically carbenicillin-resistant" isolates, which were often assumed to produce outer membranes of even lower permeability. It has been shown, however, that this hypothesis cannot explain the beta-lactam resistance of these isolates (D.M. Livermore and K.W.M. Davy, Antimicrob. Agents Chemother. 35:916-921, 1991). In this study, we examined the uptake of tetracycline, chloramphenicol, and norfloxacin by intact cells using strains showing widely different levels of intrinsic resistance. Their accumulation and the response to the addition of a proton conductor showed that even relatively susceptible strains of P. aeruginosa actively pump out these compounds from the cell and that the efflux activity becomes much stronger in strains showing higher levels of intrinsic resistance. We conclude that the efflux mechanism(s) are likely to contribute significantly to the intrinsic resistance of P. aeruginosa isolates to tetracycline, chloramphenicol, and fluoroquinolones, as does the low permeability of the outer membrane. This conclusion is supported by the observation that the hypersusceptibility to various agents of the mutant K799/61 (W. Zimmermann, Antimicrob. Agents Chemother. 18:94-100, 1980) was apparently caused by the lack of active efflux. Although the hypersusceptibility of this mutant has hitherto been assumed to be solely due to its higher outer membrane permeability, its outer membrane was shown to have a coefficient of permeability to cephaloridine that was not significantly different from that of the parent, resistant strain K799/WT. The strains with elevated intrinsic resistance overproduced two cytoplasmic membrane proteins and one outer membrane protein; at least two of these proteins appeared different from the proteins overproduced in the recently described mutant with a derepressed multidrug efflux system, MexA-MexB-OprK (K. Poole, K. Krebes, C. McNally, and S. Neshat, J. Bacteriol. 175:7363-7372, 1993).
Full text
PDF
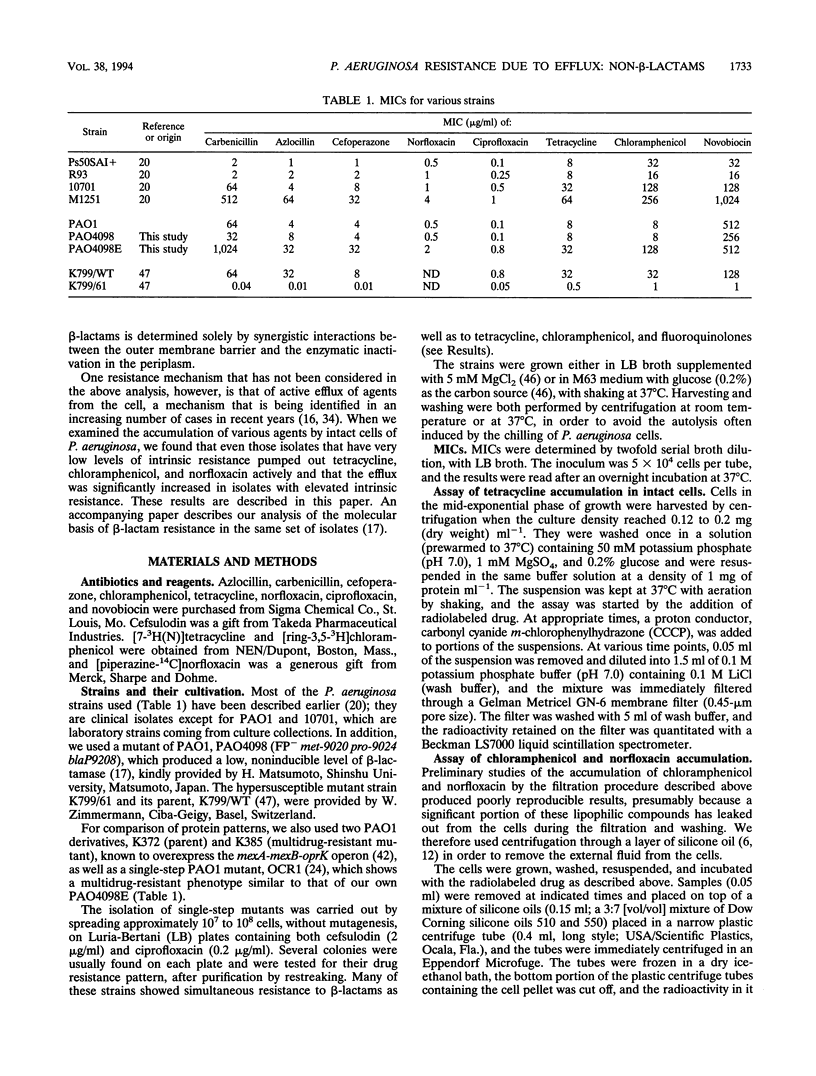
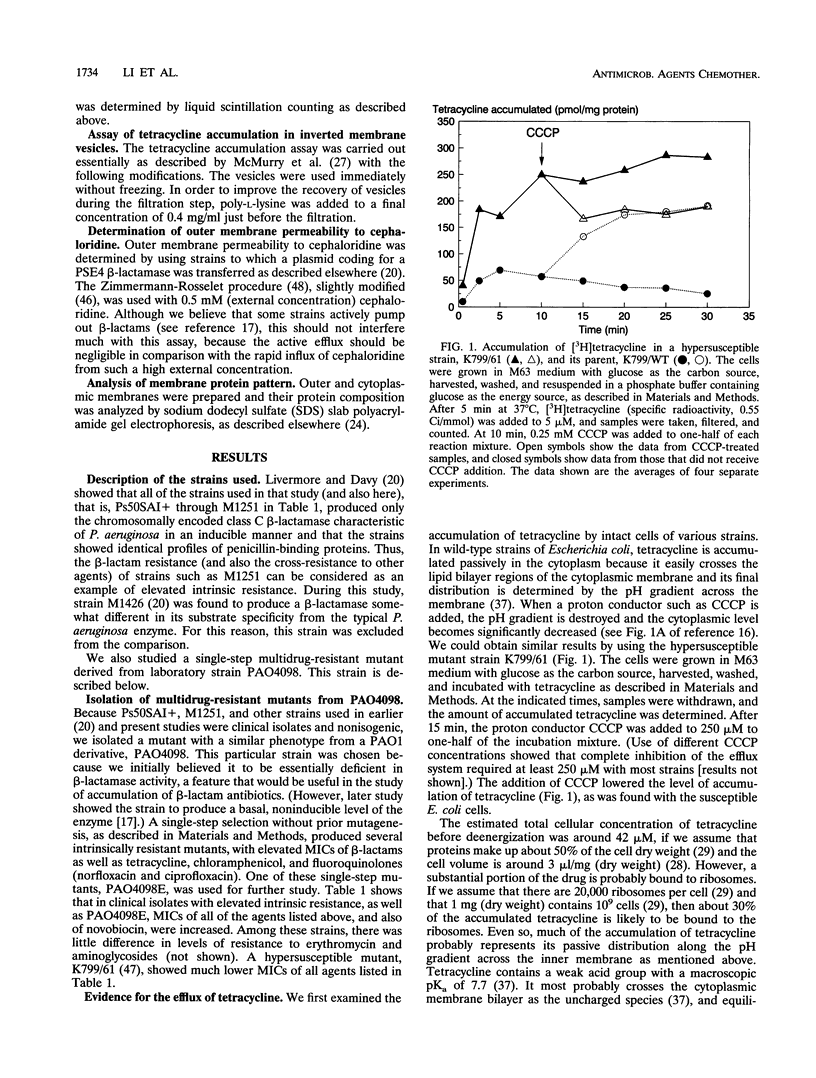
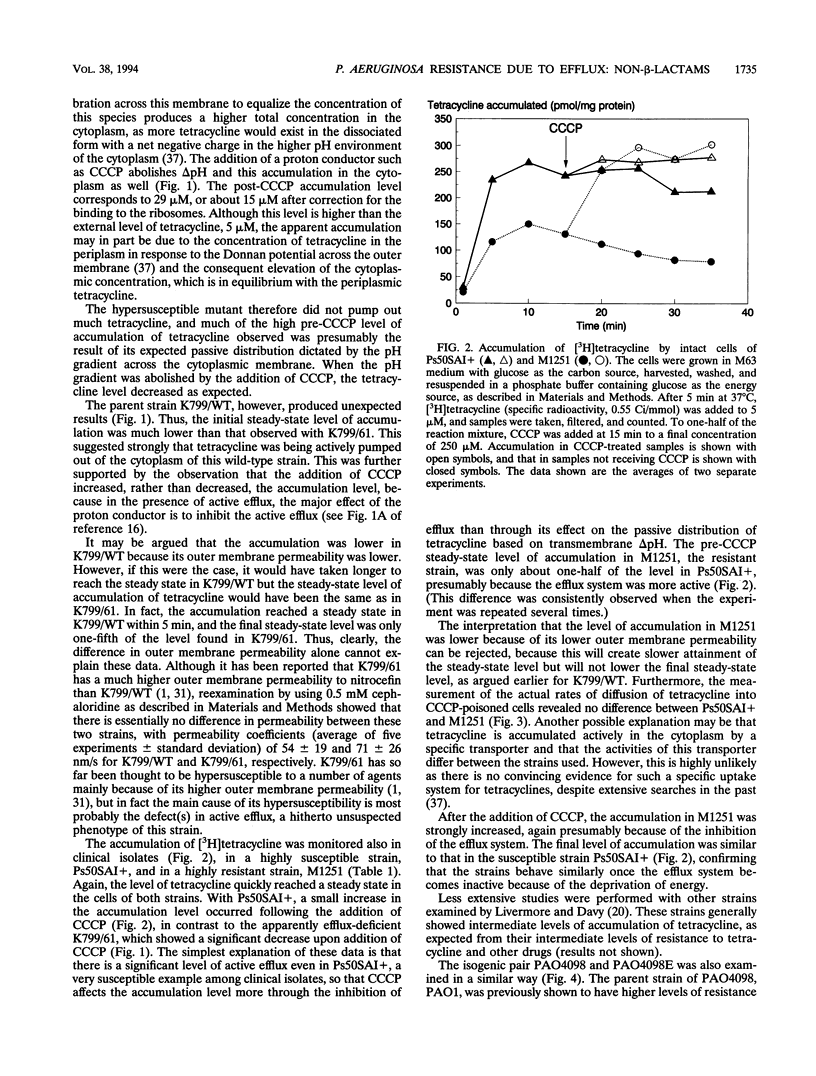
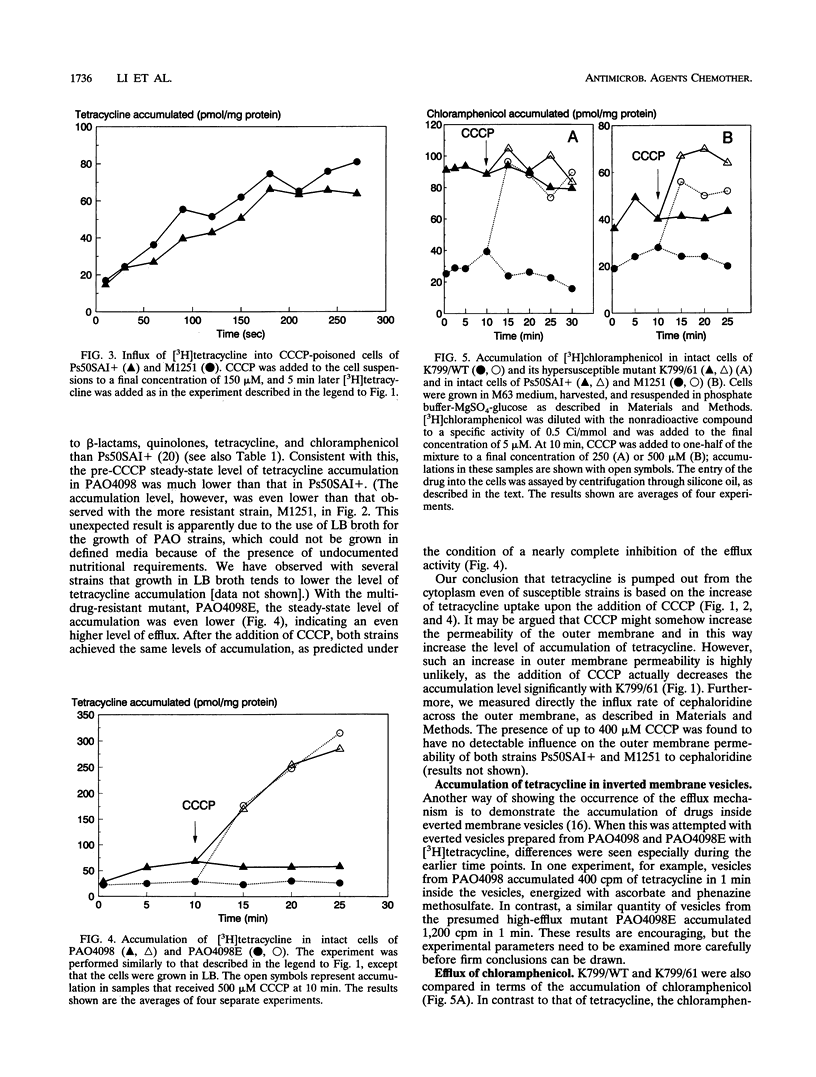
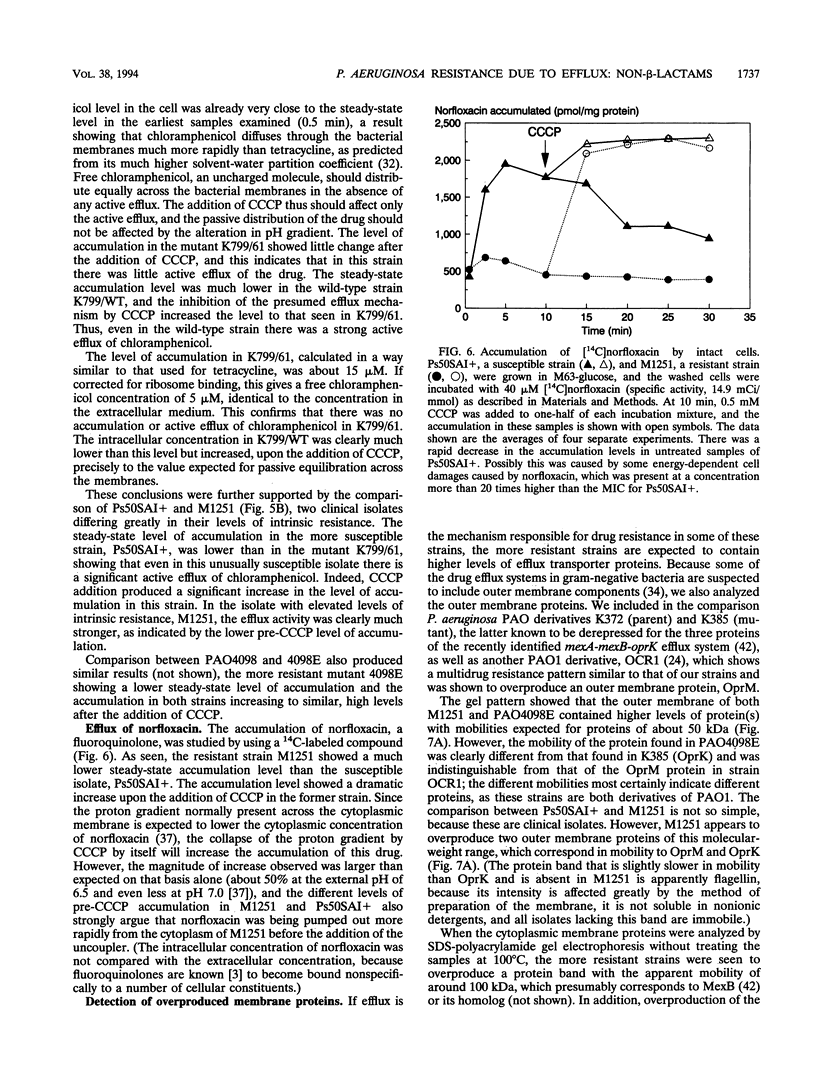
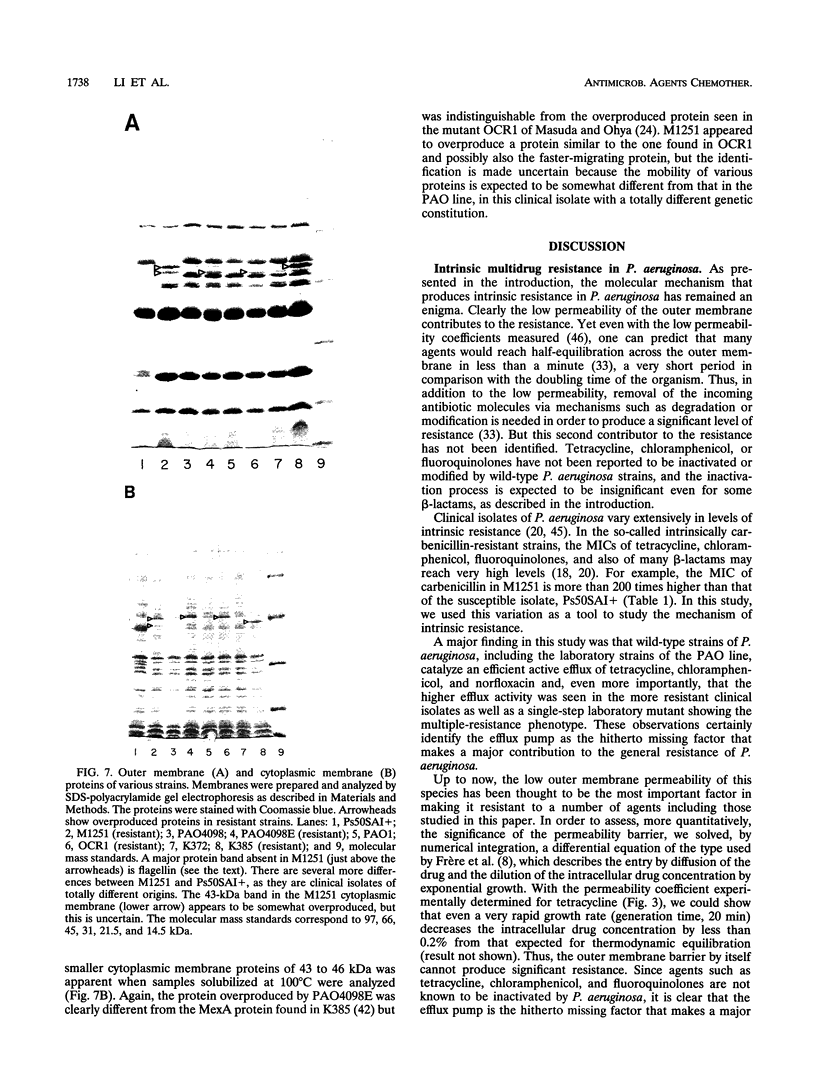
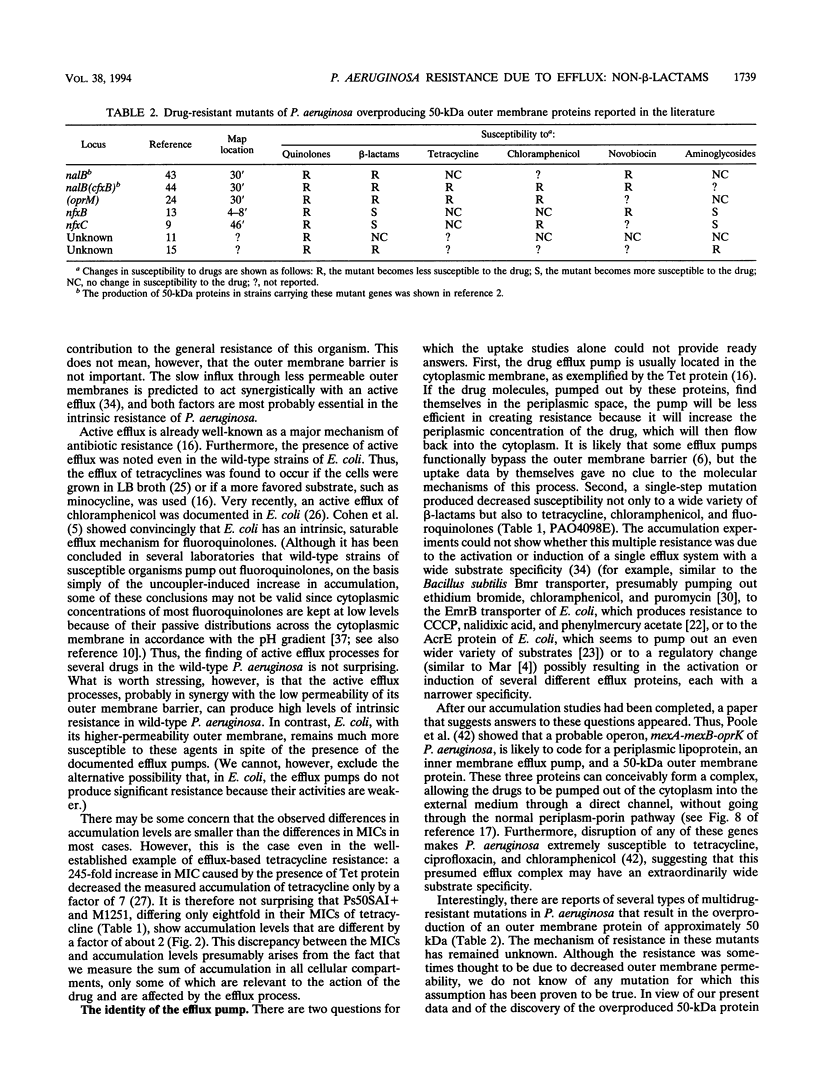


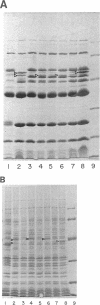
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hächler H., Levy S. B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993 Mar;175(5):1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Boulton M. G., Ross G. W. Penicillin-binding proteins of Pseudomonas aeruginosa. Comparison of two strains differing in their resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1981 Feb;7(2):127–136. doi: 10.1093/jac/7.2.127. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B., Crine M., Martin H. H. Quantitative relationship between sensitivity to beta-lactam antibiotics and beta-lactamase production in gram-negative bacteria--II. Non-steady-state treatment and progress curves. Biochem Pharmacol. 1989 May 1;38(9):1427–1433. doi: 10.1016/0006-2952(89)90181-0. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi Z. S., Smith J. M. Outer membrane changes in quinolone resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 1991 Sep;28(3):465–470. doi: 10.1093/jac/28.3.465. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Active efflux mechanisms for antimicrobial resistance. Antimicrob Agents Chemother. 1992 Apr;36(4):695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Z., Ma D., Livermore D. M., Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob Agents Chemother. 1994 Aug;38(8):1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M., Davy K. W. Invalidity for Pseudomonas aeruginosa of an accepted model of bacterial permeability to beta-lactam antibiotics. Antimicrob Agents Chemother. 1991 May;35(5):916–921. doi: 10.1128/aac.35.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M. Penicillin-binding proteins, porins and outer-membrane permeability of carbenicillin-resistant and -susceptible strains of Pseudomonas aeruginosa. J Med Microbiol. 1984 Oct;18(2):261–270. doi: 10.1099/00222615-18-2-261. [DOI] [PubMed] [Google Scholar]
- Livermore D. M., Yang Y. J. Beta-lactamase lability and inducer power of newer beta-lactam antibiotics in relation to their activity against beta-lactamase-inducibility mutants of Pseudomonas aeruginosa. J Infect Dis. 1987 Apr;155(4):775–782. doi: 10.1093/infdis/155.4.775. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. beta-Lactamases of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1991;44:215–220. doi: 10.1159/000420317. [DOI] [PubMed] [Google Scholar]
- Lomovskaya O., Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Cook D. N., Alberti M., Pon N. G., Nikaido H., Hearst J. E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993 Oct;175(19):6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992 Sep;36(9):1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Aronson D. A., Levy S. B. Susceptible Escherichia coli cells can actively excrete tetracyclines. Antimicrob Agents Chemother. 1983 Oct;24(4):544–551. doi: 10.1128/aac.24.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., George A. M., Levy S. B. Active efflux of chloramphenicol in susceptible Escherichia coli strains and in multiple-antibiotic-resistant (Mar) mutants. Antimicrob Agents Chemother. 1994 Mar;38(3):542–546. doi: 10.1128/aac.38.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974 Apr 16;43(3):533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Neyfakh A. A., Bidnenko V. E., Chen L. B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994 Apr 15;264(5157):382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Thanassi D. G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob Agents Chemother. 1993 Jul;37(7):1393–1399. doi: 10.1128/aac.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Fukasawa M., Komatsu T., Iyobe S., Mitsuhashi S. Isolation of two types of Pseudomonas aeruginosa mutants highly sensitive to a specific group of beta-lactam antibiotics and with defect in penicillin-binding proteins. J Antibiot (Tokyo) 1980 Dec;33(12):1521–1526. doi: 10.7164/antibiotics.33.1521. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Azuma A., Suzuki Y., Hashimoto Y. Factors involved in beta-lactam antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1977 Oct;12(4):537–539. doi: 10.1128/aac.12.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plésiat P., Nikaido H. Outer membranes of gram-negative bacteria are permeable to steroid probes. Mol Microbiol. 1992 May;6(10):1323–1333. doi: 10.1111/j.1365-2958.1992.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Poole K., Krebes K., McNally C., Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993 Nov;175(22):7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Livermore D. M., Lindridge M. A., Said A. A., Williams J. D. Mechanisms of beta-lactam resistance in British isolates of Pseudomonas aeruginosa. J Med Microbiol. 1984 Jun;17(3):283–293. doi: 10.1099/00222615-17-3-283. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]