Abstract
Purpose
The purpose of this study was to examine the joint effects of bereavement and caregiver intervention on caregiver depressive symptoms
Design and Methods
Alzheimer’s caregivers from a randomized trial of an enhanced caregiver support intervention versus usual care who had experienced the death of their spouse (n = 254) were repeatedly assessed with the Geriatric Depression Scale prior to and following bereavement. Random effects regression growth curve analyses examined the effects of treatment group and bereavement while controlling for other variables
Results
The death of the care recipient led to reductions in depressive symptoms for both caregiving groups. Enhanced support intervention led to lower depressive symptoms compared with controls both before and after bereavement. Post-bereavement group differences were stronger for caregivers of spouses who did not previously experience a nursing home placement. These caregivers maintained these differences for more than 1 year after bereavement. Caregivers who received the enhanced support intervention were more likely to show long-term patterns of fewer depressive symptoms before and after bereavement, suggesting resilience, whereas control caregivers were more likely to show chronic depressive symptoms before and after the death of their spouse.
Implications
Caregiver intervention has the potential to alter the long-term course of the caregiving career. Such clinical strategies may also protect caregivers against chronic depressive symptoms that would otherwise persist long after caregiving ends.
Keywords: Caregiving, Bereavement, Intervention, Resilience
The Alzheimer’s Association reports that approximately 5.1 million people in this the United States have Alzheimer’s disease (AD) and that 9.8 million caregivers provided a total of 8.5 billion hours of unpaid care to these individuals in 2005 (Alzheimer’s Association, 2007). In addition to physical assistance with activities of daily living, many of these caregivers help with instrumental activities of daily living, face challenging behavioral problems, provide financial assistance, and navigate the complex health care system in an effort to meet the health care needs of the care recipient (Alzheimer’s Association and National Alliance for Caregiving, 2004). Coupled with the financial challenges imposed by this care, many caregivers provide care at great cost to their personal and family lives, careers, and physical and mental health (Pinquart & Sörensen, 2003; Schulz & Beach, 1999). For some, the negative health effects of caregiving are sustained for months or years after the loss of the care recipient (Aneshensel, Botticello, & Yamamoto-Mitani, 2004; Bodnar & Kiecolt-Glaser, 1994).
The intensity and duration of care needed by individuals with AD has led to the conceptualization of caregiving as a “career” (Aneshensel, Pearlin, Mullan, Zarit, & Whitlatch, 1995) with the potential to last for many years (Hurley & Volicer, 2002; Schulz et al., 2003). Researchers have increasingly focused on the transitions such as the institutionalization and death of the care recipient that punctuate this experience (Burton, Zdaniuk, Schulz, Jackson, & Hirsch, 2003; Gaugler, Anderson, Zarit, & Pearlin, 2004). These transitions are frequently preceded by periods of demanding and highly stressful caregiving.
The effects of bereavement on the caregiving career are complex and can be better understood in light of the broader literature on bereavement. Bereavement is a well-documented risk factor for depression (e.g., Cole & Dendukuri, 2003). However, recent research also suggests that bereavement is associated with a wide variety of reactions, including not only grief responses but also relief, recovery, and resilience. In a prospective study of older married couples and bereavement, Bonanno and colleagues (2002) identified eight bereavement patterns among spouses after examining pre-bereavement and 6- and 18-month post-bereavement depression levels. The most common pattern (45.9%) was termed “resilient,” indicating low levels of depression at each measurement. The “chronic grief” trajectory (15.6%) was characterized by low pre-loss depression and elevated, sustained depression in bereavement. The “common grief” trajectory (10.7%), which had been described in the general bereavement literature as the most prevalent pattern of grief (Bonanno & Kaltman, 2001), resembled the chronic grief pattern until an improvement in depressive symptoms occurred at the 18-month post-loss measurement. Those on the “depressed-improved” trajectory (10.2%) demonstrated high levels of depression prior to the loss but not while bereaved, and those on the “chronic depression” trajectory (7.8%) experienced high levels of depression throughout the study. Other less common patterns included “delayed grief,” “delayed-improved,” and “improved-relapse” (see Bonanno et al., 2002).
Responses to the death of a relative with dementia can be multifaceted. As summarized in a review by Schulz, Newsom, Fleissner, Decamp, and Nieboer (1997), bereavement after dementia caregiving has the potential to lead to both chronic stress reactions as well as relief from the chronic strains of care-giving, because end-stage dementia is particularly challenging. In a recent study of caregivers for dementia patients in the last year of life, Schulz and colleagues (2003) found that more than half of caregivers felt “on duty” around the clock and reported providing care for more than 46 hr per week. Prospective longitudinal studies of depressive symptoms in dementia caregivers who have experienced bereavement have identified a variety of adaptation patterns. Bodnar and Kiecolt-Glaser (1994) found that dementia caregivers showed continued depressive symptoms even after the death of their spouses. In contrast, Schulz and colleagues (2003) reported that dementia caregivers indicated decreasing depressive symptoms after the death of the care recipient; more than 90% of caregivers viewed the death as a relief to the person with AD, and 72% viewed the death as a relief to themselves. However, a substantial percentage of caregivers (about 25%) remained depressed a year after the death, with depression while caregiving the strongest predictor of sustained depression after death (Schulz et al., 2003). Grant and colleagues (2002) found that although dementia caregivers reported decreasing depressive symptoms after bereavement, they may nevertheless have sustained elevations in blood pressure.
Going beyond analysis of mean scores among caregivers, Aneshensel and colleagues (2004) studied bereaved AD caregivers and identified four subgroups with different bereavement trajectories. Most common was the “repeatedly symptomatic” trajectory (63.7%), in which caregivers showed sustained moderate levels of depressive symptoms both while caregiving and after bereavement. Next most common was a “temporarily distressed” trajectory (17.7%), in which caregivers showed depressive symptoms while caregiving that improved after bereavement. Less common were the “repeatedly asymptomatic” trajectory (10.5%), in which caregivers experienced infrequent depressive symptoms both while caregiving and consistently through 5 years post-loss, and a “repeatedly distressed” group (8.1%) that showed sustained high levels of depressive symptoms throughout.
Research has demonstrated that psychosocial interventions (e.g., counseling, support group strategies, skills training) improve caregiver depressive symptoms (Sörensen, Pinquart, & Duberstein, 2002). It is also important to determine whether these intervention strategies have the potential to improve caregiver well-being after bereavement, a major transition in the caregiving career. We have identified only one published study to date that examined whether caregiver intervention affected bereavement responses. In the Schulz and colleagues (2003) study, which was a product of the Resources for Enhancing Alzheimer Caregiver Health (REACH) intervention trial, bereaved caregivers in active intervention conditions did not differ significantly from bereaved caregivers in control conditions on depressive symptoms after bereavement. However, the REACH interventions as a whole did not lead to significant immediate improvements in caregiver depressive symptoms (Gitlin et al., 2003), making it unlikely that REACH interventions would lead to subsequent improvements in adaptation to bereavement.
The New York University (NYU) caregiver intervention project provides an ideal opportunity to study the joint effects of caregiver intervention and bereavement on long-term caregiver outcomes, given the large sample size in this randomized intervention trial, long-term follow-up, and low levels of attrition. The NYU intervention has previously reported robust intervention effects in improving depressive symptoms that were sustained for at least 3 years after randomization (Mittelman, Roth, Coon, & Haley, 2004), suggesting that the NYU intervention might lead to sustained benefits for caregivers even after the death of the patient. In our previous analyses (Mittelman, Roth, Coon, et al., 2004) we reported a reduction in depressive symptoms after the death of the care recipient. We did not examine the complex interaction of the long-term effects of intervention, bereavement, institutionalization, and time that are necessary to understand the potential benefits of intervention that may be sustained even after the death of the person with dementia.
In the present study, we examined the effects of bereavement and caregiver intervention on the long-term course of caregiver depressive symptoms. Consistent with previous reports from our project (Mittelman, Roth, Coon, et al., 2004) we predicted that caregivers in the intervention group would have a long-term reduction in depressive symptoms compared with those in the usual care control group and that death of the care recipient would also lead to reduced depressive symptoms. In the present analyses, we predicted that caregiver intervention prior to any major transition (e.g., Gaugler, Kane, Kane, & Newcomer, 2005) would improve post-bereavement adaptation. We explored the duration of these benefits within the course of long-term follow-up. We also explored the impact of the intervention on mean levels of depressive symptoms and also on patterns of depressive responses over time after bereavement to provide a link to recent research that recognizes important types of reaction to death. In addition, because improvements in caregiver depressive symptoms can be associated with other prior factors, particularly relief of depressive symptoms after nursing home placement (Gaugler, Roth, Haley, & Mittelman, 2008), our analyses also controlled for the effects of caregiver gender, baseline depressive symptoms, and nursing home placement of the care recipient.
Methods
Sample
Participants included 254 spouse caregivers of AD patients who had experienced the death of their care recipient during involvement in the NYU Caregiver Intervention (NYUCI) study and who had provided post-bereavement outcome data. All were primarily responsible for caregiving, were caring for their spouse at home at baseline, and had at least one other family member or relative living in the vicinity. Caregivers were recruited through the NYU Aging and Dementia Research Center, media announcements, physicians, lawyers, and aging network providers including the Alzheimer’s Association and AD adult day care centers. The study received NYU School of Medicine Institutional Review Board approval and obtained written informed consent from each caregiver and other participating family members prior to study enrollment. We have previously reported that this intervention improves caregiver depressive symptoms, appraisal, and social support and delays nursing home placement, and that this study has experienced low rates of attrition over the nearly 20 years of data collection (Mittelman, Haley, Clay, & Roth, 2006; Mittelman, Roth, Coon, et al., 2004; Roth, Mittelman, Clay, Madan, & Haley, 2005). Each bereaved caregiver included in this analysis had completed at least one post-loss assessment.
Procedure
Following completion of a comprehensive, inperson baseline assessment, caregivers were assigned randomly to the enhanced counseling and support condition (n = 203) or the usual care control condition (n = 203). During the first year, participants were reinterviewed every 4 months with the same assessment tool used at baseline. After Year 1, follow-up interviews were conducted every 6 months. Interview administration continued on this schedule until the death of the care recipient unless the caregiver refused or was lost to follow-up. Up to two more follow-up interviews were conducted approximately 1 and 2 years after the death. All follow-up interviews were conducted face to face or by telephone.
Intervention
Enhanced Counseling and Support Treatment
The NYUCI comprised three components. The first component was delivered during the first 4 months of participation and involved two individual and four family counseling sessions. Family members included in the counseling sessions were designated by the spouse caregiver. The care recipient did not participate. The content of these sessions was tailored to the needs of each caregiver as determined during baseline assessment and was delivered by trained professional counselors. Goals of counseling ranged from providing education and resource information about AD and related issues to resolving family conflicts, improving communication among family members, bolstering family support for the spouse caregiver, and developing skills to manage difficult patient behaviors.
The second intervention component consisted of participation in a weekly support group for AD caregivers that was affiliated with the local chapter of the Alzheimer’s Association. Spouse caregivers were provided with information regarding support groups in their neighborhood and geographical area and were encouraged to maintain regular involvement. These groups were a source for emotional support, education, and connection with other AD caregivers.
The third component, “ad hoc” counseling, was available to caregivers at any time during their participation in the study. Ad hoc counseling, initiated by the spouse caregiver or any participating family member, was provided over the telephone or through additional face-to-face sessions to assist caregivers and families in handling crises or changes associated with the disease or the provision of care. The open-ended nature of the ad hoc counseling component offered caregivers and their families the freedom to determine the intensity and duration of follow-up support. All three components of the intervention were available to each treatment condition participant.
Usual Care Control Condition
Usual care condition participants received the standard level and types of care provided to caregivers of all AD patients at the NYU Aging and Dementia Research Center. Caregivers in the usual care group and their family members did not participate in the formal counseling provided in the treatment condition. However, they did have access to the same counselors who provided the intervention counseling and could request information, referral, and crisis counseling as needed. Additionally, no participant was prevented from utilizing outside assistance or support at any time during the course of the study. Thus, it is likely that usual care condition spouse caregivers accessed more services than are typically available in other community or medical care settings. As we have reported in greater detail in another article (Mittelman, Roth, Haley, & Zarit, 2004), treatment and control participants did not differ in their use of support groups at baseline, and over the course of the first year of the study, a substantial number (42%) of the caregivers in the control group joined support groups, compared to 58% of those in the treatment group, χ2 (1, N = 367) = 10.13, p = .0015.
Measures
Care Recipient Death
During each follow-up interview and other telephone contacts with spouse caregivers or other family members, dates of death were recorded. Dates of death were subsequently confirmed using an online version of the Social Security Death Index. We used a site available only through NYU Web servers, but there are similar public access versions available online (e.g., http://ssdi.rootsweb.ancestry.com/).
Depressive Symptoms
The Geriatric Depression Scale (Yesavage, Brink, Rose, & Adey, 1983) was administered at baseline and each follow-up interval to measure spouse caregivers’ mood and psychological well-being. The 30-item version has been widely validated, including identification of clinical cut points, with a cutoff score of 11 yielding a sensitivity of 84% and a specificity of 95% (Brink et al., 1982).
Statistical Analyses
We estimated the effects of the intervention and bereavement on depressive symptoms and tested this using a random effects regression growth curve analysis. We used a multilevel change model that estimated the longitudinal trajectories for individual participants at one level, with the intercepts and slopes of these person-specific longitudinal trajectories analyzed as the effects of between-subjects predictors at a higher order second level (Singer & Willett, 2003). This allowed us to take into account in the analytic model the nature of each individual caregiver’s participation during community, nursing home residence, and bereavement phases of the research and the timing of these transitions relative to enrollment in the study. We conducted all analyses using restricted maximum likelihood estimation as provided by SAS Proc Mixed (Littell, Milliken, Stroup, & Wolfinger, 1996).
We based the model on 254 participants (122 intervention, 132 usual care) who completed at least one assessment after baseline while providing care in the community and who completed at least one assessment after bereavement. Of the 152 participants excluded from these analyses, 131 had not experienced bereavement, 10 had completed a base-line assessment but were lost to follow-up after that point, and 11 were already in the bereavement stage at the first post-baseline assessment. We collected and analyzed in these models a total of 2,953 observations over time (including the baseline observations for the 254 caregivers included in these analyses). This included 1,799 observations during the community caregiving phase, 659 observations after a nursing home placement but before bereavement, and 495 observations after the death of the care recipient.
The model included two predictors for time: one that measured the amount of time elapsed since the date of the baseline assessment, and one for the amount of time elapsed since the death of the care recipient. Both metrics for time were originally calculated in days and converted to years by dividing by 365.25. We further modified the years since baseline measure by subtracting 1, which resulted in a “centered” measure that indicated the time before or after the 1-year post-baseline point. The centering of time allowed us to scale the model so that the main effect tests (i.e., treatment group, gender) represented a comparison at the 1-year post-baseline point. We set the years since death variable to be equal to 0 for any observations before death. Given these two measures for time, the main effect for years since baseline tested whether the linear slope across time was significantly different from 0, and the main effect for time since death tested whether this linear slope across time changed significantly after the death of the care recipient.
We also included time-varying indicators for care recipient nursing home placement and for bereavement as predictors in the model. We set these indicators to 0 for all observations before care recipient placement or death and to 1 for any observations that were made after care recipient placement or death. These predictors allowed us to test whether there was an abrupt change in the level of depression after nursing home placement or death.
We included three time-invariant predictors in the analytic model: baseline depression, caregiver gender, and intervention group. Baseline depression score was mean-centered such that the overall mean was subtracted from each participant’s score, resulting in a centered score that was a raw deviation from the mean and equal to 0 for someone who scored at the mean. We coded caregiver gender as 1 for female and 0 for male. We included baseline depression and caregiver gender as covariates to control for the effects of random imbalances at baseline. We coded intervention group as 1 for enhanced care and 0 for usual care.
In addition to the main effects for the time-varying and time-invariant predictors described above, we included 6 two-way interaction effects in the analytic model. A Placement × Bereavement interaction effect allowed us to test whether the change in caregiver depression following care recipient death was modulated by a previous nursing home placement for that care recipient. The model also included both baseline depression and intervention group interaction effects with years since baseline. The Group × Years Since Baseline term tested whether linear rates of change before bereavement differed between intervention (enhanced support) and usual care participants. Also included was a term for Group × Years After Death effect, which tested whether the linear slope in depression across time after death differed between the enhanced support and usual care groups. We also included Group × Placement and Group × Bereavement interaction terms to test for a difference in the change in depression after placement or death between groups.
We conducted additional, more descriptive analyses based on the patterns described by Bonanno and colleagues (2002). We identified patterns of depression scores over time using data collected at 1 year post-randomization and at the first and second bereavement assessments for the 174 caregivers who completed both post-bereavement assessments. For each time point, caregivers were described as experiencing either high (H) or low (L) depressive symptoms using the clinical threshold on the Geriatric Depression Scale, with scores greater than 11 suggesting clinically significant depressive symptomatology. Eight patterns were identified: resilient (LLL); chronically depressed (HHH); depressed improved (HLL); depressed, delayed improved (HHL); chronic grief (LHH); delayed grief (LLH); common grief (LHL); and improved, relapse (HLH). We used chi-square tests to compare the frequencies of the LLL, HHH, and HLL patterns between the intervention and usual care groups. Our method of identifying patterns is different than that used by Bonanno and colleagues (2002) but is based on the use of clinically significant cut scores rather than indices of change as used in the previous work. We used Fisher’s exact tests for the other less frequent patterns, for which low expected cell frequencies were common.
Results
Descriptive Information
Table 1 summarizes descriptive information on the participants. In spite of the random assignment, there were some imbalances on demographic variables and baseline depression. Statistical comparisons of the groups using t tests for continuous variables (caregiver age, patient age, baseline depression score) and chi-square tests for categorical variables (caregiver gender, global deterioration scale) showed that there were gender and dementia severity imbalances at baseline, with a higher proportion of husband caregivers randomly assigned to the intervention group, χ2(1, N = 254) = 4.21, p = .04; and a higher proportion of severe dementia cases randomly assigned to the usual care group, χ2(2, N = 254) = 9.26, p = .01. There was also a group imbalance on baseline depression score, t(252) = 2.03, p = .04. Dementia severity was a time-varying variable that progressed similarly across the intervention and usual care groups, and this reduced its potential as a confounder of the intervention effect over time. Gender and baseline depression were included as time-invariant covariates in the analytic model.
Table 1.
Baseline Demographic Data for the Intervention and Usual Care Groups
| Variable | Intervention | Usual Care |
|---|---|---|
| n | 122 | 132 |
| Caregiver age (M ± SD) | 71.0 ± 8.4 | 71.1 ± 8.9 |
| Care recipient age (M ± SD) | 73.8 ± 8.7 | 75.4 ± 8.0 |
| Caregiver gender (% women)a | 63.1 | 75.0 |
| Care recipient severity of dementia (%)a | ||
| Global Deterioration Scale = 4 | 34.4 | 25.8 |
| Global Deterioration Scale = 5 | 47.5 | 39.4 |
| Global Deterioration Scale ≥ 6 | 18.0 | 34.9 |
| Baseline caregiver depressive symptomsb (M ± SD) |
9.4 ± 5.7 | 11.1 ± 7.2 |
Note: Groups were compared at baseline on all variables and significant differences (p < .05) were revealed by
chi-square tests or
independent samples t test as indicated.
Table 2 presents the statistical results of the multilevel growth curve model. We found significant effects for both time from baseline and time from bereavement, indicating that the linear decrease in depression was significant across time and that this rate of decline was stronger after the care recipient’s death than before. The placement and bereavement effects were also statistically significant, indicating that there was an abrupt decrease in caregiver depression after both nursing home placement and care recipient death. The Placement × Bereavement interaction further indicated that the depression decrease after bereavement was significantly less for caregivers who had previously experienced a reduction in depression following nursing home placement. The final five rows in Table 2 report the results for tests involving the intervention condition versus the usual care condition. The estimate of −1.32 for the main effect for group indicates that depression was, on average, 1.32 units lower for the intervention group than the usual care group at 1 year post-baseline and before any placement or bereavement events. The groups did not differ significantly in the rates of change over time in depression before care recipient death or in the immediate changes due to nursing home placement or death. However, intervention and usual care groups did show significantly different slopes after patient death, such that depressive symptoms decreased at a steeper rate after death for the participants who received usual care than for those who received the intervention (p = .0088).
Table 2.
Random Effects Regression Model of Caregiver Depressive Symptoms
| Effect | Estimate | SE | t | df | p |
|---|---|---|---|---|---|
| Intercept | 11.27 | 0.56 | 20.24 | 250 | <.0001 |
| Years after baseline (centered at 1 year) | −0.27 | 0.13 | −2.13 | 251 | .0343 |
| Years after death | −1.35 | 0.38 | −3.55 | 2184 | .0004 |
| Placement (0 = before nursing home placement and for those who never placed, 1 = after placement) |
−2.22 | 0.39 | −5.70 | 2184 | <.0001 |
| Bereavement (0 = before death, 1 = after death) |
−1.32 | 0.66 | −2.01 | 2184 | .0444 |
| Placement × Bereavement | 1.21 | 0.44 | 2.74 | 2184 | .0062 |
| Caregiver gender (0 = ale, 1 = female) | −0.52 | 0.57 | −0.91 | 2184 | .3609 |
| Depression at baseline (mean centered) | 0.69 | 0.04 | 16.50 | 2184 | <.0001 |
| Baseline Depression × Years After Baseline | −0.08 | 0.01 | −6.98 | 2184 | <.0001 |
| Group (0 = usual care, 1 = intervention) | −1.32 | 0.53 | −2.47 | 2184 | .0138 |
| Group × Years After Baseline | −0.15 | 0.18 | −0.82 | 2184 | .4116 |
| Group × Years After Death | 1.47 | 0.56 | 2.62 | 2184 | .0088 |
| Group × Placement | 0.95 | 0.56 | 1.69 | 2184 | .0902 |
| Group × Bereavement | −1.23 | 0.89 | −1.38 | 2184 | .1674 |
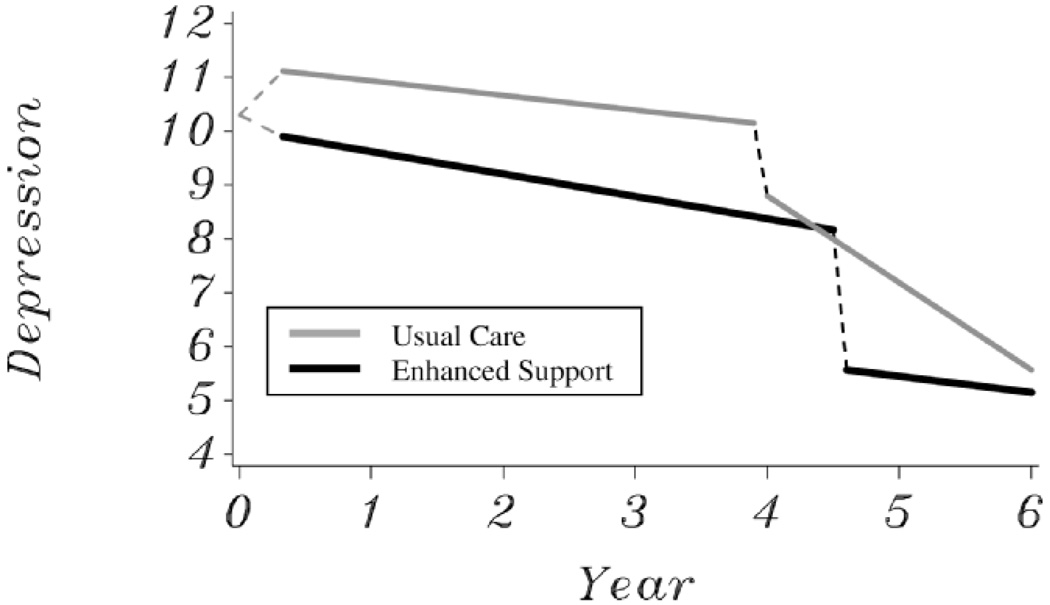
We examined model-predicted average trajectories for intervention and usual care caregivers, and Figure 1 illustrates trajectories for dyads for which no nursing home placement effects occurred (i.e., placement effects were fixed to 0). The effects of nursing home placement transitions are described in detail in another manuscript from this project (Gaugler et al., 2008). The death of the care recipient occurred at an average of 4.62 and 3.97 years after baseline for the intervention and usual care groups, respectively, and these means serve as the bereavement transition points illustrated in Figure 1. We compared covariate-adjusted means between the groups across these trajectories, and intervention depressive symptom levels were significantly lower than usual care scores at all points after baseline and before care recipient death. For those caregivers who had not experienced a previous nursing home placement, group differences in caregiver depression after care recipient death remained significant and up until 1.08 years (or 394 days) after death. At this point, the more rapid decrease in symptoms observed after death for the usual care group approached the low levels of depressive symptoms previously achieved by the intervention group.
Figure 1.
Model-predicted trajectories of caregiver depressive symptoms after treatment and bereavement in the enhanced support (black line) and usual care (gray line) groups.
Patterns of Depressive Symptoms
As shown in Table 3, the resilient pattern (n = 90; 52%), indicating low depressive symptoms while caregiving and in bereavement, was most common among participants in the NYUCI. Resilience was also significantly more prevalent in the intervention group (60%) than among controls (42.9%). Nearly a quarter of caregivers (n = 38) whose pattern is described as depressed improved experienced a drop in depressive symptoms from a high level while providing care to a level below the clinical threshold in bereavement. We found this pattern in comparable levels among treatment and control conditions.
Table 3.
Patterns of Depressive Symptoms in Caregivers, Overall and by Group
| All | Intervention | Usual Care | |||||
|---|---|---|---|---|---|---|---|
| Pattern | n | % | n | % | n | % | p |
| Resilient (LLL) | 90 | 51.7 | 54 | 60.0 | 36 | 42.9 | .0234 |
| Depressed improved (HLL) | 38 | 21.8 | 19 | 21.1 | 19 | 22.6 | .8099 |
| Chronically depressed (HHH) | 22 | 12.6 | 7 | 7.8 | 15 | 17.9 | .0439 |
| Depressed, delayed improved (HHL) | 10 | 5.8 | 2 | 2.2 | 8 | 9.5 | .0512 |
| Delayed grief (LLH) | 6 | 3.5 | 3 | 3.3 | 3 | 3.6 | 1.000 |
| Common grief (LHL) | 4 | 2.3 | 2 | 2.2 | 2 | 2.4 | 1.000 |
| Chronic grief (LHH) | 2 | 1.2 | 2 | 2.2 | 0 | 0.0 | .4977 |
| Improved, relapse (HLH) | 2 | 1.2 | 1 | 1.1 | 1 | 1.2 | 1.000 |
Note: L = low; H = high.
Caregivers in the usual care group were more than twice as likely (17.9%) to be chronically depressed than caregivers who received treatment (7.8%), a statistically significant difference. Participants receiving usual care also tended to be more likely to show delayed improvement in depressive symptoms than intervention caregivers (9.5% vs 2.2%, respectively) and the Fisher’s exact test of this effect closely approached the conventional statistical significance level (p = .0512). Other patterns including delayed grief, common grief, chronic grief, and improved with relapse were reported by fewer than 5% of caregivers.
Discussion
Our results yield important information about the effects of bereavement following dementia caregiving and the potential for caregiver intervention to affect caregivers over the long course of a caregiving career. The analyses show that the death of the spouse with dementia leads to decreases in depressive symptoms among caregivers in both the treatment and usual care groups, and that this decrease is largest if nursing home placement has not yet occurred. However, the most noteworthy finding is that the beneficial impact of enhanced caregiver support delivered relatively early in the caregiving career can lead to improvements in both mean levels and patterns of depressive symptoms that persist beyond the time of the death of the patient, often many years later. In some cases these effects can last for more than a year after the cessation of caregiving due to care recipient death. The random effects regression analyses show that the death of the spouse with dementia leads to mean decreases in caregiver depressive symptoms both for treatment and control caregivers. In addition, intervention effects in reducing caregiver depressive symptoms were sustained even after death of the care recipient, especially for caregivers who had not previously received relief from depression due to the nursing home placement of the care recipient. Eventually, the additive effects of nursing home placement, death of the care recipient, and time after these transition events lead to comparable levels of depressive symptoms across the two groups. The rate of decrease in depressive symptoms was significantly larger for the usual care participants after the death of the care recipient, which means they eventually approached the previously lower post-bereavement depressive symptom levels of the intervention participants.
Examination of patterns of depressive symptoms before and after death of the care recipient also yields important findings. Similar to the results reported by Bonanno and colleagues (2002), resilience (or depression scores that did not reach clinically significant levels at any point in the study) was the most common pattern observed. However, resilience was significantly more common in caregivers receiving the intervention (60%) compared with those in the control condition (42.9%). This finding, coupled with the finding that chronic depressive symptoms were more than twice as common in controls as in caregivers who received supportive treatment, suggests that caregiver intervention has the potential to alter the course of the long-term caregiving career for a large subgroup of caregivers.
Our results show that, when examining the effects of intervention and transitional events such as bereavement, it is valuable to consider analyses that not only examine mean levels of change but also identify subgroups that exhibit different patterns of change over time. In the present analyses, these two sets of results lead to comparable conclusions—a multicomponent psychosocial intervention for caregivers can not only reduce depression at major transition points such as bereavement but can also enhance resilience and reduce the risk of chronic, clinically significant depressive symptoms over the lengthy caregiving career.
Our results also highlight the importance of considering the problems of subgroups of caregivers who have continued problems with depressive symptoms even after their caregiving roles have ended. In the present project, 17.9% of controls (but only 7.8% of those receiving the intervention) had high depression scores at all three measurement points, demonstrating chronic depression. Although events such as death of the care recipient and cessation of the caregiving role do lead to some degree of relief (Schulz et al., 2003), a sizable number of caregivers may have chronic difficulties. Aneshensel and colleagues (2004) found that high levels of role over-load before bereavement was a risk factor for poor bereavement outcomes and that social support and high caregiver self-esteem were protective factors. Our previous results have shown that the beneficial effects of the NYUCI on caregiver depressive symptoms are primarily mediated by intervention-driven improvements in caregivers’ perceived satisfaction with social support (Roth et al., 2005). This suggests that an enhancement in social support while caregiving may provide a long-term and beneficial resource for caregivers that is useful even after caregiving ends.
Although our study is unique in terms of its long-term follow-up of participants in a randomized caregiver intervention trial, we should note a number of limitations. Although we examined patterns of caregiver depressive symptoms using a clinically relevant approach (i.e., whether caregivers were at or above levels of clinical significance on the Geriatric Depression Scale), this approach was not exactly comparable to the trajectory analyses that Bonanno and colleagues (2002) or Aneshensel and associates (2004) utilized. Thus, sample comparisons of the bereavement patterns across studies should be made with caution. In addition, we have very little information about how the NYUCI may have altered a number of variables relevant to end-of-life care and bereavement, including whether the caregiver intervention altered utilization of hospice or other services or altered end-of-life decision making. We should note that although group differences were statistically significant, the magnitude of these differences was sometimes small. This concern is offset by the fact that our control participants were likely to have had relatively high levels of treatment (e.g., high support group participation), so the comparison in this study was not with a truly untreated control group. These matters deserve greater attention in future studies.
Conclusion
Our results suggest that the NYUCI improves caregiver depressive symptoms not only during active caregiving but also during caregivers’ adaptation to bereavement. It is important that other intervention studies gather data on the long-term impact of intervention not only in the community but across important transitions such as bereavement. Caregivers who gain knowledge, skills, and supports through caregiver intervention may find these resources of value not only while providing active caregiving assistance but also as they face the challenges of coping with the death of a loved one. Translational efforts to disseminate effective caregiver interventions that reduce the risk of bereaved caregivers suffering chronic depression, or that address those needs during bereavement, are important priorities for this field.
Acknowledgments
This study was funded by the National Institute of Mental Health (R01 MH 42216) and the National Institute on Aging (R01 AG14634). Additional funding was provided through the Alzheimer’s Disease Core Center (P30-AG08051). William E. Haley was also supported by the Florida Alzheimer’s Disease Research Center (P50-AG025711).
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures, 2007. 2007 Retrieved May 25, 2007, from http://www.alz.org/national/documents/Report_2007FactsAndFigures.pdf.
- Alzheimer’s Association and National Alliance for Caregiving. Families care: Alzheimer’s caregiving in the United States, 2004. 2004 Retrieved May 25, 2007, from http://www.caregiving.org/data/alzcaregivers04.pdf.
- Aneshensel CS, Botticello AL, Yamamoto-Mitani N. When caregiving ends: The course of depressive symptoms after bereavement. Journal of Health and Social Behavior. 2004;45:422–440. doi: 10.1177/002214650404500405. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Pearlin LI, Mullan JT, Zarit SH, Whitlatch CJ. Profiles in caregiving: The unexpected career. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Bodnar JC, Kiecolt-Glaser JK. Caregiver depression after bereavement: Chronic stress isn’t over when it’s over. Psychology and Aging. 1994;9:372–380. doi: 10.1037//0882-7974.9.3.372. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Kaltman S. The varieties of grief experience. Clinical Psychology Review. 2001;21:705–734. doi: 10.1016/s0272-7358(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, et al. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. Journal of Personality and Social Psychology. 2002;83:1150–1164. doi: 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]
- Brink TL, Yesavage JA, Owen L, Heersema PH, Adey M, Rose TL. Screening tests for geriatric depression. Clinical Gerontologist. 1982;1:37–44. [Google Scholar]
- Burton LC, Zdaniuk B, Schulz R, Jackson S, Hirsch C. Transitions in spousal caregiving. The Gerontologist. 2003;43:230–241. doi: 10.1093/geront/43.2.230. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. American Journal of Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in the nursing home: Effects on stress and well-being. Aging & Mental Health. 2004;8:65–75. doi: 10.1080/13607860310001613356. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Kane RL, Kane RA, Newcomer R. Early community-based service utilization and its effects on institutionalization in dementia caregiving. The Gerontologist. 2005;45:177–185. doi: 10.1093/geront/45.2.177. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counseling and support reduce Alzheimer’s caregivers’ burden and depressive symptoms during the transition to institutionalization? Results from the NYU intervention study. Journal of the American Geriatrics Society. 2008;56:421–428. doi: 10.1111/j.1532-5415.2007.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D, et al. Effect of multicomponent interventions on caregiver burden and depression: The REACH multisite initiative at 6-month follow-up. Psychology and Aging. 2003;18:361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Adler KA, Patterson TL, Dimsdale JE, Ziegler MG, Irwin MR. Health consequences of Alzheimer’s caregiving transitions: Effects of placement and bereavement. Psychosomatic Medicine. 2002;64:477–486. doi: 10.1097/00006842-200205000-00012. [DOI] [PubMed] [Google Scholar]
- Hurley AC, Volicer L. Alzheimer disease: “It’s okay, Mama, if you want to go, it’s okay.”. Journal of the American Medical Association. 2002;288:2324–2331. doi: 10.1001/jama.288.18.2324. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in Alzheimer’s caregivers. American Journal of Psychiatry. 2004;161:850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: Results of a randomized trial. Journal of Gerontology: Psychological Sciences. 2004;59B:P27–P34. doi: 10.1093/geronb/59.1.p27. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Roth DL, Mittelman MS, Clay OJ, Madan A, Haley WE. Changes in social support as mediators of the impact of a psychosocial intervention for spouse caregivers of persons with Alzheimer’s disease. Psychology and Aging. 2005;20:634–644. doi: 10.1037/0882-7974.20.4.634. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, Mendelsohn AB, Haley WE, Mahoney D, Allen RS, Zhang S, et al. End of life care and the effects of bereavement among family caregivers of persons with dementia. New England Journal of Medicine. 2003;349:1936–1942. doi: 10.1056/NEJMsa035373. [DOI] [PubMed] [Google Scholar]
- Schulz R, Newsom JT, Fleissner K, Decamp AR, Nieboer AP. The effects of bereavement after family caregiving. Aging & Mental Health. 1997;1:269–282. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. The Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Adey M. The Geriatric Depression Rating scale: Comparison with other self-report and psychiatric rating scales. In: Crook T, Ferris SH, Bartus R, editors. Assessment in geriatric psychopharmacology. New Canaan, CT: Mark Powley Associates; 1983. pp. 153–165. [Google Scholar]