Abstract
Background
Mass levels of lipoprotein-associated phospholipase A2 (Lp-PLA2), a leukocyte-derived enzyme involved in the metabolism of low-density lipoprotein to pro-inflammatory mediators, are associated with prognosis after stroke. Lp-PLA2 mass correlates only moderately with levels of Lp-PLA2 activity. The relationship of Lp-PLA2 activity to risk of stroke recurrence is unknown. We hypothesized that Lp-PLA2 activity levels would predict risk of recurrence.
Methods
In the population-based Northern Manhattan Stroke Study, first ischemic stroke patients ≥40 years were followed for recurrent stroke. Levels of Lp-PLA2 activity were assessed in 467 patients, and categorized by quartile. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (95% CI) for risk of recurrent stroke associated with marker quartiles after adjusting for demographics, vascular risk factors, and high-sensitivity C-reactive protein (hsCRP).
Results
Mean age was 68.9 ± 12.7 years; 54.6% were women; 53.3% Hispanic, 27.2% black, and 17.8% white. Median follow-up was 4.0 years, and there were 80 recurrent strokes. Compared to the lowest quartile of Lp-PLA2 activity, those in the highest had an increased risk of recurrent stroke (adjusted HR 2.54, 95% CI 1.01–6.39).
Conclusion
Stroke patients withLp-PLA2 activity levels in the highest quartile, compared to those in the lowest quartile, had an increased risk of recurrence after first ischemic stroke. Further studies are warranted to determine whether this biomarker has clinical utility in determining high-risk populations of stroke survivors, and whether anti-inflammatory strategies that reduce levels of activity of Lp-PLA2 reduce the risk of stroke recurrence.
Key Words: Anti-inflammatory strategies, C-reactive protein, Ischemic stroke, Lipoprotein-associated phospholipase A2, Pro-inflammatory mediators, Recurrent stroke
Introduction
Inflammation appears to play an important role in atherosclerosis and its clinical sequelae, including myocardial infarction (MI) and stroke [1]. Peripheral blood markers of inflammation, including leukocyte count, high-sensitivity C-reactive protein (hsCRP), and others, are associated with carotid atherosclerosis and predict incident stroke [2,3,4,5,6,7,8,9,10,11,12]. The activity of lipoprotein-associated phospholipase A2 (Lp-PLA2), a leukocyte-derived enzyme involved in the metabolism of low-density lipoprotein (LDL) to pro-inflammatory mediators, has also been shown in prospective studies to predict incident stroke independently of hsCRP [13, 14].
The role of elevated levels of markers of inflammation in predicting prognosis after a first ischemic stroke is less clear, although growing evidence suggests a potential role for several inflammatory biomarkers [15, 16]. We have previously shown that leukocyte count [17] predicts recurrent stroke or death after a first ischemic stroke, and that levels of Lp-PLA2 mass predict risk of recurrent stroke [18]. We have also reported that hsCRP predicts death after stroke [18]. Recently, a secondary analysis of the PROVE IT trial (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction) [19] demonstrated that elevated levels of Lp-PLA2 activity, but not mass, measured 30 days after acute coronary syndromes were associated with recurrent cardiovascular events.
We hypothesized that relative elevations in Lp-PLA2 activity at the time of a first ischemic stroke would be associated with an increased risk of recurrent stroke among an elderly, urban, multi-ethnic population after adjusting for conventional stroke risk factors and levels of hsCRP.
Patients and Methods
The Northern Manhattan Stroke Study includes a population-based incident ischemic stroke follow-up study designed to determine predictors of stroke recurrence and prognosis in a multi-ethnic, urban population. Northern Manhattan consists of the area north of 145th Street, south of 218th Street, bordered on the west by the Hudson River, and on the east by the Harlem River. The race-ethnic mixture of the community consists of approximately 60% Hispanic, 20% non-Hispanic black, and 20% non-Hispanic white residents [20, 21].
Selection of the Stroke Cohort
The methods of patient identification and enrollment have been described previously [22, 23]. Briefly, stroke patients were enrolled if they: (1) were diagnosed with a first stroke; (2) were age 40 and over, and (3) resided in Northern Manhattan for ≥3 months in a household with a telephone. For this analysis, only ischemic stroke cases were included. Over 80% of the patients with acute ischemic stroke in Northern Manhattan are hospitalized at the Columbia University Medical Center (CUMC). Subjects hospitalized at other local hospitals were identified through active surveillance of admissions to those hospitals and through agreements with local physicians. Approximately 5% of incident ischemic stroke patients in Northern Manhattan are not hospitalized [23]. Evaluation of patients was performed at the hospital; those subjects either not hospitalized or hospitalized elsewhere were evaluated in the outpatient research clinic. The study was approved by the CUMC Institutional Review Board. All participants gave consent directly or through a surrogate when appropriate.
Index Evaluation of Subjects
Data were collected through interviews by trained research assistants, and physical and neurological examinations were conducted by study neurologists. When possible, data were obtained directly from subjects using the standardized data collection instruments. When the subject was unable to provide answers, a proxy knowledgeable about the subject's history was interviewed. Assessments were conducted in English or Spanish depending upon the primary language of the participant. Race/ethnicity was based upon self-identification through a series of interview questions modeled after the US census and conforming to the standard definitions outlined by Directive 15 [24]. All participants identifying themselves as Hispanic were classified as such. All participants classifying themselves as white without any Hispanic origin, or black without any Hispanic origin were classified as white, non-Hispanic, or black, non-Hispanic residents, respectively.
Standardized questions were adapted from the Behavioral Risk Factor Surveillance System [25] of the Centers for Disease Control and Prevention. Standard techniques were used to measure blood pressure, height, weight, and fasting glucose and lipid panels as described previously [22, 23]. Hypertension was defined as in prior publications [22, 23], and diabetes mellitus was defined by a fasting blood glucose level ≥126 mg/dl, the subject's self-report of such a history, or insulin or oral hypoglycemic use.
Stroke severity was assessed using the National Institutes of Health Stroke Scale. Assessment of stroke subtype using modified TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria [26] was determined by a consensus of stroke neurologists, using all available information, as described previously [27].
Assessment of Lp-PLA2 Activity and hsCRP
Blood samples were collected at the time of hospitalization or clinic visit in 5-ml serum separator tubes by a trained phlebotomist. Samples were centrifuged at 3,000 g for 15 min and then the serum was aliquotted into 2-ml Eppendorf tubes. Samples were then stored at −80°C until analyses were run. Serum samples were assayed for levels of Lp-PLA2 activity using a colorimetric assay (diaDexus, South San Francisco, Calif., USA) [28, 29]. hsCRP was measured using an enzyme-linked immunoassay (BioCheck, Foster City, Calif., USA). Assays were run at a central laboratory at diaDexus. Laboratory personnel were blinded to all patient clinical data and outcomes.
Quality control was maintained by the laboratory using standard procedures, and 24% of the total samples were run in duplicate. The average coefficient of variation was 2.74%. In total, 98.4% of samples produced coefficients of variation ≤10% between duplicates.
Follow-Up and Outcome Assessment
Follow-up evaluations were conducted at 6 months by telephone and then annually in person for 5 years. Information on vital status, functional status, and intercurrent symptoms, illness, or hospitalization was collected, as well as measurement of vital signs, and physical and neurological examination. Patients unable or unwilling to come to the CUMC were visited by a member of the research staff, and the evaluation was conducted at home or in an alternative place of residence (e.g. nursing home). An ongoing surveillance system of admissions to the CUMC and other local hospitals, described previously [30], was also used to identify study participants who experienced recurrent stroke, MI, hospitalization, or death. When available, medical records were reviewed for all outcome events including death. All outcome events were reviewed by a research assistant. MI was validated by review by a study cardiologist, and strokes by a study neurologist. Deaths were also validated by a study physician.
Statistical Analyses
Descriptive statistics were calculated among the cohort of stroke patients. Means for continuous variables and proportions for dichotomous variables were compared using t tests and χ2 tests, as appropriate. Values for hsCRP were log transformed. Correlations between Lp-PLA2 activity levels and other prognostic factors were also calculated. After examining the distributions, Lp-PLA2 activity levels were categorized by quartile for further analyses. Cox proportional hazard models were then constructed to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the effect of these markers on independent risks of recurrent stroke and on a combined ischemic event outcome of recurrent stroke, MI, or vascular death. Time to first event was analyzed with censoring at the time to either non-vascular death or last follow-up. Unadjusted models, and models adjusted for demographic characteristics (age, sex, and race/ethnicity) and risk factors (coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, atrial fibrillation, and smoking), and hsCRP quartile were calculated. Because a previous study had indicated that the effect of Lp-PLA2 mass differed in those with LDL ≥130 mg/dl [31], an interaction term for LDL ≥130 mg/dl was also included. The final models were tested to ensure that they satisfied assumptions of proportionality. Analyses including death were additionally adjusted by stroke severity. Because this was a post hoc analysis of a previously assembled cohort, power was not formally calculated prospectively. Statistical analyses were conducted using SAS software (version 8.2; SAS Institute, Cary, N.C., USA).
Results
Study Population
The total Northern Manhattan Stroke Study cohort included 655 incident ischemic stroke patients, as described previously [17]. Measurements of Lp-PLA2 activity were available for this analysis in 467 participants. The distribution of sociodemographic factors, comorbid vascular diseases, and conventional atherosclerotic risk factors is shown in table 1. Differences between this sample and the overall cohort have been described previously [18]. Briefly, the sample with Lp-PLA2 activity measurements available was slightly younger than those of the patients who did not have these measurements made: 68.9 ±12.7 compared to 71.9 ±12.4 years (p = 0.006). There were no significant differences between the groups in sex, race/ethnicity, or any risk factors. By logistic modeling, the probability of selection was also shown to be independent of recurrent stroke, conditioning on other covariates (age, sex, race/ethnicity, diabetes mellitus, hypertension, hyperlipidemia, atrial fibrillation, current smoking, and coronary artery disease).
Table 1.
Characteristics of the participants
| Participants, n | 467 |
| Demographics | |
| Age, years | 68.9 ± 12.7 |
| Male | 212 (45.4) |
| High school education | 160 (34.9) |
| Non-Hispanic white | 83 (17.8) |
| Non-Hispanic black | 127 (27.2) |
| Hispanic | 249 (53.3) |
| Other | 8 (1.7) |
| Risk factors | |
| Hypertension | 317 (67.9) |
| Diabetes mellitus | 150 (32.2) |
| History of MI | 74 (15.9) |
| History of coronary artery disease | 160 (34.3) |
| History of congestive heart failure | 62 (13.3) |
| History of atrial fibrillation | 50 (10.7) |
| History of peripheral arterial disease | 108 (23.2) |
| Current smoking | 101 (22.7) |
| Ever smoked | 250 (53.8) |
| History of hypercholesterolemia | 171 (36.9) |
| Total cholesterol, mg/dl (n = 461) | 192.2 ± 45.0 |
| LDL, mg/dl (n = 452) | 121.3 ± 39.3 |
| HDL, mg/dl (n = 459) | 39.7 ± 12.0 |
| LDL >130 mg/dl (3.37 mmol/1; n = 452) | 170 (37.6) |
| HDL <40 mg/dl (1.04 mmol/1; n = 459) | 274 (58.7) |
| Stroke: etiologic subtypes (n = 464) | |
| Atherosclerotic | 77 (16.5) |
| Lacunar | 109 (23.3) |
| Cardioembolic | 86 (18.4) |
| Cryptogenic | 195 (41.7) |
| Stroke severity (n = 455) | |
| NIHSS score 0–5 | 237 (52.1) |
| NIHSS score 6–13 | 154 (33.9) |
| NIHSS score ≥14 | 64 (14.1) |
Hypertension was defined as a systolic blood pressure recording ≥140 mm Hg or a diastolic blood pressure recording ≥90 mm Hg, or the patient's self-report of a history of hypertension or an-tihypertensive use. Diabetes mellitus was defined by a fasting blood glucose level >126 mg/dl, the patient's self-report of such a history, or insulin or hypoglycémie use. Not all data were available for all participants. Cryptogenic subtype includes 180 cryptogenic strokes after full evaluation, 14 conflicting mechanisms, and 1 other mechanism. HDL = High-density lipoprotein; NIHSS = National Institutes of Health Stroke Scale. Means ± SD and numbers (%) of patients are shown.
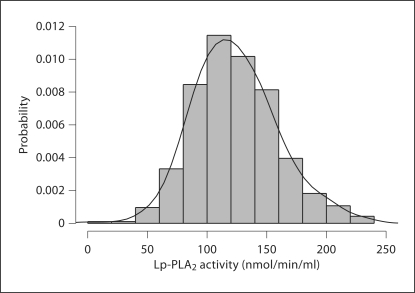
Blood samples were drawn at the time of admission (≤72 h after stroke onset in 83.7% of patients and ≤6 days in 90.0% of patients). Lp-PLA2 activity levels were normally distributed (mean 123.9 ± 35.2 nmol/min/ml; median 120.4 nmol/min/ml; fig. 1). Lp-PLA2 activity was moderately correlated with Lp-PLA2 mass (R = 0.60, p < 0.0001). Lp-PLA2 activity levels after stroke were associated with age, sex, race/ethnicity, and a history of diabetes mellitus, but not with stroke severity, history of coronary artery disease, hypertension, atrial fibrillation, or current smoking (table 2).
Fig. 1.
Distribution of Lp-PLA2 activity levels in the cohort.
Table 2.
Lp-PLA2 activity levels and patient characteristics
| n | Lp-PLA2 activity |
||
|---|---|---|---|
| nmol/min/ml | p value | ||
| Overall | 123.9 ± 35.2 | – | |
| Age | |||
| <70 years | 243 | 119.3 ± 34.2 | 0.0034 |
| >70 years | 224 | 128.8 ± 35.7 | |
| Sex | |||
| Men | 212 | 130.0 ± 33.1 | 0.0005 |
| Women | 255 | 118.7 ± 36.1 | |
| Race/ethnicity | |||
| NH white | 83 | 143.0 ± 34.9 | <0.0001 |
| NH black | 127 | 119.7 ± 34.9 | |
| Hispanic | 249 | 119.3 ± 33.7 | |
| Other | 8 | 131.8 ± 26.5 | |
| Risk factors | |||
| Diabetes | 316 | 126.6 ± 35.8 | 0.015 |
| No diabetes | 150 | 118.1 ± 33.6 | |
| Hypertension | 317 | 121.8 ± 34.6 | 0.066 |
| No hypertension | 150 | 128.2 ± 36.1 | |
| Hyperlipidemia | 147 | 122.8 ± 32.9 | 0.692 |
| No hyperlipidemia | 317 | 124.2 ± 36.4 | |
| Currently smoking | 101 | 125.6 ± 39.3 | 0.536 |
| Not currently smoking | 345 | 123.0 ± 34.6 | |
| Atrial fibrillation | 50 | 126.8 ± 32.5 | 0.526 |
| No atrial fibrillation | 416 | 123.5 ± 35.6 | |
| History of CAD | 160 | 127.9 ± 35.0 | 0.075 |
| No history of CAD | 307 | 121.8 ± 35.2 | |
| Stroke severity | |||
| NIHSS score <6 | 237 | 122.8 ± 32.6 | 0.676 |
| NIHSS score 6–13 | 154 | 124.9 ± 36.5 | |
| NIHSS score >14 | 64 | 126.8 ± 42.2 | |
NH = Non-Hispanic; CAD = coronary artery disease; NIHSS = National Institutes of Health Stroke Scale.
Lp-PLA2 Activity as a Predictor of Outcome
Median follow-up was 4.0 years. Outcomes included 80 recurrent strokes, including 15 fatal recurrent strokes. In addition, there were 18 MIs, and 53 non-stroke-related vascular deaths. In an unadjusted model using the lowest quartile as a reference group, the highest quartile of Lp-PLA2 activity was significantly associated with risk of recurrent stroke (HR 2.09, 95% CI 1.07–4.08; table 3). The second and third quartiles were not associated with any increased risk (table 3).
Table 3.
Lp-PLA2 activity levels as predictors of outcome after first ischémie stroke
| Lp-PLA2 activity |
||||
|---|---|---|---|---|
| quartile 1 | quartile 2 | quartile 3 | quartile 4 | |
| 0–98.7 nmol/min/ml | 98.8–120.4 nmol/min/ml | 120.5–145.4 nmol/min/ml | ≥145.5 nmol/min/ml | |
| Recurrent stroke, HR (95% CI) | ||||
| Unadjusted | 1.0 | 1.66 (0.83–3.31) | 1.57 (0.78–3.16) | 2.09 (1.07–4.08) |
| Adjusted1 | 1.0 | 1.19 (0.47–3.01) | 0.91 (0.30–2.78) | 2.54 (1.01–6.39) |
| Recurrent stroke/MI/vascular death, HR (95% CI) | ||||
| Unadjusted | 1.0 | 1.51 (0.88–2.59) | 1.45 (0.84–2.50) | 1.76 (1.03–2.99) |
| Adjusted2 | 1.0 | 1.24 (0.61–2.53) | 1.04 (0.45–2.40) | 1.69 (0.76–3.76) |
Adjusted for age, sex, race/ethnicity, history of hypertension, diabetes mellitus, hyperlipidemia, smoking, coronary artery disease, hsCRP, LDL, and the interaction with LDL ≥130 mg/dl.
Adjusted for age, sex, race/ethnicity, history of hypertension, diabetes mellitus, hyperlipidemia, smoking, coronary artery disease, hsCRP, LDL, stroke severity, and the interaction with LDL >130 mg/dl.
Because a prior study had indicated that the effect of Lp-PLA2 mass in predicting prognosis after cardiac events was attenuated in those with LDL ≥130 mg/dl [31], evidence of an interaction with LDL was sought. There was a suggestion of an interaction between Lp-PLA2 and LDL ≥130 mg/dl (p = 0.10), and an interaction term was therefore kept in the final model. After adjusting for age, sex, race/ethnicity, history of hypertension, diabetes mellitus, hyperlipidemia, smoking, coronary artery disease, hsCRP, and the interaction with LDL ≥130 mg/dl, those in the highest quartile of Lp-PLA2 activity continued to have an increased risk of recurrent stroke (adjusted HR 2.54, 95% CI 1.01–6.39). Further adjusting for time from stroke onset to time of blood sampling did not change the results. While there was evidence of an effect of Lp-PLA2 activity on stroke recurrence risk in those with LDL <130 mg/dl, no definite evidence of an effect in those with LDL ≥130 mg/dl was found (p = 0.313).
Receiver operator curves (ROC) were constructed and area under the curve (AUC) calculated to assess for the additional information gained by inclusion of Lp-PLA2 activity in prediction of risk of stroke recurrence (fig. 2). These curves were also compared to ROC curves for models including Lp-PLA2 mass. Addition of Lp-PLA2 activity to the model including all other risk factors but Lp-PLA2 activity led to a slight increase in AUC from 0.646 to 0.668. The increase in AUC was less than that for inclusion of Lp-PLA2 mass levels, which increased from 0.612 to 0.652.
Fig. 2.
a ROC for model containing age, sex, race/ethnicity, history of hypertension, diabetes mellitus, hyperlipidemia, smoking, coronary artery disease, hsCRP, and LDL. b ROC for model containing covariates in a and Lp-PLA2 and the interaction between LDL ≥130 mg/dl and Lp-PLA2.
Only 12% of patients in this sample were taking cholesterol-lowering medications, including statins, prior to their stroke, based on the interviews at the time of entry into the study. We did not have systematic data collected on use of statins after stroke, during follow-up. Analyses including cholesterol-lowering medication use at the time of stroke as a covariate showed minimal change in the magnitude of the HR (2.36, 95% CI 0.86–6.51). In addition, stratified analyses were performed to assess the differential effects of Lp-PLA2 in atherosclerotic and non-atherosclerotic subtypes of stroke. While the number of patients with stroke subtypes was too small to draw definitive conclusions, there was a suggestion of a greater effect on risk of stroke recurrence among those with atherosclerotic stroke (adjusted HR 1.81, 95% CI 0.45–7.25) versus non-atherosclerotic stroke (adjusted HR 0.84, 95% CI 0.31–2.26).
hsCRP was not associated with an increased risk of recurrent stroke (adjusted HR 0.72, 95% CI 0.36–1.45) in the fully adjusted model.
Although in an unadjusted model Lp-PLA2 activity was associated with the composite secondary outcome of recurrent stroke, MI, or vascular death, activity levels were not associated with the secondary outcome after adjusting for other covariates, including stroke severity (adjusted HR for highest versus lowest quartile 1.69, 95% CI 0.76–3.76).
Discussion
Inflammatory markerspredictincident ischemic events, including MI and stroke, in many studies, but their ability to predict prognosis after ischemic stroke remains uncertain [32]. We have previously reported that levels of hsCRP predict mortality after stroke and that Lp-PLA2 mass levels predict recurrent stroke and other ischemic events [18]. In the present study, we found that Lp-PLA2 activity levels in the top quartile also predict recurrent stroke, but not ischemic events overall. Patients in the highest quartile of Lp-PLA2 activity had approximately 2.5 times the risk of stroke recurrence as those in the first quartile, even after adjusting for other risk factors, though there is a 95% chance that the true increased risk could be ∼1.01–6.39 times as high. The effect on risk appeared to be greater for those with atherosclerotic stroke, although numbers of patients with stroke subtypes were too small to draw definitive conclusions about the effect in subtypes.
Lp-PLA2, an enzyme derived from leukocytes, particularly macrophages, is responsible for metabolism of LDL to the pro-inflammatory mediators lysophosphatidylcholine and oxidized fatty acids [33]. Lysophosphatidylcholine increases expression of vascular adhesion molecules, upregulates cytokines and CD40 ligand, and stimulates macrophage proliferation [34]. Levels of Lp-PLA2 activity have been associated with increased risk of incident ischemic cardiac and cerebrovascular events in epidemiological studies [13]. Our study provides evidence that this novel marker of vascular inflammatory activity is associated with risk of recurrent stroke in patients after first stroke. Levels of Lp-PLA2 activity, like those of Lp-PLA2 mass, were not markedly affected by stroke severity in our population.
Most studies of Lp-PLA2 have focused on enzyme mass levels rather than activity. The current commercially available assay tests mass only. Lp-PLA2 mass and activity levels are incompletely correlated, however, probably because the assays measure different populations of the enzyme in serum related to binding to specific lipoprotein subfractions. The assay we used, moreover, has a high concentration of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, which acts as a detergent and minimizes measurement error due to presence of other lipases [28]. Other studies have found correlations ranging from r = 0.36 in the large PROVE IT trial [19] to r = 0.89 in an earlier, smaller study among men only [35]. The correlation in our study (r = 0.60) is intermediate between these values. These differences are likely attributable to the different assays used to measure Lp-PLA2 activity. In our cohort, mean levels of Lp-PLA2 activity were higher than those in studies using radiometric [13, 19] rather than colorimetric assays, but the mean value was very similar to that using the same colorimetric assay in a German population [28]. The correlation between mass and activity in our study (R = 0.60) was also very similar to that observed in the same German population (R = 0.573) [28]. Activity levels were significantly different between men and women in our analysis, consistent with other studies [13].
Few studies have assessed the association of outcomes with both Lp-PLA2 mass and activity [19, 36]. In one study in which both measurements were made, mass but not activity was significantly associated with calcified coronary plaques [36]. In secondary analysis of a large cardiac prevention trial (PROVE IT), activity levels measured 30 days after an acute coronary event were associated with outcome, but mass levels measured either immediately or 30 days after the event were not [19]. Possible reasons for differences between mass and activity levels as prognostic markers include acute changes in activity levels in the setting of lipoprotein changes.
The absence of an effect of measurements of Lp-PLA2 activity immediately after an event on prognosis in some [19], but not all [37], cardiac studies could reflect acute changes in Lp-PLA2 activity levels, as with LDL levels, after acute coronary events because Lp-PLA2 co-localizes with LDL. LDL levels do not appear to be influenced as strongly by stroke, however [38]. We did not have levels available at 30 days or other time points to compare.
Recently, studies in other populations have also demonstrated that Lp-PLA2 activity levels measured in cardiac patients predict long-term risk of recurrent coronary ischemic events independently of other lipid and inflammatory biomarkers, including hsCRP and LDL [29, 39]. Mass levels were associated with a slightly greater magnitude of risk than Lp-PLA2 activity levels in those studies, however, and remained significant after adjusting for other biomarkers. Lp-PLA2 mass was minimally more predictive of recurrent stroke than was Lp-PLA2 activity in our population, based on ROC analyses. Both markers, moreover, provided only a minimal incremental increase in information about risk compared with other risk factors. In this context, our results are similar to those of analyses of multiple biomarkers conducted in large epidemiological studies, in which Lp-PLA2 mass was one of the only biomarkers to provide incremental risk information, albeit modest [40]. Nonetheless, because stroke is a prevalent, debilitating, and preventable disease, we would argue that any additional prognostic risk information can serve as a valuable adjunct to already identified risk factors.
Although further studies are needed, both to define the optimal role of Lp-PLA2 testing in clinical practice and to determine the relative merits of mass versus activity testing, at present measurement of mass levels would seem clinically preferable for several reasons. These include the greater magnitude of effect for Lp-PLA2 mass levels in most studies, their independent predictive value, their ability to predict a wider variety of adverse vascular events, and the availability of a commercially available, standardized assay.
Lp-PLA2 is found predominantly on LDL particles, and there is some evidence of effect modification by levels of LDL. In the ARIC (Atherosclerosis Risk in Communities) study, for example, the increased risk of incident heart disease associated with Lp-PLA2 was limited to those with LDL levels <130 mg/dl [14]. In other studies, particularly for stroke, there was no definite interaction between Lp-PLA2 and LDL levels [13, 14, 41]. We found evidence of a possible interaction of Lp-PLA2 activity with LDL levels in predicting risk of recurrent stroke, similar to what was seen for incident coronary disease in ARIC. In our population, Lp-PLA2 activity levels predicted risk of recurrent stroke in those with LDL levels <130 mg/dl, but not in those with LDL ≥130 mg/dl. Lp-PLA2 may be a less informative biomarker when LDL is ≥130 mg/dl because at higher levels of LDL, the LDL effect overshadows any increased risk associated with Lp-PLA2. The clinical utility of Lp-PLA2 may therefore be greater when LDL is lower. Use of inflammatory biomarkers for prognostication among patients without otherwise-defined high-risk conditions is consistent with recommended guidelines on their use from a recent consensus conference [4]. Further studies in larger populations are needed to better define the extent of interactions between Lp-PLA2 and lipid levels in stroke prognosis.
Our study has several limitations. We did not have blood available for analysis in all stroke patients in our cohort, but the differences between our entire cohort and the sample analyzed in this study were minimal. We also did not have available data at multiple times after stroke. Further studies are needed to determine whether assessment of Lp-PLA2 levels at longer intervals provide improved prognostic information. We also did not collect blood samples at uniform time intervals after stroke. The use of non-standardized timing of assessments of Lp-PLA2, if random and unassociated with outcomes, would be expected to bias the results toward the null. Our study could therefore underestimate the effect of an association with outcomes. Finally, we did not have systematic data on the use of statins after stroke in this population.
In summary, our data support an association between Lp-PLA2 activity levels in the top quartile and stroke recurrence. Further prospective studies on the role of Lp-PLA2 activity and mass in stroke prognosis might lead to the use of these markers to improve prediction of stroke or other vascular events after stroke. Identification of those patients at increased risk could justify targeted or more aggressive treatment in these patients using other prevention modalities, such as weight loss, blood pressure reduction, or other proven risk reduction strategies. In addition, treatments that directly reduce levels of Lp-PLA2 could potentially be tested in clinical trials to prevent incident and recurrent stroke. For example, statins may reduce Lp-PLA2 levels [42]. Other trials, moreover, are investigating whether the risk of atherosclerotic disease is modified by use of direct inhibitors of Lp-PLA2[43, 44].
Conflict of Interest
M.S.V.E. receives research funding from diaDexus, Inc., and BMS-Sanofi Partnership, and honoraria for speaking from diaDexus, Inc., (modest) and BMS-Sanofi Partnership and Boehringer-Ingelheim (significant); R.L.S. receives honoraria for speaking from BMS-Sanofi Partnership and Boehringer-Ingelheim (modest), and serves as a consultant for BMS-Sanofi Partnership, Boehringer-Ingelheim, Merck, Wyeth, and GlaxoSmithKline.
Acknowledgements
This work was supported by grants from the American Heart Association (Kathleen Scott Research Fellowship, M.S.V.E.), National Institute of Neurological Disorders and Stroke (R01 NS27517 and R01 NS29993, R.L.S.; and K23 42912 and R01 NS48134, M.S.V.E.), and research funding for performance of blood assays from diaDexus.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 3.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in leukocyte count predicts first cerebral infarction. Neurology. 2005;64:2121–2125. doi: 10.1212/01.WNL.0000165989.12122.49. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease. Application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992;267:1253–1256. [PubMed] [Google Scholar]
- 6.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, Muir KW, Paciucci A, Papa F, Roncacci S, Sander D, Sander K, Smith CJ, Stefanini A, Weber D. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S-J, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 12.Grau AJ, Buggle F, Becher H, Werle E, Hacke W. The association of leukocyte count, fibrinogen and C-reactive protein with vascular risk factors and ischemic vascular diseases. Thromb Res. 1996;82:245–255. doi: 10.1016/0049-3848(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 13.Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 14.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Myerson M, Wu KK, Sharrett AR, Boerwinkle E. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos M, Serena J. Applicability of biomarkers in ischemic stroke. Cerebrovasc Dis. 2007;24(suppl 1):7–15. doi: 10.1159/000107374. [DOI] [PubMed] [Google Scholar]
- 16.Chamorro Á. Role of inflammation in stroke and atherothrombosis. Cerebrovasc Dis. 2004;17(suppl 3):1–5. doi: 10.1159/000075297. [DOI] [PubMed] [Google Scholar]
- 17.Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 19.O'Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation. 2006;113:1745–1752. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 20.United States Census of Population and Housing, 1990 (Public Use Microdata Sample). www.census.gov
- 21.Garfield R, Abramson D, editors. Washington Heights/Inwood: The Health of a Community. The Health of the Public Program. New York: Columbia University; 1995. [Google Scholar]
- 22.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Elkind MS, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Office of Management and Budget Race and Ethnic Standards for Federal Statistics and Administrative Reporting (Directive No. 15) Federal Register. 1978;43:19269. [PubMed] [Google Scholar]
- 25.Gentry EM, Kalsbeek WD, Hegelin G, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 26.Adams HP, Jr, Woolson RF, Clarke W, et al. Design of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Control Clin Trials. 1997;18:358–377. doi: 10.1016/s0197-2456(97)00012-3. [DOI] [PubMed] [Google Scholar]
- 27.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997;48:1204–1211. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka T, Yamaguchi M, Soda Y, Kishimoto T, Tago A, Toyosato M, Mizuno K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin Chim Acta. 2000;296:151–161. doi: 10.1016/s0009-8981(00)00216-3. [DOI] [PubMed] [Google Scholar]
- 29.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–1593. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 30.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 31.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 32.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, Muir KW, Paciucci A, Papa F, Roncacci S, Sander D, Sander K, Smith CJ, Stefanini A, Weber D. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 33.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA, Leake DS, Milliner KJ, Patterson RA, Suckling KE, Tew DG, Hickey DM. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 34.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 35.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413–419. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 36.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Jr, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: the CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25:216–221. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]
- 37.Gerber Y, McConnell JP, Jaffe AS, Weston SA, Killian JM, Roger VL. Lipoprotein-associated phospholipase A2 and prognosis after myocardial infarction in the community. Arterioscler Thromb Vasc Biol. 2006;26:2517–2522. doi: 10.1161/01.ATV.0000240406.89440.0c. [DOI] [PubMed] [Google Scholar]
- 38.Kargman DE, Tuck C, Berglund L, Lin IF, Mukherjee RS, Thompson EV, Jones J, Boden-Albala B, Paik MC, Sacco RL. Lipid and lipoprotein levels remain stable in acute ischemic stroke: the Northern Manhattan Stroke Study. Atherosclerosis. 1998;139:391–399. doi: 10.1016/s0021-9150(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 39.Corsetti JP, Rainwater DL, Moss AJ, Zareba W, Sparks CE. High lipoprotein-associated phospholipase A2 is a risk factor for recurrent coronary events in postinfarction patients. Clin Chem. 2006;52:1331–1338. doi: 10.1373/clinchem.2006.066845. [DOI] [PubMed] [Google Scholar]
- 40.Folsom AR, Chambless LE, Ballantyne CM, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med. 2006;166:1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 41.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. E Heart J. 2005;26:137–144. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer EJ, McNamara JR, Asztalos BF, Tayler T, Daly JA, Gleason JL, Seman LJ, Ferrari A, Rubenstein JJ. Effects of atorvastatin versus other statins on fasting and postprandial C-reactive protein and lipoprotein-associated phospholipase A2 in patients with coronary heart disease versus control subjects. Am J Cardiol. 2005;95:1025–1032. doi: 10.1016/j.amjcard.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Blackie JA, Bloomer JC, Brown MJ, Cheng HY, Hammond B, Hickey DM, Ife RJ, Leach CA, Lewis VA, Macphee CH, Milliner KJ, Moores KE, Pinto IL, Smith SA, Stansfield IG, Stanway SJ, Taylor MA, Theobald CJ. The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg Med Chem Lett. 2003;13:1067–1070. doi: 10.1016/s0960-894x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 44.Blackie JA, Bloomer JC, Brown MJ, Cheng HY, Elliott RL, Hammond B, Hickey DM, Ife RJ, Leach CA, Lewis VA, Macphee CH, Milliner KJ, Moores KE, Pinto IL, Smith SA, Stansfield IG, Stanway SJ, Taylor MA, Theobald CJ, Whittaker CM. The discovery of SB-435495. A potent, orally active inhibitor of lipoprotein-associated phospholipase A2 for evaluation in man. Bioorg Med Chem Lett. 2002;12:2603–2606. doi: 10.1016/s0960-894x(02)00473-0. [DOI] [PubMed] [Google Scholar]