Abstract
The structural organization of the cytoskeleton determines its viscoelastic response which is crucial for the correct functionality of living cells. Both the mechanical response and microstructure of the cytoskeleton are regulated on a microscopic level by the local activation of different actin binding and/or bundling proteins (ABPs). Misregulations in the expression of these ABPs or mutations in their sequence can entail severe cellular dysfunctions and diseases. Here, we study the structural and viscoelastic properties of reconstituted actin networks formed by the ABP espin and compare the obtained network properties to those of other bundled actin networks. Moreover, we quantify the impact of physiologically relevant espin mutations on the viscoelastic properties of these cytoskeletal networks.
1. Introduction
Actin filaments are key structural and mechanical components in the cytoskeleton of eukaryotic cells. These filaments are organized by actin binding proteins (ABPs) into supramolecular assemblies such as bundles or cross-linked networks [1–3]. In order to reduce the overwhelming complexity found in living cells, it is a main strategy in the field of cytoskeletal mechanics to reconstitute actin filament assemblies with well-defined properties [4]. Indeed, this approach has been very successful for our understanding of how cross-linking of semi-flexible actin filaments into isotropically cross-linked networks determines the ensuing viscoelastic properties [5–9]. In contrast, the physical principles which lead to the formation of more complicated structural arrangements [10–14] are less well understood. Although the mechanical properties of single actin bundles have been described for several ABP systems [15, 16] and considerable progress has been made in quantitatively describing viscoelastic properties of purely bundled actin/fascin networks [17, 18], an overall understanding of bundled actin networks has still to be achieved. This calls for a detailed investigation of other bundled actin systems to allow for a thorough test of theoretical predictions or simulations on bundled cytoskeletal networks [19, 20]. Espins constitute a familiy of actin bundling proteins which are mainly found in the stereocilia of chochlear hair cells - key components for the mechanotransduction of sound during the hearing process [21]. Thus, genetic mutations in the espin protein are reported to prevent the correct organization of actin filaments into parallel bundles [22] and are known to be associated with deafness in vivo [23–25].
In this article we analyze the structural and viscoelastic behavior of reconstituted actin/espin networks. We compare bundle networks formed by wildtype (WT) espin to actin filament assemblies which are organized by physiologically relevant espin mutants. Our findings show that WT espin forms cross-linked bundle networks whose properties are highly similar to those of actin bundle networks formed by fascin. Mutations in the F-actin-binding domain of espin not only prevent the correct formation of actin bundles but also significantly weaken the viscoelastic properties of the formed networks.
2. Results and Discussion
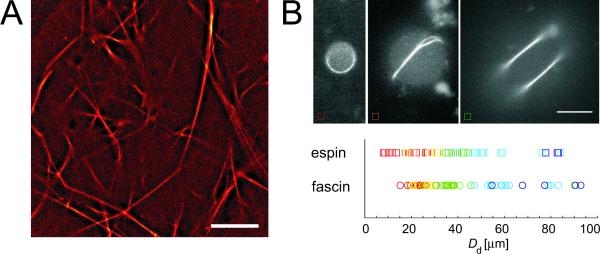
Espin is reported to bundle actin filaments at high ratios between the cross-linker and actin, [22]. Thus, we first analyze the microstructure of reconstituted actin/espin networks at high espin concentrations. Espin forms long and straight bundles which assemble into a well-ordered network (fig. 1A). The overall structure of this bundle network closely resembles the network structure of purely bundled actin/fascin networks [17]. This underlines the generic trend of small ABPs showing a high bundling propensity [11, 26]. For a further quantification of this striking resemblance of actin/espin and actin/fascin bundles we study the formation of actin bundle ring structures in confined geometries employing an emulsion droplet systems [16, 27]. As depicted in fig. 1B, espin forms a continuous actin bundle ring at small droplet diameters. For larger emulsion droplets, however, the thickness of these bundle rings does not grow further. Instead, side branches or secondary rings are formed. The critical droplet diameter at which the first formation of a bundle side branch occurs is very similar for actin/espin and actin/fascin structures (yellow/orange symbols in fig. 1B). This suggests that these two bundle systems have comparable finite thicknesses in the range of ~20 actin filaments/bundle [28].
Figure 1.
(A) Overall network structure for an actin/espin network at R = 1 (cactin = 0.4 mg/ml). Espin forms a bundle network with well-ordered straight and long bundles. Scale bar denotes 10 μm. (B) Epi-fluorescence micrographs of TRITC phalloidin-labeled F-actin/espin bundles (R = 1, cactin = 0.4 mg/ml) confined in emulsion droplets. The colors depicted in the diagram represent the different structures observed for espin (squares) and fascin (circles): For small-droplet diameters Dd filaments organize into a single ring (red), in larger droplets forks and sidebranches appear (yellow), and in very large droplets more complicated structures are found (green and blue). Scale bar: 10 μm.
While in the emulsion droplet system the formation of actin bundles is facilitated by the confinement effect of the spherical geometry [27], a higher cross-linker concentration is necessary in bulk in order to induce a transition into a bundle network. In fact, the bundling transition for actin/espin networks is reported to occur at a similar concentration regime as for fascin [17, 22] underlining the generic nature of this structural transition as parameterized in [17]. It has been well established that such a structural transition always entails significant changes in the viscoelastic network response [26]. Thus, in the following part of the manuscript we address the influence of espin and its mutants on the viscoelastic response of reconstituted actin/espin networks.
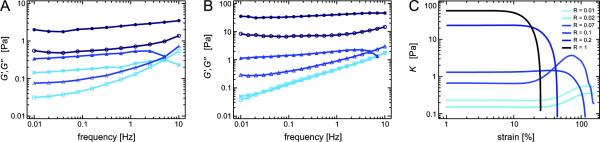
Depending on the type and concentration of the cross-linking molecule, actin networks show different mechanical properties [26]. While the cross-linking molecules HMM [6] and scruin [10] as well as fascin [17] and α-actinin [14] in the bundle phase strongly enhance the network elasticity in the linear regime, a comparably small effect is observed for the bundling protein filamin [13]. As shown in fig. 2A and B, addition of espin results in a strong increase of the network elasticity. The elastic modulus is enhanced up to 1000fold at 0.4 mg/ml actin while at a lower actin concentration (0.2 mg/ml actin) this effect is less pronounced. In addition, the shape of the frequency dependent viscoelastic moduli G′(f) and G″(f) drastically changes: For both actin concentrations investigated here, G′(f) becomes gradually flatter with increasing espin concentrations. Moreover, at a high espin concentrations a weak minimum in G″(f) appears. Both effects have also been reported for actin networks formed by the ABP fascin. However, for actin/fascin networks the minimum in G″(f) is more pronounced.
Figure 2.
Viscoelastic response for actin networks cross-linked by wild type (WT) espin. (A), (B): Frequency spectra of actin/espin networks for different espin concentrations (R =0.01 (squares), R = 0.1 (triangles) and R = 1 (circles)) at (A) 0.2 mg/ml and (B) 0.4 mg/ml actin, respectively. Closed symbols represent G′(f), open symbols represent G″(f). WT espin increases the network elasticity and induces a flattening of the frequency spectrum. (C) Non-linear stiffness as a function of strain for actin/WT espin networks (0.4 mg/ml actin) at a shear rate of . With increasing WT espin concentration the non-linear response changes from strain hardening to strain weakening.
In the non-linear regime, i.e. at high deformations, the viscoelastic network response depends on many parameters including the cross-linker density, the shear rate and the cross-linker microstructure [26]. For instance, actin solutions and weakly cross-linked actin networks show a strain hardening behavior at sufficently high shear rates [3, 13, 29] whereas strain weakening is observed for fascin networks at low shear rates or high fascin concentrations [18]. We now investigate the non-linear properties of actin/espin networks by applying a constant shear rate of 12.5 %/s and evaluating the resulting stress-strain relation as described in [30]. Similar to actin/fascin networks, actin/espin networks show a strain hardening response at low espin concentrations up to R = 0.07 while strain weakening is observed for high espin concentrations, i.e. for R = 0.1 and above (fig. 2C). Thus, the microstructure, the onset of bundle formation as well as the linear and the non-linear viscoelastic response of actin/espin networks resemble the behavior observed for actin/fascin networks as described in [17, 18]. There, it was suggested that the high tunability of this non-linear network response arises from forced cross-linker unbinding events occuring between distinct actin bundles.
Yet, the absence of a pronounced minimum in the viscous dissipation of actin/espin networks suggests that the density of cross-links between distinct espin bundles might be rather low [8, 9, 18] – that is, if the small espin protein is able to effectively cross-link distinct bundles at all.
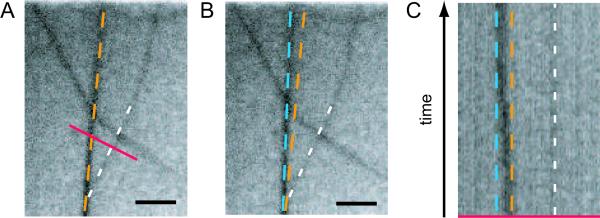
To clarify this question we investigate distinct bundle/bundle intersections as putative loci for cross-link points more in detail. For bundle networks formed by filamin it has been demonstrated that the network acquires internal stress during polymerization [31]. Due to the complicated branched structure of actin/filamin bundle networks, this internal stress cannot be released by transient cross-linker unbinding events – resulting in an kinetically trapped network. However, for the much simpler network morphology observed for actin/espin bundle networks, such a stress-induced bundle unbinding and rebinding event might be observable by microscopy. In fig. 3A, a bundle/bundle intersection is depicted right before such an unbinding and rebinding event occured for the left-hand bundle. As can be seen from the corresponding kymograph (fig. 3C), unbinding of the bundle/bundle cross-link is followed by an intermediate state of diffusive search for a new binding site until the bundle finally locks in into a new stable position (fig. 3B). This demonstrates that espin is indeed capable of forming bundle/bundle cross-links in reconstituted actin networks. However, such events are extremely rare to observe which underlines the low degree of bundle/bundle cross-linking as already inferred from macrorheology.
Figure 3.
Unbinding and rebinding of a bundle/bundle cross-link point visualized by fluorescence microscopy. Two neighboring bundle/bundle intersection points are shown at time t = 0 (A) and t = 28 s (B). (C) Kymograph showing a time lapse of 28 s for the red section depicted in (A). The position of the left bundle switches from its original position (orange) to a new final position (blue) while the position of the right bundle remains constant (white). Scale bars represent 2 μm.
For isotropically cross-linked actin networks it has been demonstrated that the viscoelastic network properties are largely set by the interaction potential between the F-actin-binding domain of the cross-linker and the actin filament [14]. The F-actin-binding domains of espin are crucial for the correct formation of cross-links within actin/espin bundles [22]. In the remaining part of this article we aim to address the question how physiologically relevant mutations in the actin binding sites of wild type (WT) espin affect the viscoelastic response of reconstituted actin/espin networks.
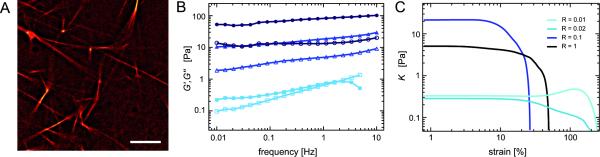
The mildest form of a mutation which can occur in a protein is a point mutation. The first espin mutant investigated here, hE3AdelK, shows exactly such a point mutation in the actin binding domain of espin, in a region which is believed to encompass one of its two F-actin binding sites [25, 32]. In [22] it has been shown that at molar ratios below R = 0.1, where WT espin can effectively organize actin into hexagonally packed bundles, hE3AdelK only induces the formation of aggregates with a short-range positional ordering. Only at high concentrations of hE3AdelK, highly ordered bundles are formed (fig. 4A). Nevertheless, the effect of hE3AdelK on the static network elasticity is surprisingly similar to what is observed for WT espin: The increase of the apparent plateau modulus G0 as a function of the cross-linker concentration as well as the maximal observed network stiffness are comparable in magnitude(compare fig. 2B and fig. 4B). This suggests that the structural properties of single actin/espin bundles have a rather small effect on the macroscopic network stiffness. At high cross-linker concentrations, where both WT espin and hE3ADelK induce the formation of bundles, also the viscoelastic spectra of actin/hE3AdelK networks resemble the spectra observed for actin/WT espin (fig. 4B): G′(f) is rather independent of frequency and G″(f) exhibits a shallow minimum. Moreover, the espin mutant modulates the non-linear network response in a similar way as observed for WT espin: no strain hardening is detectable any more at high cross-linker concentrations (fig. 4C). However, this effect occurs already at a lower cross-linker concentration R = 0.02 – at this R value strain hardening is still observed in networks cross-linked by WT espin.
Figure 4.
(A) hE3AdelK induces the formation of a bundle network which resembles the networks formed by wildtype espin (R = 1, cactin = 0.4 mg/ml). Scale bar denotes 10 μm. (B) Frequency spectra of actin/hE3AdelK networks for different hE3AdelK concentrations (R =0.01 (squares), R = 0.1 (triangles) and R = 0.5 (circles)) at 0.4 mg/ml actin. Closed symbols represent G′(f), open symbols represent G″(f). Also hE3AdelK espin increases the network elasticity and induces a flattening of the frequency spectrum. (C) Non-linear stiffness as a function of strain for actin/hE3AdelK espin networks (0.4 mg/ml actin) at a shear rate of . With increasing hE3AdelK concentration the non-linear response changes from strain hardening to strain weakening.
Altogether, the viscoelastic properties of hE3AdelK/actin networks are very similar to those of WT espin/actin networks. However, much stronger alterations in the viscoelastic network response can be expected for a frame shift mutation which affects a F-actin-binding binding domain. Such a frame shift mutation should completely abolish the specific binding of the corresponding espin domain towards actin.
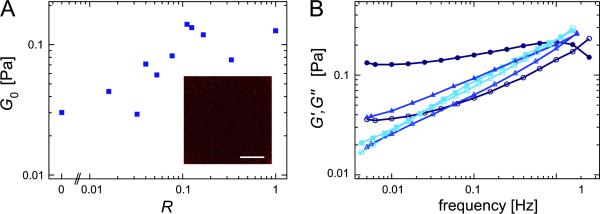
Surprisingly, it has been reported from X-ray scattering experiments that the frame shift espin mutant hE3AdelCt is still able to mediate a perceivable interaction between actin filaments [22]. Such an interaction is also detectable in the linear viscoelastic response of actin/hE3AdelCt networks. As depicted in fig. 5A, the apparent plateau elasticity G0 := G′(10 mHz) of an entangled actin solution (0.2 mg/ml actin) can be increased by the addition of hE3AdelCt up to 5fold which is a comparable effect as observed for the cross-linker filamin at this actin concentration [13]. This is supported by our finding that also the frequency spectra of actin/hE3AdelCt networks gradually change from a power law-like shape at R = 0 to a rather flat spectrum at high R (fig. 5B) as generically observed for cross-linked systems [6, 9, 33]. WT espin is known to be monomeric in solution [32]. Thus, one might speculate that the frame shift espin mutant could dimerize by its mutated domains forming an relatively effective cross-linking (but not bundling) ABP. An alternative explanation for the unexpected cross-linking ability of hE3AdelCt could be that WT espin contains an additional third actin binding site which is unaffected by the frame shift mutation. Finally, non-specific binding to actin might also account for the observed behavior in the viscoelastic frequency response.
Figure 5.
(A) Apparent plateau moduli G0 := G′(10 mHz) as a function of the relative cross-linker concentration for hE3AdelCt. The inset shows an actin network in the presence of hE3AdelCt (R = 1 cactin = 0.2 mg/ml). No bundles are detectable. Scale bar denotes 10 μm. (B) Frequency spectra of actin/hE3AdelCt networks for different hE3AdelCt concentrations (R =0 (squares), R = 0.016 (triangles) and R = 1 (circles)) at 0.2 mg/ml actin. Closed symbols represent G′(f), open symbols represent G″(f). Surprisingly, even hE3AdelCt slightly increases the network elasticity and induces a flattening of the frequency spectrum.
3. Conclusions
In summary, we have shown that the microstructure as well as the linear and non-linear viscoelastic properties of actin/WT espin networks closely resemble those reported for purely bundled actin networks formed by fascin. The main difference between these two bundle networks seems to be given by a lower degree of bundle interconnectivity in the actin/espin system. Furthermore we have analyzed the impact of two physiologically relevant espin mutations on the viscoelastic network response. While the point mutation investigated here is known to disturb the packing order of actin/espin bundles we find that the ensuing viscoelastic properties of the bundle network are rather insensitive to this mutation. For the much more drastic frame shift mutation we detect a weak but significant cross-linking activity which is surprising given that one F-actin-binding domain is eliminated [22]. In conclusion, the data presented here nicely fit into the current understanding of how the cross-linker properties dictate the structure and viscoelastic reponse of reconstituted actin networks. However, the results obtained for the two espin mutants demonstrate that a detailed correlation of cross-linker properties, bundle organization, network structure and mechanical properties is far from being trivial. Yet, investigating a broad spectrum of actin cross-linking proteins as well as associated mutants using both experimental and theoretical means might enable us to understand the molecular basis of cytoskeleton related diseases and predict the impact of dysfunctional actin cross-linkers in vivo.
Experimental Section
Materials
G-actin is obtained from rabbit skeletal muscle and stored in lyophilized form at −21 °C [34]. The G-actin solution is prepared by dissolving lyophilized actin in deionized water and dialyzing against G-buffer (2 mM Tris, 0.2 mM ATP, 0.2 mM CaCl2, 0.2 mM DTT and 0.005 % NaN3, pH 8) at 4 °C. The G-actin solution is kept at 4 °C and used within ten days. The average length of the actin filaments is controlled to 21 μm by adjusting the the molar ratio between actin and gelsolin [35] obtained from bovine plasma serum following [36]. Human espin 3A (MW: 30.9 kDa) and its two mutants hE3AdelK (MW: 30.9 kDa) and hE3AdelCt (MW: 29.8 kDa) are expressed in bacteria and purified as described in [22].
Methods
The viscoelastic response of actin/espin networks is determined by measuring the frequency-dependent viscoelastic moduli G′(f) and G″(f) with a stress-controlled rheometer (Physica MCR 301, Anton Paar, Graz, Austria) over a frequency range of three decades. Polymerization is initiated by adding 10 % volume 10× F-buffer (20 mM Tris, 5 mM ATP, 20 mM MgCl2, 2 mM CaCl2, 1 M KCl, 2 mM DTT, pH 7.5). Approximately 480 μl sample volume is loaded within 1 min into the rheometer using a 50 mm plate-plate geometry with 160 μm plate separation. Actin polymerization is carried out in situ, measurements are taken after full polymerization. To ensure linear response only small torques (≈ 0.5 μNm) are applied. In all experiments the the relative cross-linker concentration is controlled as a key parameter. To investigate the network structures, actin was labelled with phalloidin-TRITC (Sigma-Aldrich, Germany). Pictures and movies were acquired on an Axiovert 200 microscope (Zeiss, Oberkochen, Germany). Emulsion droplets containing F-actin/epsin bundles were prepared as described previously [16, 27].
Acknowledgements
We thank M. Rusp for the actin preparation. This work was supported by Deutsche Forschungsgemeinschaft through the DFG-Cluster of Excellence Nanosystems Initiative Munich and the National Institutes of Health (NIH) grant R01 DC004314 (to JRB). O. Lieleg acknowledges a postdoc fellowship from the German Academic Exchange Service (DAAD).
References
- [1].Tempel M, Isenberg G, Sackmann E. Phys. Rev. E. 1996;54:1802. doi: 10.1103/physreve.54.1802. [DOI] [PubMed] [Google Scholar]
- [2].Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Science. 2004;304:1301. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- [3].Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Proc. Natl Acad. Sci. USA. 2006;103:1762. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bausch AR, Kroy K. Nature Phys. 2006;2:231. [Google Scholar]
- [5].MacKintosh FC, Käas J, Janmey PA. Phys. Rev. Lett. 1995;75:4425. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- [6].Tharmann R, Claessens MMAE, Bausch AR. Phys. Rev. Lett. 2007;98:088103. doi: 10.1103/PhysRevLett.98.088103. [DOI] [PubMed] [Google Scholar]
- [7].Luan Y, Lieleg O, Wagner B, Bausch AR. Biophys. J. 2008;94:688693. doi: 10.1529/biophysj.107.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lieleg O, Claessens MMAE, Luan Y, Bausch AR. Phys. Rev. Lett. 2008;101:108101. doi: 10.1103/PhysRevLett.101.108101. [DOI] [PubMed] [Google Scholar]
- [9].Lieleg O, Schmoller KM, Claessens MMAE, Bausch AR. Biophys. J. 2009;96:4725. doi: 10.1016/j.bpj.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA. Proc. Natl Acad. Sci. USA. 2004;101:9636. doi: 10.1073/pnas.0308733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wagner B, Tharmann R, Haase I, Fischer M, Bausch AR. Proc. Natl Acad. Sci. USA. 2006;103:13974. doi: 10.1073/pnas.0510190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmoller K, Lieleg O, Bausch A. Phys. Rev. Lett. 2008;101:118102. doi: 10.1103/PhysRevLett.101.118102. [DOI] [PubMed] [Google Scholar]
- [13].Schmoller KM, Lieleg O, Bausch AR. Biophys. J. 2009;97:83. doi: 10.1016/j.bpj.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lieleg O, Schmoller KM, Cyron CJ, Luan Y, Wall WA, Bausch AR. Soft Matter. 2009;5:1796. [Google Scholar]
- [15].Shin JH, Mahadevan L, So PT, Matsudaira P. J. Mol. Biol. 2004;337:255. doi: 10.1016/j.jmb.2004.01.028. [DOI] [PubMed] [Google Scholar]
- [16].Claessens MMAE, Bathe M, Frey E, Bausch AR. Nature Mater. 2006;5:748. doi: 10.1038/nmat1718. [DOI] [PubMed] [Google Scholar]
- [17].Lieleg O, Claessens MMAE, Heussinger C, Frey E, Bausch AR. Phys. Rev. Lett. 2007;99:088102. doi: 10.1103/PhysRevLett.99.088102. [DOI] [PubMed] [Google Scholar]
- [18].Lieleg O, Bausch AR. Phys. Rev. Lett. 2007;99:158105. doi: 10.1103/PhysRevLett.99.158105. [DOI] [PubMed] [Google Scholar]
- [19].Heussinger C, Bathe M, Frey E. Phys. Rev. Lett. 2007;99:048101. doi: 10.1103/PhysRevLett.99.048101. [DOI] [PubMed] [Google Scholar]
- [20].Astroem J, Sunil Kumar PB, Vattulainen I, Karttunen M. Phys. Rev. E. 2008;77:051913. doi: 10.1103/PhysRevE.77.051913. [DOI] [PubMed] [Google Scholar]
- [21].Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. J. Cell Biol. 2004;164:887. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Purdy KR, Bartles JR, Wong GCL. Phys. Rev. Lett. 2007;98:058105. doi: 10.1103/PhysRevLett.98.058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng LL, Sekerkova G, Tilney L, Mugnaini E, Bartles JR. Cell. 2000;102:377. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Naz S, Griffith AJ, Riazuddin S, Hampton LL, Battey JF, Khan SN, Riazuddin S, Wilcox ER, Friedman TB. J. Med. Genet. 2004;41:591. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Donaudy F, Zheng L, Ficarella R, Ballana E, Carella M, Melchionda S, Estivill X, Bartles JR, Gasparini P. J. Med. Genet. 2006;43:157. doi: 10.1136/jmg.2005.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lieleg O, Claessens MMAE, Bausch AR. Soft Matter. (submitted) [Google Scholar]
- [27].Claessens MMAE, Tharmann R, Kroy K, Bausch AR. Nature Phys. 2006;2:186. [Google Scholar]
- [28].Claessens MMAE, Semmrich C, Ramos L, Bausch AR. Proc. Natl Acad. Sci. USA. 2008;105:6590. doi: 10.1073/pnas.0711149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Semmrich C, Glaser J, Merkel R, Bausch AR, Kroy K. Proc. Natl Acad. Sci. USA. 2007;104(51):20199. doi: 10.1073/pnas.0705513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Semmrich C, Larsen RJ, Bausch AR. Soft Matter. 2008;4:1675. doi: 10.1039/b800989a. [DOI] [PubMed] [Google Scholar]
- [31].Schmoller KM, Lieleg O, Bausch AR. Soft Matter. 2008;4:2365. [Google Scholar]
- [32].Bartles JR, Zheng L, Li A, Wierda A, Chen B. J. Cell Biol. 1998;143(1):107. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira PA, Weitz DA. Phys. Rev. Lett. 2004;93(18):188102. doi: 10.1103/PhysRevLett.93.188102. [DOI] [PubMed] [Google Scholar]
- [34].Spudich JA, Watt S. J. Biol. Chem. 1971;246:4866. [PubMed] [Google Scholar]
- [35].Janmey PA, Peetermans J, Zaner KS, Stossel PS, Tanaka T. J. Biol Chem. 1986;261(18):8357. [PubMed] [Google Scholar]
- [36].Kurokawa H, Fujii W, Ohmi K, Sakurai T, Nonomura Y. Biochem. Bioph. Res. Co. 1990;168(2):451. doi: 10.1016/0006-291x(90)92342-w. [DOI] [PubMed] [Google Scholar]