Abstract
The present study compared the acute dose effects of the benzodiazepine triazolam and the anticholinergic scopolamine on metamemory (knowledge and awareness of one's own memory) in a two-phase paradigm designed to assess effects on both monitoring and control components of metamemory in both semantic (general knowledge) and episodic memory (cued recall) tasks. Placebo and two doses each of triazolam (0.125, 0.25 mg/70 kg, oral) and scopolamine (0.25, 0.50 mg/70 kg, subcutaneous) were administered to 80 healthy volunteers (16/group) in a double-blind, double-dummy, independent groups design. Both triazolam and scopolamine impaired episodic memory (quantity and accuracy) but not semantic memory. Results suggested that both drugs impaired monitoring as reflected in absolute accuracy measures (impaired calibration in the direction of overconfidence) and control sensitivity (the relationship between confidence and behavior). Overall, the results did not provide evidence for differences between triazolam and scopolamine in memory or metamemory. In addition to the clinical relevance of the observed effects, this study adds to the accumulating body of cognitive psychopharmacological research illustrating the usefulness of drug-induced amnesia as a vehicle to explore memory and metamemory.
Metamemory, which refers to an individual's knowledge and awareness of his/her own memory (Flavell, 1971; Metcalfe & Shimamura, 1994), plays an important role in memory performance in many everyday situations. Metamemory can be thought of as encompassing two components: Monitoring, which consists of processes involved in an individual's self-assessment of his/her memory, and control, which refers to ways in which an individual can regulate cognitive processing when given the opportunity to do so (Nelson & Narens, 1990, 1994). One can take as an example an eyewitness in a courtroom who swears to “tell the truth, the whole truth, and nothing but the truth.” Given that memory is a reconstructive process, the witness presumably must first assess the features of his/her memory of the event being discussed (monitoring) and then determine which features of that memory to report in court (i.e., which features reach the subjective threshold of “truth”) (control).
It is well established that benzodiazepine (e.g., diazepam: Valium®, lorazepam: Ativan®; triazolam: Halcion®) and anticholinergic (e.g., scopolamine) drugs induce temporary amnesia when administered acutely to healthy volunteers (for reviews, see Curran, 1991, 2000; Kopelman, 1986; Polster, 1993). The effects of memory-impairing drugs on metamemory have both clinical and theoretical significance. From a clinical perspective, if a drug produces memory impairment, it is essential to know the degree to which patients being prescribed that drug are aware of the impairment and can control their behavior accordingly. Memory impairment without awareness of that impairment can lead to risky behavior with potentially dangerous consequences. From a theoretical perspective, memory-impairing drugs may be useful tools for exploring the relationship between memory and metamemory, and the relationship among different aspects of metamemory. Several researchers (Duka et al., 1996; Hirshman et al., 2001; Mintzer & Griffiths, 2001a; Polster, 1993) have argued that, like neuropsychological studies of brain-damaged patients, which have played a critical role in advancing the understanding of normal and abnormal memory mechanisms, investigation of drug-induced amnesia can also be a powerful tool for elucidating memory mechanisms. In fact, investigation of drug-induced amnesia has several distinct advantages over traditional studies of amnesic patients; most importantly, unlike the memory deficits found in amnesic patients, effects of drugs on memory processes are reversible and can be empirically manipulated in a dose-related fashion in controlled laboratory experiments with large numbers of healthy volunteers. Findings of selective effects of drugs on particular aspects of memory (or metamemory) performance but not other aspects can provide converging evidence with data from non-pharmacological studies for the dissociability of specific processes or sub-components (cf. Hirshman et al., 2001; Hirshman et al., 2002; Mintzer, 2003; Mintzer & Griffiths, 2001a).
The present experiment was designed to use the benzodiazepine triazolam (Halcion®) and the anticholinergic drug scopolamine to explore the processes underlying metamemory using a two-phase paradigm developed by Koriat and Goldsmith (1996) to test effects on both monitoring and control components of metamemory. Koriat and Goldsmith's original paradigm used a general knowledge task designed to assess semantic memory (memory for culturally-shared knowledge, such as meanings of words and facts, not associated with a specific spatial and temporal context; Tulving, 1972, 1983). A series of 60 general-knowledge questions (e.g., “What is the name of a large hairy spider that lives near bananas?”) were presented to participants in two consecutive phases. During the first phase (forced report), participants were instructed to answer every question, even if they had to guess, and to make a retrospective confidence judgment after every response: “How confident are you that the response you just made is correct? (from 0 = definitely is not correct to 100 = definitely is correct).” During the second phase (free report), participants were presented with the same 60 questions but were allowed to choose whether or not to provide a response to each question; accurate responding was reinforced with a moderate incentive payoff and omitted responses were not penalized. In addition to the semantic memory task, the present experiment also tested drug effects on metamnemonic monitoring and control in a task introduced by Kelley and colleagues (Kelley & Sahaykan, 2003; Rhodes and Kelley, 2005) that used a two-phase paradigm similar to Koriat and Goldsmith but within an episodic memory rather than a semantic memory task. Episodic memory refers to memory for a personally experienced event, associated with a specific spatial and temporal context. In lieu of the general knowledge test, the episodic memory task involved a cued-recall test (e.g., turkey – op _ _ a) for word pairs (e.g., turkey - opera) that were presented during an initial study phase.
This two-phase (i.e., forced and free report) paradigm enables drug effects to be tested on several different measures of memory (semantic or episodic memory), metamnemonic monitoring, and metamnemonic control. Memory was evaluated both by measures of quantity and accuracy. Quantity is calculated as the proportion of correct responses out of the total number of items presented, whereas accuracy is calculated as the proportion of correct responses out of the total number of responses provided by the participant. While laboratory experiments typically have focused on quantity as a measure of memory, in some everyday situations, accuracy may be more important than quantity. Going back to the example of an eyewitness, if five people were present at a crime scene and the eyewitness recalls three people and those three people were actually present at the scene, quantity would be 60% (3 correct out of the 5 that were present) and accuracy would be 100% (3 correct out of the 3 recalled). However, if the eyewitness instead recalls four people but only three of the four were actually present, quantity would still be 60% (3 correct out of the 5 that were present), but accuracy would be reduced to 75% (3 correct out of the 4 recalled), raising doubts about the trustworthiness of the witness's memory.
The pattern of effects that is typically observed in this two-phase paradigm is that accuracy is higher in the free report phase (in which participants are given the opportunity to control whether or not to provide a response to each item), relative to the forced report phase (in which participants must provide a response to each item). This increase in accuracy comes at the cost of quantity such that quantity is lower in the free report phase relative to the forced report phase because participants improve their accuracy in the free report phase by choosing only to respond to a subset of items. The ability to increase accuracy in the free report phase by controlling response reflects metamemory because participants must be aware of the likelihood that they will answer an item correctly in order to appropriately determine whether or not to provide a response to that item. As described next, this paradigm also enables the calculation of several specific measures of metamnemonic monitoring and control.
The monitoring judgments (i.e., the confidence ratings made after every response) were evaluated by comparing participants' confidence ratings to their actual memory performance (on the general knowledge or cued recall test) using measures of both relative accuracy (i.e., resolution) and absolute accuracy (i.e., calibration). Relative accuracy refers to the degree to which a participant accurately judges the level of performance for one item relative to another, and is high if a participant more often assigns higher confidence ratings to items that are correct than to items that are not correct on the target test. Absolute accuracy refers to the degree to which a participant accurately judges the actual percentage of correct responses overall for a set of items. Thus, absolute accuracy is higher if a participant's ratings closely match the percentage of correct responses (e.g., no items given a rating of 0 are correct, 20% of items given a rating of 20% are correct, etc.) than if the ratings overestimate or underestimate the percentage of correct responses. The two-phase paradigm enables effects on metamnemonic control to be tested on an item-by-item basis by examining the relationship for each participant between confidence ratings for each item during the forced-report phase and the participant's decision to respond or not respond to that item during the free-report phase, where they are able to control responding.
In addition to using a pharmacological manipulation to explore the cognitive mechanisms underlying different aspects of metamemory, the present study was also designed to enhance the understanding of the neurochemical mechanisms underlying metamemory by comparing two drugs that both impair memory but that act via different neurochemical mechanisms. Benzodiazepines facilitate the action of gamma-aminobutyric acid (GABA) by acting as agonists at specific sites on the GABAA receptor complex (Mohler & Okada, 1977; Squires & Braestrup, 1977), while scopolamine inhibits the action of acetylcholine by acting as an antagonist at muscarinic cholinergic receptors (Ketchum et al., 1973).
To our knowledge, the effects of benzodiazepines and scopolamine have not been previously compared with respect to the control component of metamemory. However, in a placebo-controlled, independent groups study in healthy volunteers in our laboratory (Mintzer & Griffiths, 2005), the dose effects of the benzodiazepine lorazepam and scopolamine were directly compared on the monitoring component of metamemory using a different episodic memory paradigm from that in the present study (judgment of learning paradigm). Participants were asked to make prospective monitoring judgments during an initial study phase regarding the probability that they would recall word pairs (e.g., pencil-bear) on a future memory test, as well as retrospective confidence ratings during the test regarding the probability that they correctly recalled the word pairs. Lorazepam and scopolamine produced a similar pattern of effects in which both drugs impaired the absolute accuracy measure of monitoring but spared the relative accuracy measure. However, no differences between the drugs were observed. Based on these results, we might hypothesize that triazolam and scopolamine would produce comparable effects on metamnemonic monitoring in the present study. However, results of a performance estimation task in another study in our laboratory (Mintzer & Griffiths, 2003) suggest that lorazepam-treated participants may be less aware of their drug-induced performance impairment than scopolamine-treated participants, which might be hypothesized to translate into less accurate monitoring of memory performance/impairment under certain conditions. Specifically, when asked to predict their level of performance by making a global estimate prior to performing the digit symbol substitution test (DSST; a measure of cognitive/psychomotor performance), participants in the lorazepam but not the scopolamine condition underestimated the degree of performance impairment. Additional support for the notion that benzodiazepine-treated participants are less aware of their drug-induced impairment than scopolamine-treated participants comes from participant ratings of subjective state in previous studies in our laboratory (Mintzer & Griffiths, 2003, 2005). While the drugs produced comparable deficits in psychomotor performance, participant ratings of overall strength of drug effect were lower in the lorazepam condition relative to scopolamine, suggesting that participants in the lorazepam condition were less aware of the degree to which they were being impacted by the drug.
In summary, the present study directly compared the dose effects of the benzodiazepine triazolam and the anticholinergic drug scopolamine on metamemory in a two-phase paradigm designed to assess effects on both monitoring and control components in both semantic and episodic memory tasks. Placebo and two doses each of triazolam (0.125, 0.25 mg/70 kg, oral) and scopolamine (0.25, 0.50 mg/70 kg, subcutaneous) were administered to participants in a double-blind, double-dummy, independent groups design. To facilitate direct comparison of triazolam and scopolamine at corresponding dose levels, doses of the two drugs were carefully selected to produce comparable decrements in psychomotor performance based on previous studies in our laboratory with each of the drugs. Dose was manipulated between subjects because of concerns that repeated exposure of participants to the metamemory tasks in a repeated measures design could compromise our ability to interpret the results, based on evidence that participants may shift strategies with repeated exposure (cf. Koriat, 1997).
Method
Participants
Eighty adult volunteers, recruited from local colleges and from the community-at-large in Baltimore, MD, were included in an independent groups design with five drug conditions (placebo, 0.125, 0.25 mg/70 kg triazolam, 0.25, 0.50 mg/70 kg scopolamine) (16 per condition); three additional volunteers participated but were replaced by other participants because the original participants had positive urine drug tests on the day of the session. Assignment of participants to condition was random with the constraint that the five conditions were balanced with respect to sex (9 males and 7 females per drug condition), age (mean ± S.E.M. in the placebo, 0.125, 0.25 mg/70 kg triazolam, 0.25, and 0.50 mg/70 kg scopolamine conditions, respectively: 25.6 ± 1.9, 28.9 ± 2.8, 25.1 ± 1.8, 28.5 ± 2.2, and 25.3 ± 2.1 years), years of education (mean ± S.E.M. in the five drug conditions, as listed above, respectively: 15.5 ± .80, 14.7 ± .62, 15.3 ± .84, 16.1 ± .64, and 15.7 ± .81 years), and number of correct responses on a general knowledge test conducted at screening (see below) (mean ± S.E.M. in the five drug conditions, as listed above, respectively: 35.8 ± 2.5, 34.1 ± 2.3, 37.3 ± 2.0, 37.9 ± 2.6, and 36.4 ± 1.8 responses). The groups were balanced using a minimization procedure in which each participant was assigned to the drug condition that minimized the differences among conditions in number of males, number in each of three age range categories, number in each of three level of education categories, and number in each of three general knowledge score categories (cf. Pocock, 1983). The five groups did not differ with respect to body weight. No participant reported a history of abuse of psychoactive drugs or a history of prescription or non-medical use of a benzodiazepine or anticholinergic drug.
Participants were requested to obtain a regular night's sleep before the experimental session and to refrain from using alcohol for a period of 12 hours before and 12 hours after the session. Before drug administration, participants were tested for the presence of various drugs in urine (benzodiazepines, opioids, methadone, and cocaine) using an EMIT system (Syva Co., Palo Alto, CA) and the presence of alcohol in expired air using a breathalyzer test. Use of tobacco cigarettes or other nicotine products was not permitted during the experimental session (although there was no restriction on use of these products before or after the session). Only nine participants reported smoking cigarettes (three in the placebo condition, two each in the 0.125 and 0.25 mg/70 kg triazolam conditions, and one each in the 0.25 and 0.50 mg/70 kg scopolamine conditions) and all reported smoking less than one pack per day. Based on concerns that caffeine withdrawal effects might interfere with task performance, participants were allowed their normal consumption of caffeinated products before the experimental session. All participants were in good health (as determined by medical history and personal interview) with no contraindications to benzodiazepine or anticholinergic drugs. Individuals with current or past histories of significant psychiatric disorders were excluded. In the female participants, urine pregnancy tests conducted during screening were negative. The study was approved by the Institutional Review Board of the Johns Hopkins Bayview Medical Center. Participants gave their written informed consent before beginning the study and were paid for their participation.
General Procedure
Participants completed a single outpatient experimental session at the Behavioral Pharmacology Research Unit of Johns Hopkins University School of Medicine. Single doses of placebo, 0.125 mg/70 kg triazolam, 0.25 mg/70 kg triazolam, 0.25 mg/70 kg scopolamine, and 0.50 mg/70 kg scopolamine were administered in a double-blind, double-dummy, independent groups design in which each participant received both an oral dose (of either triazolam or lactose placebo; capsules ingested with approximately 150 ml of water) and a subcutaneous injection (of either scopolamine or saline placebo; administered in a constant volume of 1 ml in the upper right arm). Scopolamine was administered subcutaneously as in previous studies due to its more variable absorption rate after oral administration (Bishop et al., 1996; Mintzer & Griffiths, 2001b, 2003, 2005; Wesnes et al., 1988). In order to synchronize the time to peak effects of triazolam and scopolamine based on pharmacokinetic and behavioral data, a staggered dosing regimen was used in which the oral dose was administered 30 min earlier than the subcutaneous dose. Triazolam doses were prepared from commercially available 0.25 mg tablets (Halcion; Pharmacia and UpJohn Company, Bridgewater, NJ, USA). Tablets were crushed, and doses were adjusted for participant body weight and dispensed in size 0 opaque capsules. Scopolamine doses (expressed as salt) were prepared from scopolamine hydrobromide trihydrate, USP (Spectrum Pharmacy Products, Gardena, CA); doses were adjusted for participant body weight and dissolved in saline to a volume of 1 ml.
The primary dependent measures involved the two metamemory tasks (described in detail below). Participants also completed other tasks before drug administration and at various timepoints after drug administration. These included psychomotor performance tasks (the circular lights task in which participants pressed a series of 16 buttons as rapidly as possible in response to the randomly sequenced illumination of their associated lights, and a standing balance task in which participants were asked to stand upright on one foot with their eyes closed and arms extended to the side at shoulder height, for a maximum of 30 sec on each foot; Mintzer et al., 1997), ratings of subjective state, and other cognitive tasks (data not presented). Data from the psychomotor performance tasks were used to verify the comparability of the selected doses of triazolam and scopolamine. It was expected that triazolam and scopolamine would produce similar decrements on these tasks at corresponding dose levels (i.e., low: 0.125 mg/70 kg triazolam, 0.25 mg/70 kg scopolamine; high: 0.25 mg/70 kg triazolam, 0.50 mg/70 kg scopolamine). The subjective rating of strength of drug effect was used as a measure of participants' awareness of the magnitude of their drug effect and to test the reliability of previously observed differences between benzodiazepines and scopolamine in awareness of drug effect. In response to the question: “How strong a drug effect are you feeling right now?” participants made a rating on a scale with five response options (coded numerically from 0 to 4, respectively): no drug effect at all, possible mild effect but not sure, definite mild effect, moderately strong effect, and very strong effect.
Metamemory Tasks: Procedures
The cued recall and general knowledge tasks used a similar two-phase paradigm to test drug effects on metamnemonic monitoring and control within episodic memory and semantic memory tasks respectively. They were administered on an Apple Macintosh microcomputer (Apple Computer, Cupertino, CA). The tasks began 80 min after capsule administration (i.e., 50 min after subcutaneous injection) so that both tasks would be conducted within the period of peak effects for triazolam and scopolamine. The cued recall task was always administered first, with a 5-min rest period between tasks. To ensure that participants understood the tasks, they were required to repeat the instructions in detail to the research assistant before beginning each task.
Cued recall task
The procedures were similar to those used by Kelley and Sahakyan (2003) and Rhodes and Kelley (2005), and the stimuli were identical to those used by Rhodes and Kelley (2005). There were 75 word pairs (cue-target; e.g., turkey-opera), 50 of which were unrelated pairs and 25 of which were related filler pairs (e.g., morning-evening). Half of the unrelated word pairs were presented as deceptive items (25) that had potentially interfering competitors that were associatively related to the target and shared the same first two letters and last letter with the target (e.g., “nurse-dollar”, with an interfering competitor “doctor”). The remaining half of the unrelated word pairs were presented as control items (25), which were created from the deceptive items by pairing the target word with an unrelated cue word (e.g., “clock-dollar”). Thus, all unrelated word pairs could be presented either as deceptive or control items. The 50 unrelated word pairs were randomly divided into two sets of 25 each for counterbalancing purposes such that across participants within each drug condition, each set appeared equally often as deceptive and control items. Deceptive items are particularly challenging because the interfering competitor (e.g., doctor) is more strongly associated to the cue word (e.g., nurse) than is the studied target word (e.g., dollar) and therefore will come to mind naturally and fluently, and this fluency may be mistaken by the participant as familiarity from the study phase. Thus, increased monitoring is needed to avoid simply responding with the interfering competitor. In the framework of a dual-process model of memory in which a distinction is made between familiarity-based recognition (i.e., a general feeling of familiarity that leads to an inference that an item was presented) and recollection-based recognition (i.e., a specific recollection of the episode in which an item was presented) (e.g., Atkinson & Juola, 1974; Jacoby, 1991; Mandler, 1980), recollection rather than familiarity would be needed to respond accurately to deceptive items. Given that benzodiazepines and scopolamine both have been shown to produce relatively greater impairment in recollection versus familiarity-based recognition (Curran et al., 1993; Hirshman et al., 2002; Mintzer, 2003; Mintzer & Griffiths, 2001b), drug-induced memory impairment might be expected to be relatively greater for deceptive versus control items.
During the study phase, 60 word-pairs (20 of each type: control, deceptive, related filler) appeared on the computer screen one at a time for 8 s each and participants were asked to study them for a subsequent memory test. To ensure that participants maintained focus on the word presentation, participants were asked to press a “next item” button between successive items. After the study phase, participants briefly completed subjective ratings. Participants then completed the test phase, which involved the presentation of a total of 75 items (60 items that had been presented during the study phase, plus 5 new items of each type: control, deceptive, related filler). In addition to counterbalancing for presentation as control vs. deceptive as described above, presentation of items as old vs. new was randomized across participants. The test phase consisted of two phases, done in succession item by item. During the first phase (forced report), participants were presented with the cue word and three letters of the target word (e.g., turkey – op _ _ a). They were told that some of the word pairs appeared in the study phase and some did not and that if they think the cue word appeared in the study phase, they are to recall the target word and to type it in, even if they have to guess. They were told that if they think the cue word did not appear earlier in the session, they are to type in the word `new.' Immediately after recalling or guessing the word or typing “new,” participants rated the likelihood that the response they produced was correct using a visual analog scale from 0% (definitely not correct) to 100%( definitely correct). Participants then were tested on the same item in a second phase (free report). In the free report phase, participants were again presented with the item (e.g., turkey – op _ _ a) along with their response from the forced report phase. They were told that in this phase, they could choose whether or not they want to use that response towards their bonus pay (by using the computer mouse to click on a button labeled “yes” or “no”) and that they would not be penalized (but neither would they receive any bonus) for omitted items. Accurate responding was reinforced by paying the participant 25 cents for each correct answer (for a possible total of $18.75) and penalizing the same amount for each incorrect answer. The amount of money that participants earned in this task was added to their total pay at the end of the study and participants were assured that they would not have to pay any losses.
General knowledge task
The procedures were similar to those used by Koriat and Goldsmith (1996) and involved two successive test phases that centered on 45 general knowledge questions. The questions were selected based on initial piloting in our laboratory from a pool of 300 general knowledge questions developed by Nelson and Narens (1980) on which normative data were collected. The questions were formulated such that the correct answer was always a single word or a proper name (e.g., Question: What is the name of a large hairy spider that lives near bananas? Answer: Tarantula). The five drug condition groups were balanced with respect to participants' general knowledge by administering a unique set of 60 questions from this pool during the initial screening interview (see Participants above). The order of questions was randomized for each participant in each phase of the task. In the first phase (forced report), the 45 questions appeared on the computer screen one at a time and participants were required to provide an answer to every question by typing in a one-word response, even if they had to guess. Immediately after responding to each question, participants were asked to rate the likelihood that their answer was correct, using a 0 (definitely not correct) to 100% (definitely correct) visual analog scale. The question and the participant's response remained on the screen until the participant made a confidence rating and there were no time constraints. In the second phase (free report), participants were presented with the same 45 questions but were told that they could choose whether to provide an answer to a given question (by typing in a one-word response) or not to provide an answer (by typing in the word “pass) and that they would not be penalized (but neither would they receive any bonus) for omitted items. Accurate responding was reinforced by paying the participant 25 cents for each correct answer (for a total of $11.25 per session) and penalizing the same amount for each incorrect answer. The amount of money that participants earned in this task was added to their total pay at the end of the study and participants were assured that they would not have to pay any losses.
Metamemory Tasks: Measures
The outcome measures for these tasks are identical to those described by Koriat and Goldsmith (1996).
Memory
Memory performance in the cued recall task (episodic memory) and general knowledge task (semantic memory) was evaluated by both measures of quantity and accuracy. Quantity was calculated as the proportion of correct responses divided by the total number of items presented. In the cued recall task, for both the forced report and free report phases the total number of items consisted of the 20 control and 20 deceptive word pairs presented in the study phase (as in the studies by Kelley and colleagues, data from the related fillers and new items were not included in the analyses). In the general knowledge task, for both the forced report and free report phases the total number of items consisted of the 45 questions. Accuracy is calculated as the proportion of correct responses out of the total number of responses provided by the participant. For the forced report phase in which participants were required to make a response to every item, the total number of responses consisted of the 20 control and 20 deceptive word pairs in the cued recall task, and the 45 questions in the general knowledge task. For the free report phase in which participants were allowed to choose whether or not to use each response towards their bonus pay (cued recall)/ provide a response to each item (general knowledge), the total number of responses (i.e., responses used towards bonus pay/non-“pass” responses) varied across participants.
Metamemory (monitoring)
Monitoring effectiveness was evaluated by both relative and absolute accuracy measures as described below. Because these analyses involve the relationship between participants' responses in the forced report and free report phases, it should be noted that for the general knowledge task (in which it was possible based on the procedures for participants to provide different responses to the same question in the forced report and free report phases), data from items for which a participant's responses differed between phases were excluded. Importantly, only 3.4 % of the responses differed between phases. The confidence ratings were grouped into 12 categories: 0, .01–.10, ,11–.20…, .91–.99, 1.0.
As recommended by Koriat and Goldsmith (1996), two measures of relative accuracy (resolution) were calculated: the Goodman-Kruskal gamma correlation (Goodman & Kruskal, 1954) commonly used by metamemory researchers (cf. Nelson, 1984) and the adjusted normalized discrimination index (ANDI) (cf. Yaniv et al., 1991). Gamma as a measure of the relative accuracy of monitoring is a correlation between confidence ratings and correctness on the memory test (cued recall or general knowledge), and can range from 1 (complete concordance between confidence ratings and correctness as reflected in high ratings given to correct responses/low ratings given to incorrect responses) to −1 (complete discordance between ratings and correctness). ANDI is the proportion of variance in correctness on the memory test that is explained by the participant's confidence ratings. Absolute accuracy was measured in two ways. First, calibration scores were calculated for each participant as the weighted mean of the absolute differences between mean estimated probability of correctness (i.e., confidence rating) and the actual proportion correct on the memory test for each confidence rating category (Oskamp, 1962); higher scores indicate greater deviation from perfect calibration (i.e., worse calibration). Second, calibration curves were constructed in which items in each confidence rating category were aggregated, and the actual proportion correct on the memory test was plotted against the estimated proportion correct (cf. Lichtenstein et al., 1982). Deviations from perfect calibration (indicated by the main diagonal line) can be assessed by visual inspection of the curves.
Metamemory (control)
Control sensitivity was measured by examining the relationship for each participant between confidence rating category for each item during the forced-report phase and the participant's decision to use that response towards bonus pay (cued recall)/ provide a response to that item (general knowledge) in the free report phase using gamma correlations and ANDI (see Monitoring above). In addition, the response criterion (Prc) used by participants to determine whether or not to use a response towards bonus pay/provide a response to an item was estimated for each participant using the computational procedure described below. Response criterion is defined as the confidence rating category that meets the following decision rule: Any item receiving a confidence rating equal to or greater than the response criterion is used towards bonus pay/receives a non-“pass” response, while any item receiving a confidence rating below the criterion is not used towards bonus pay/receives a “pass” response. Thus, each confidence rating category (assessed probability; Pa) used by the participant was considered a potential Prc. Hits were defined as those items used towards bonus pay/receiving a non-“pass” response for which Pa ≥ Prc and correct rejections were defined as those items not used towards bonus pay/receiving a “pass” response for which Pa < Prc. The Prc estimate selected for each participant was the value (i.e., confidence rating category) that maximized the proportion of hits and correct rejections combined (the fit ratio).
Data Analysis
Data for the circular lights task were missing for one participant at one timepoint due to experimenter error. In addition, data from several participants (3 for cued recall relative accuracy, 4 for cued recall control sensitivity, and 1 for general knowledge relative accuracy and control sensitivity) were excluded from gamma and ANDI analyses due to insufficiently distributed data. Data were analyzed using PROC MIXED in SAS (SAS Institute Inc., Cary, NC). Data from the psychomotor performance tasks (percentages of predrug values) and participant ratings (raw values) were analyzed by a mixed design analysis of variance (ANOVA) with drug condition (placebo and four active drug conditions) as the between-subjects factor, and time (see Figure 1 for specific timepoints) as the within-subject factor. Peak effects for psychomotor performance tasks (defined for each participant as the minimum value observed after drug administration) and participant ratings (defined for each participant as the maximum value observed after drug administration) were also analyzed (ANOVA with drug condition as the factor). Data from the cued-recall task were analyzed by a 5 × 2 mixed design ANOVA with drug condition as the between-subjects factor and item type (deceptive vs. control) as the within-subject factor for monitoring and control measures, and by a 5 × 2 × 2 mixed design with an additional within-subject factor of phase (forced vs. free report) for memory measures. Data from the general knowledge task were analyzed by a one-way ANOVA with drug condition as the between-subjects factor for monitoring and control measures, and by a 5 × 2 mixed design ANOVA with drug condition as the between-subjects factor and phase (forced vs. free report) as the within subjects factor for memory measures. Significant main effects and interactions were followed up with simple effects tests as appropriate and modified Bonferroni corrections were used (cf. Keppel, 1991). For all statistical tests, p ≤ 0.05 was considered significant.
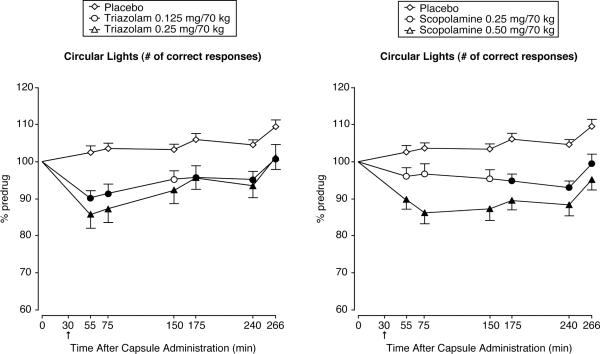
Figure 1.
Triazolam (left panel) and scopolamine (right panel) time-course functions for performance on the circular lights task (mean number of correct responses during a 60-sec trial). X axis: time in min after capsule administration; arrow indicates time of subcutaneous injection; 0 indicates predrug. Brackets show 1 S.E.M.; for clarity of presentation, either the top or lower bracket is omitted. A filled symbol indicates an active drug that is significantly different (p ≤ 0.05) from the corresponding placebo value at the same timepoint.
Results
Data from the psychomotor performance tasks used to verify the comparability of the triazolam and scopolamine doses will be presented first, followed by data from the participant rating of strength of drug effect used to measure awareness of the magnitude of drug effect, followed by data from the cued recall and general knowledge tasks.
Psychomotor Performance
As expected, triazolam and scopolamine produced similar dose- and time-related decrements in performance on the circular lights (Figure 1) and balance (data not shown) tasks relative to placebo at corresponding dose levels.
For circular lights, the ANOVA revealed a significant interaction between drug condition and time [F (4, 75) = 2.24]. Simple effects tests indicated no significant differences between corresponding doses of triazolam and scopolamine at any timepoint. The high doses of both drugs were significantly different from placebo at all post-drug timepoints. The low doses of both drugs were significantly different from placebo at the last three post-drug timepoints, but only low dose triazolam was also significantly different from placebo at the first two timepoints. For balance, the ANOVA revealed no significant interaction between drug condition and time. For both circular lights and balance, the ANOVA on peak effects data revealed a significant effect of drug condition [Fs (4, 75) ≥ 2.80]. Simple effects tested revealed no significant differences between corresponding doses of triazolam and scopolamine, supporting the comparability of the selected doses. For circular lights, the high doses of both triazolam (M = 81.98, S.E.M. = 3.78) and scopolamine (M = 81.24, S.E.M. = 2.71) were significantly different from placebo (M = 98.52, S.E.M. = 1.23), while for balance, both the low (triazolam M = 54.07, S.E.M. = 6.47; scopolamine M = 49.65, S.E.M. = 6.76) and high (triazolam M = 33.43, S.E.M. = 6.09; scopolamine M = 43.64, S.E.M. = 9.34) doses of triazolam and scopolamine were significantly different from placebo (M = 65.65, S.E.M. = 6.66).
Participant Ratings of Strength of Drug Effect
Both triazolam and scopolamine produced orderly dose- and time-related increases in participant ratings of strength of drug effect relative to placebo, and mean ratings were higher for scopolamine than triazolam at corresponding dose levels. The ANOVA revealed no significant interaction between drug condition and time. Thus, peak effects data are shown in Figure 2. The ANOVA on peak ratings of strength of drug effect revealed a significant effect of drug condition [F (4, 75) = 17.66], and simple effects tests confirmed that mean ratings were significantly higher relative to placebo in each of the active drug conditions, and significantly higher for scopolamine relative to triazolam at low dose levels; the difference between high dose scopolamine and triazolam was only marginally significant after Bonferroni correction.
Figure 2.
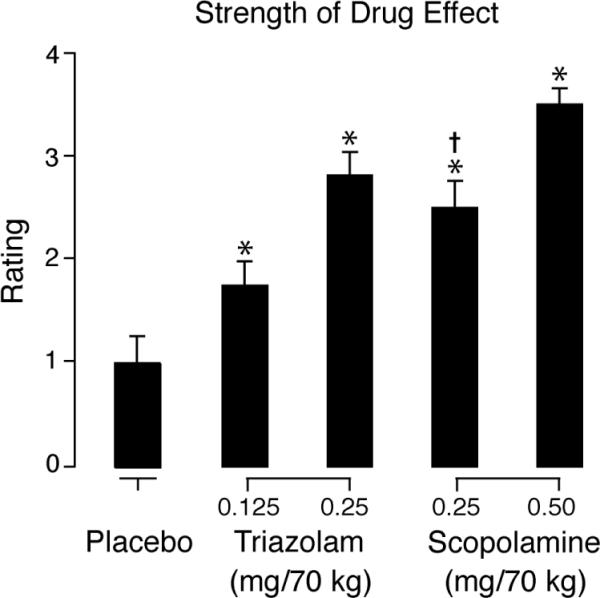
Mean peak participant ratings of strength of drug effect as a function of drug condition. Brackets show 1 S.E.M. An asterisk (*) indicates an active drug value that is significantly different from placebo. A dagger (†) indicates a scopolamine value that is significantly different from the value of the corresponding triazolam dose.
Cued Recall Task: Memory
Data from the memory measures are shown in Figure 3 (collapsed across item type because there were no significant interactions between drug condition and item type). Triazolam and scopolamine produced similar dose-related decrements relative to placebo in both accuracy and quantity measures of memory.
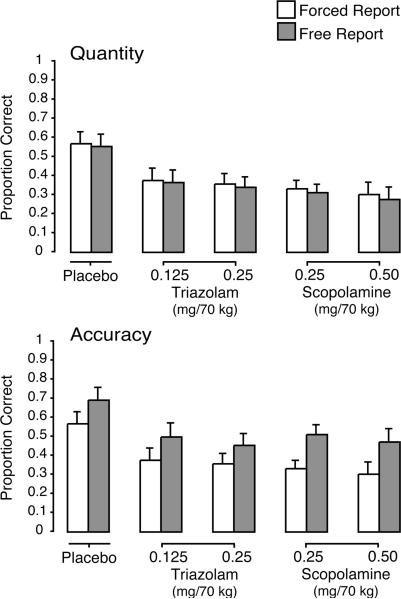
Figure 3.
Mean quantity and accuracy on the cued-recall task as a function of drug condition and phase (forced vs. free report). Brackets show 1 S.E.M. For both quantity and accuracy, all active doses were significantly different from placebo, with no significant differences between corresponding doses of triazolam and scopolamine; free report was significantly different from forced report.
The ANOVA revealed a significant main effect of drug condition for both accuracy and quantity [Fs (4, 75) ≥ 3.01], and simple effects tests indicated that both accuracy and quantity were significantly lower in each of the active drug conditions relative to placebo, with no significant differences between corresponding doses of triazolam and scopolamine. There were no significant interactions between drug condition and any of the other factors. There was a significant main effect of phase for both accuracy and quantity [Fs (1, 75) ≥ 38.71], replicating the pattern of higher accuracy and lower quantity in the free report vs. forced report phase observed in previous studies with this paradigm. There was also a significant main effect of item type [Fs (1, 75) ≥ 39.26], such that accuracy and quantity were both higher for control items (accuracy M = 0.54, S.E.M. = 0.02; quantity M = 0.43, S.E.M. = 0.02) relative to deceptive items (accuracy M = 0.38, S.E.M. = 0.02; quantity M = 0.33, S.E.M. = 0.02), and a significant phase by item type interaction [Fs (1, 75) ≥ 23.05].
Cued Recall Task: Metamemory (monitoring)
Data from the metamemory monitoring measures are shown in Table 1 (collapsed across item type because there were no significant interactions between drug condition and item type).
Table 1.
Mean scores (standard error of the mean in parentheses) on metamemory measures as a function of drug condition
| Placebo | 0.125 mg/70 kg Triazolam | 0.25 mg/70 kg Triazolam | 0.25 mg/70 kg Scopolamine | 0.50 mg/70 kg Scopolamine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (S.E.M.) | Mean | (S.E.M.) | Mean | (S.E.M.) | Mean | (S.E.M.) | Mean | (S.E.M.) | |
| Cued recall task | ||||||||||
| Metamemory (monitoring) | ||||||||||
| Relative accuracy: Gamma | 0.82 | (0.06) | 0.76 | (0.07) | 0.74 | (0.08) | 0.77 | (0.04) | 0.70 | (0.06) |
| Relative accuracy: ANDIa | 0.34 | (0.05) | 0.25 | (0.05) | 0.20 | (0.05) | 0.09* | (0.03) | 0.17* | (0.04) |
| Absolute accuracy: Calibrationb | 0.26 | (0.03) | 0.37 | (0.04) | 0.45* | (0.04) | 0.39* | (0.03) | 0.40* | (0.03) |
| Metamemory (control) | ||||||||||
| Control sensitivity: Gammab | 0.96 | (0.01) | 0.85 | (0.03) | 0.75* | (0.09) | 0.77* | (0.05) | 0.87 | (0.03) |
| Control sensitivity: ANDIa | 0.55 | (0.05) | 0.40 | (0.06) | 0.30* | (0.04) | 0.26* | (0.05) | 0.33* | (0.05) |
| Response criterion (Prc) | 0.77 | (0.03) | 0.66 | (0.04) | 0.71 | (0.04) | 0.73 | (0.03) | 0.72 | (0.04) |
| General knowledge task | ||||||||||
| Metamemory (monitoring) | ||||||||||
| Relative accuracy: Gamma | 0.79 | (0.03) | 0.84 | (0.03) | 0.74 | (0.04) | 0.84 | (0.02) | 0.75 | (0.04) |
| Relative accuracy: ANDI | 0.30 | (0.04) | 0.34 | (0.04) | 0.27 | (0.04) | 0.32 | (0.05) | 0.26 | (0.03) |
| Absolute accuracy: Calibration | 0.15 | (0.01) | 0.17 | (0.02) | 0.17 | (0.02) | 0.15 | (0.02) | 0.18 | (0.01) |
| Metamemory (control) | ||||||||||
| Control sensitivity: Gamma | 0.93 | (0.02) | 0.94 | (0.01) | 0.92 | (0.02) | 0.91 | (0.02) | 0.87 | (0.06) |
| Control sensitivity: ANDI | 0.58 | (0.05) | 0.57 | (0.04) | 0.53 | (0.04) | 0.49 | (0.05) | 0.50 | (0.06) |
| Response criterion (Prc) | 0.71 | (0.05) | 0.70 | (0.05) | 0.72 | (0.06) | 0.72 | (0.05) | 0.68 | (0.06) |
indicates a significant main effect of drug condition (p ≤ 0.05).
indicates a marginally significant main effect of drug condition (0.05 < p < 0.10).
indicates a significant difference between active drug and placebo (p ≤ 0.05).
Relative accuracy
There were no significant effects of drug condition or item type on relative accuracy as measured by gamma correlations. In contrast, the ANOVA on ANDI revealed significant main effects of drug condition [F (4, 75) = 3.01] and item type [F (1,72) = 4.05] [mean ANDI scores were higher for control (M = 0.23, S.E.M. = 0.03) vs. deceptive (M = 0.18, S.E.M. = 0.03) items]. Simple effects tests indicated that mean ANDI scores were significantly lower for both scopolamine doses relative to placebo.
In order to explore effects on relative accuracy further, we examined mean confidence ratings for correct versus incorrect items; good relative accuracy would be indicated by higher confidence ratings for correct relative to incorrect items. The ANOVA on confidence ratings revealed a significant main effect of correctness [F (1,75) = 353.59] [as expected, mean confidence ratings were higher for correct (M = 87.07, S.E.M. = 1.27) vs. incorrect (M = 56.74, S.E.M. = 1.50) items]. There were also significant interactions between correctness and item type [F (1,72) = 22.19] and between correctness and drug condition [F (4,75) = 2.67]. Simple effects tests indicated that confidence ratings were significantly higher for correct vs. incorrect items (indicating good relative accuracy) in all drug conditions. The only significant effect involving drug condition consisted of lower confidence ratings for correct items in the high dose scopolamine condition (M = 81.54, S.E.M. = 3.19) relative to placebo (M = 92.33, S.E.M. = 2.63) (consistent with the finding of lower ANDI scores), and this difference was only marginally significant after Bonferroni correction.
Absolute accuracy
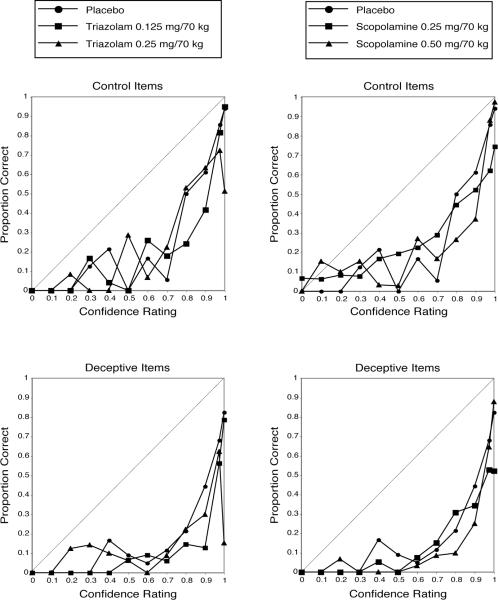
The ANOVA on calibration scores revealed a marginally significant (p = 0.058) main effect of drug condition [F (4, 75) = 2.39] and a significant main effect of item type [F (1, 75) = 96.82] (mean calibration scores/deviation from perfect calibration were higher for deceptive vs. control items). Simple effects tests indicated that mean calibration scores (i.e., deviation from perfect calibration) were significantly higher relative to placebo in all active drug conditions except low dose triazolam. Visual inspection of the calibration curves shown in Figure 4 confirms the pattern of worse calibration for deceptive vs. control items and indicates that the miscalibration for deceptive items is in the direction of greater overconfidence (i.e., values below the main diagonal). The pattern as a function of drug condition is less clear based on visual inspection of the curves; the clearest observable effect is greater overconfidence (i.e., values below the main diagonal) relative to placebo for high dose triazolam, such that there was a particularly low level of accuracy associated with the highest confidence rating category (1.0).
Figure 4.
Calibration curves of mean actual proportion correct on the cued-recall task for items receiving each confidence-rating category as a function of drug condition and item type (deceptive vs. control). Main diagonal represents perfect absolute accuracy.
Cued Recall Task: Metamemory (control)
Data from the metamemory control measures are shown in Table 1 (collapsed across item type because there were no significant interactions between drug condition and item type).
Control sensitivity
There were significant main effects of drug condition [F (4, 74) = 3.00] and item type [F (1, 71) = 4.94] for control sensitivity (i.e., the relationship between confidence rating category for a response and the participant's decision to use that response towards bonus pay) as measured by ANDI. Simple effects tests indicated that mean ANDI scores were significantly lower for high dose triazolam and low and high dose scopolamine relative to placebo. Mean ANDI scores were significantly lower for deceptive (M = 0.34, S.EM. = 0.03) vs. control (M = 0.39, S.EM. = 0.03) items. Although control sensitivity as measured by gamma correlations showed a pattern of effects as a function of drug condition similar to that observed for ANDI (mean gamma scores were significantly lower for high dose triazolam and low dose scopolamine relative to placebo), the main effect of drug condition was only marginally significant (p = 0.082) for gamma [F (4, 74) = 2.16]. The effect of item type was not significant for gamma.
In order to explore effects on control sensitivity further, we examined mean hit rate (proportion of items given confidence ratings higher than the response criterion, that were used towards bonus pay) and correct rejection rate (proportion of items given confidence ratings lower than the response criterion, that were not used towards bonus pay). There was a marginally significant main effect of drug condition on correct rejection rate [F (4, 72) = 2.35, p = 0.062] and simple effects tests indicated that mean correct rejection rate was significantly lower for low dose scopolamine (the condition that also showed the lowest control sensitivity with ANDI) (M = 0.81, S.EM. = 0.03) relative to placebo (M = 0.92, S.EM. = 0.02). In other words, in the low dose scopolamine condition, participants were more likely to use towards their bonus pay items to which they had given low confidence ratings (i.e., items they should not have used towards their bonus pay if they were more sensitive to their confidence ratings in making the bonus pay decision) relative to placebo.
Response criterion
There was no significant effect of drug condition on the response criterion (Prc) used by participants to determine whether or not to use a response towards bonus pay. There was a significant effect of item type on Prc [F (1, 75) = 7.82] such that Prc was significantly lower for deceptive (M = 0.69, S.EM. = 0.02) vs. control (M = 0.75, S.EM. = 0.02) items.
General Knowledge Task: Memory
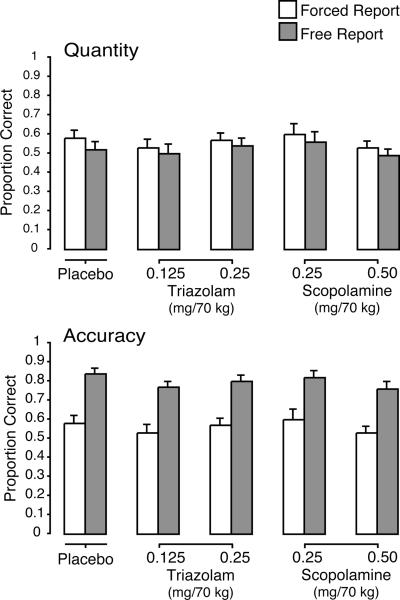
In contrast to the effects on episodic memory (cued recall), there was no significant effect of drug condition for either accuracy or quantity on the general knowledge task (semantic memory) (see Figure 5). There was a significant effect of phase for both accuracy and quantity [Fs (1, 75) ≥ 71.73], again replicating the pattern of higher accuracy and lower quantity in the free report vs. forced report phase observed in previous studies with this paradigm.
Figure 5.
Mean quantity and accuracy on the general knowledge task as a function of drug condition and phase (forced vs. free report). Brackets show 1 S.E.M. For both quantity and accuracy, free report was significantly different from forced report, and there were no differences among drug conditions.
General Knowledge Task: Metamemory (monitoring)
As can be seen in Table 1, there were no significant effects of drug condition on measures of relative accuracy (gamma correlations; ANDI) or absolute accuracy (calibration scores); calibration curves provide evidence for relatively good calibration (with slight overconfidence) in all conditions with no clear pattern of differences among drug conditions (curves not shown).
General Knowledge Task: Metamemory (control)
There were no significant effects of drug condition on control sensitivity (gamma correlations; ANDI) or response criterion estimates (Prc) (Table 1).
Discussion
This double-blind, double-dummy, placebo-controlled, independent groups study directly compared the acute dose effects of triazolam and scopolamine on measures of memory and metamemory within both episodic memory (cued recall) and semantic memory (general knowledge) paradigms.
Effects on Memory
The finding in the episodic memory task that triazolam and scopolamine produced dose-related decrements in quantity and accuracy relative to placebo replicates the well-established effect of impaired episodic memory with each of these drugs (cf. Curran, 1991, 2000; Kopelman, 1986). However, while previous studies have measured quantity, this is the first study to our knowledge to also measure accuracy. As discussed earlier, in some everyday situations such as eyewitness testimony, accuracy may be particularly relevant. Thus, impaired accuracy following drug administration may have implications for assessing the reliability of the testimony of a witness who was under the influence of drugs during the time of the target event. Interestingly, despite the observed impairment, the typical pattern of higher accuracy and lower quantity in the free report vs. forced report phase was maintained in the active drug conditions, suggesting that drug-impaired participants are able to use their metamemory to increase their accuracy when given the opportunity to choose which items to use towards their bonus pay.
In contrast to the episodic memory results, neither triazolam nor scopolamine impaired accuracy or quantity in the semantic memory task. For both benzodiazepines and scopolamine, the results of previous studies that have included semantic memory tasks are inconsistent, with some studies showing drug-induced impairment (e.g., Bacon et al., 1998, 2007; Caine et al., 1981; Massin-Krauss et al., 2002; Molchan et al., 1992; Merritt et al., 2006) and others showing no impairment (e.g., File et al., 1992; Hirshman et al., 2003; Kopelman & Corn, 1988; Pompeia et al., 2002). The finding that triazolam did not impair semantic memory specifically in the general knowledge task replicates results from a recently completed study in our laboratory that examined triazolam's effects using the same task (Kleykamp et al., 2009). However, it should be noted that Bacon and colleagues have found impairment with the benzodiazepine lorazepam in several studies with a similar general knowledge task (Bacon et al., 1998, 2007; Massin-Krauss et al., 2002). In a study from that group of researchers that used the Koriat & Goldsmith general knowledge paradigm with task parameters similar to those in the present study, impairment was observed with lorazepam in accuracy but not quantity (Massin-Krauss et al., 2002). Further research is needed to explore these conflicting results. To our knowledge, this is the first study to test the effects of scopolamine on a general knowledge task.
One possible criticism of the conclusion of a differential drug effect on episodic vs. semantic memory is that the general knowledge task was administered after the cued recall task, raising the possibility that the absence of effect on semantic memory may be simply an artifact of the presence of a weaker drug effect at that later time relative to drug administration. However, we believe this possibility is unlikely for the following reasons. First, it took participants only approximately 70 min to complete both tasks (including the 5-min break between tasks). This period is within the expected window of peak effects for both triazolam and scopolamine (Ebert et al., 1998; Friedman et al., 1986). Second, data from subjective and psychomotor performance measures taken immediately after participants completed the general knowledge task indicate the presence of strong drug effects for both triazolam and scopolamine (see Figure 1, 150 min post-capsule timepoint).
As expected, accuracy and quantity in the episodic memory task were both lower for deceptive items relative to control items. As mentioned earlier, given that success on deceptive items would seem to require use of recollection-based processes rather than relying on familiarity, and that benzodiazepines and scopolamine both have been shown to produce relatively greater impairment in recollection versus familiarity-based recognition, we predicted that drug-induced memory impairment might be relatively greater for deceptive versus control items. There was no significant interaction between drug condition and item type on either accuracy or quantity, suggesting that our prediction was not supported. However, give the relatively small sample size, we cannot rule out the possibility that there was not sufficient statistical power to detect this effect.
Effects on Metamemory
In the semantic memory task, no drug effects were observed on any measure of metamnemonic monitoring or control. This finding is consistent with the results of another recently completed study in our laboratory with the same general knowledge paradigm in which triazolam also produced no impairment in metamemory (Kleykamp et al., 2008). However, the results of these two studies from our laboratory differ from those of the Massin-Krauss et al. (2002) study mentioned above; in that study, lorazepam impaired monitoring as measured by absolute accuracy (miscalibration in the direction of overconfidence) and control as measured by control sensitivity. One important distinction to be made among these studies as noted above is that those from our laboratory did not observe benzodiazepine-related impairment of semantic memory on the general knowledge task, whereas the Massin-Krauss et al. (2002) study that reported drug-related impairment of metamemory also found drug-related semantic memory impairment. Therefore, one possibility is that the presence of memory impairment may be related to the detection of metamemory impairments.
Drug-related decrements in aspects of both monitoring and control were observed in the episodic memory task. First, both triazolam and scopolamine impaired the absolute accuracy of monitoring as reflected in increased calibration scores (i.e., deviation from perfect calibration between confidence ratings and actual proportion correct; although the main effect of drug condition was only marginally significant), and visual inspection of the calibration curves (Figure 4) suggests that the deviation was primarily in the direction of increased overconfidence relative to placebo. The finding of drug-related impairment in absolute accuracy in the direction of overconfidence is consistent with the results of previous metamemory studies with both benzodiazepines (Izaute & Bacon, 2005; Massin-Krauss et al., 2002; Merritt et al., 2005; Mintzer & Griffiths, 2005) and scopolamine (Mintzer & Griffiths, 2005) using different paradigms, and thus appears to be a reliable effect across paradigms. From a clinical perspective, overconfidence or memory impairment without awareness of that impairment can lead to risky behavior with potentially dangerous consequences.
Second, the relative accuracy of monitoring (i.e., the degree to which a participant accurately judges the level of performance for one item relative to another) appeared to be impaired by scopolamine but not triazolam, although the reliability of this effect is weakened by findings that this effect was significant for the ANDI measure but not for gamma, and that confidence ratings were significantly higher for correct versus incorrect items (indicating good relative accuracy) in all drug conditions. In previous studies with different paradigms, neither benzodiazepines (Izaute & Bacon, 2005; Massin-Krauss et al., 2002; Merritt et al., 2005; Mintzer & Griffiths, 2005) nor scopolamine (Mintzer & Griffiths, 2005) impaired relative accuracy measures, suggesting that this aspect of metamemory monitoring may be relatively spared despite reliable impairment in absolute accuracy. We speculate that relative accuracy depends on the participant's ability to assess cues related to individual item characteristics, whereas absolute accuracy depends more heavily on assessment of cues related to the participant's overall current state and competence (including drug-induced impairment). Specifically, if a participant is aware of drug-induced impairment, he/she may lower all ratings in proportion to the perceived level of impairment yielding a good match between ratings and actual proportion correct, whereas to the extent that a participant lacks awareness, ratings will be miscalibrated in the direction of overconfidence. Thus, findings across studies that absolute accuracy is more impaired than relative accuracy suggest that the ability to assess cues related to individual item characteristics is relatively unimpaired in drug-induced amnesia, whereas the ability to assess cues related to the participant's current state (which requires an awareness of the magnitude of drug effect) is impaired.
Third, results from the episodic memory task suggest that control sensitivity or the relationship between confidence rating and the participant's decision to use a response towards bonus pay is impaired by triazolam and scopolamine. However, it should be noted that only the ANDI measure showed a significant effect; although gamma showed a similar pattern of effects, the effect of drug condition was only marginally significant. The finding of drug-related impairment in control sensitivity is consistent with the results of the only other study that has examined drug effects on control (Massin-Krauss et al., 2002). Massin-Krauss et al. (2002) reported that lorazepam impaired control sensitivity within the general knowledge metamemory paradigm. Given that an individual's behavior is directly determined by control sensitivity, the finding of impaired control sensitivity may have important clinical implications. Massin-Krauss et al. (2002) have suggested that the benzodiazepine-induced impairment in control sensitivity may be related to the impairment in inhibitory control that has been observed with benzodiazepines. In other words, under the influence of a benzodiazepine, participants' behavior may not match their confidence judgments because they have difficulty exercising inhibitory control over their behavior. The finding that the response criterion used by participants (another aspect of control) did not show drug-related impairment in the present study is also consistent with the results of the Massin-Krauss et al study.
The present study was also designed to enhance the understanding of the neurochemical mechanisms underlying metamemory. Doses of the benzodiazepine triazolam and the anticholinergic scopolamine were carefully selected to produce comparable decrements in psychomotor performance based on previous studies. Indeed, data from the psychomotor tasks confirm the comparability of corresponding doses of the two drugs (Figure 1), and cued-recall performance data also indicate that triazolam and scopolamine produced similar decrements in episodic memory (Figure 3). Overall, the present study did not provide evidence for differences between triazolam and scopolamine in metamemory. Thus, these results are consistent with those of a previous study in our laboratory using a different episodic memory paradigm (judgment of learning paradigm). In that study, lorazepam and scopolamine produced a similar pattern of effects in which both drugs impaired the absolute accuracy measure of monitoring but spared the relative accuracy measure.
However, one puzzling result in both the present study and in Mintzer and Griffiths (2005) is that participant ratings suggest that benzodiazepine-treated participants were less aware of the degree to which they were being impacted by the drug than scopolamine-treated participants, which might be hypothesized to translate into less accurate monitoring of memory performance/impairment. Specifically, ratings of strength of drug effect used to assess participant's awareness of the magnitude of drug effect were higher for scopolamine relative to lorazepam in Mintzer & Griffiths (2005) and for scopolamine relative to triazolam in the present study (see Figure 2). One possible explanation for these results is that the type of awareness being tapped by the strength of drug effect rating (e.g., awareness of changes in physical or mood state) is different from that being tapped by metamnemonic monitoring judgments (e.g., awareness of changes in memory functioning), and that the difference between benzodiazepines and scopolamine is specific to the type of awareness being tapped by the strength of drug effect rating. Alternatively, it is possible that the observed difference in strength of drug effect ratings does not in fact reflect differences in awareness at all, but is simply an artifact of the different constellation of side effects produced by the two drugs. Specifically, it is possible to argue that some of the symptoms produced by scopolamine (e.g., very dry mouth and difficulty swallowing) may be qualitatively different from those encountered previously by drug-naïve research volunteers and thus more likely to elicit higher ratings of strength of drug effect. In contrast, the side effects produced by benzodiazepines (e.g., dizziness, drowsiness) may be more similar to effects previously experienced by drug-naïve volunteers and thus less likely to elicit higher ratings of drug strength. However, it should be noted that this explanation would not account for previously observed differences in participant ratings of drug strength for benzodiazepines vs. the barbiturate pentobarbital at doses of the two drugs that produced comparable effects on observer ratings of drug effect and psychomotor performance (Roache & Griffiths, 1985) because the constellation of effects of barbiturates appears qualitatively similar to that of benzodiazepines. Further research will be necessary to explore these possibilities.
In conclusion, results of the present study provide evidence for pharmacological dissociations between different types of memory (i.e., episodic vs. semantic memory), and among different aspects of metamemory within an episodic memory task. Thus, in addition to the clinical relevance of the observed effects of triazolam and scopolamine on episodic memory and aspects of metamnemonic monitoring and control, this study adds to the accumulating body of cognitive psychopharmacological research illustrating the usefulness of drug-induced amnesia as a vehicle to explore memory and metamemory.
Acknowledgments
This project was supported by National Institute on Drug Abuse Research Grant DA-11936. Portions of these data were presented at the annual meeting of the Cognitive Neuroscience Society. The authors thank Crystal Barnhouser and Kristina Burns for protocol management and technical assistance, John Yingling for computer programming assistance and technical support, and Paul Nuzzo for assistance with data analysis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
References
- Atkinson RC, Juolo JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Luce RD, Suppes P, editors. Contemporary developments in mathematical psychology: Vol. I. Learning, memory and thinking. Freeman; New York: 1974. pp. 243–293. [Google Scholar]
- Bacon E, Danion JM, Kauffmann-Muller F, Schelstraete MA, Bruant A, Sellal F, Grange D. Confidence level and feeling of knowing for episodic and semantic memory: An investigation of lorazepam effects on metamemory. Psychopharmacology. 1998;138:318–325. doi: 10.1007/s002130050677. [DOI] [PubMed] [Google Scholar]
- Bacon E, Schwartz BL, Paire-Ficout L, Izaute M. Dissociation between the cognitive process and the phenomenological experience of TOT: Effect of the anxiolytic drug lorazepam on TOT states. Consciousness and Cognition. 2007;16:360–373. doi: 10.1016/j.concog.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bishop KI, Curran HV, Lader M. Do scopolamine and lorazepam have dissociable effects on human memory systems? A dose-response study with normal volunteers. Experimental and Clinical Psychopharmacology. 1996;4:292–299. [Google Scholar]
- Caine ED, Weingartner H, Ludlow CL, Cudahy EA, Wehry S. Qualitative analysis of scopolamine-induced amnesia. Psychopharmacology. 1981;74:74–80. doi: 10.1007/BF00431761. [DOI] [PubMed] [Google Scholar]
- Curran HV. Benzodiazepines, memory, and mood: A review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- Curran HV. Psychopharmacological approaches to human memory. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. 2nd edn. MIT Press; Boston: 2000. pp. 797–804. [Google Scholar]
- Curran HV, Gardiner JM, Java RI, Allen D. Effects of lorazepam upon recollective experience in recognition memory. Psychopharmacology. 1993;110:374–378. doi: 10.1007/BF02251297. [DOI] [PubMed] [Google Scholar]
- Duka T, Curran HV, Rusted JM, Weingartner HJ. Perspectives on cognitive psychopharmacology research. Behavioural Pharmacology. 1996;7:401–410. [PubMed] [Google Scholar]
- Ebert U, Siepmann MD, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. Journal of Clinical Pharmacology. 1998;38:720–726. doi: 10.1002/j.1552-4604.1998.tb04812.x. [DOI] [PubMed] [Google Scholar]
- File SE, Sharma R, Shaffer J. Is lorazepam-induced amnesia specific to the type of memory or to the task used to assess it? Journal of Psychopharmacology. 1992;61:76–80. doi: 10.1177/026988119200600114. [DOI] [PubMed] [Google Scholar]
- Flavell JH. What is memory development the development of? Human Development. 1971;14:272–278. [Google Scholar]
- Friedman H, Greenblatt DJ, Burstein ES, Harmatz JS, Shader RI. Population study of triazolam pharmacokinetics. British Journal of Clinical Pharmacology. 1986;22:639–642. doi: 10.1111/j.1365-2125.1986.tb02951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross-classifications. Journal of the American Statistical Association. 1954;49:732–764. [Google Scholar]
- Hirshman E, Passannante A, Arndt J. Midazolam amnesia and conceptual processing in implicit memory. Journal of Experimental Psychology: General. 2001;130:453–465. doi: 10.1037//0096-3445.130.3.453. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Fisher J, Henthorn T, Arndt J, Passannante A. Midazolam amnesia and dual process models of the word frequency mirror effect. Journal of Memory and Language. 2002;47:499–516. [Google Scholar]
- Hirshman E, Fisher J, Henthorn T, Arndt J, Passannante A. Midazolam amnesia and retrieval from semantic memory: Developing methods to test theories of implicit memory. Brain and Cognition. 2003;53:427–432. doi: 10.1016/s0278-2626(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Izaute M, Bacon E. Specific effects of an amnesic drug: Effect of lorazepam on study time allocation and on judgment of learning. Neuropsychopharmacology. 2005;30:196–204. doi: 10.1038/sj.npp.1300564. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Kelley CM, Sahakyan L. Memory, monitoring, and control in the attainment of memory accuracy. Journal of Memory and Language. 2003;48:704–721. [Google Scholar]
- Ketchum JS, Sidell FR, Crowell EG, Aghajanian GK, Haines AH. Atropine, scopolamine, and ditran: Comparative pharmacology and antagonists in man. Psychopharmacologia. 1973;28:121–145. doi: 10.1007/BF00421398. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. 70th annual scientific meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. 2008. [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation of memory accuracy. Psychological Review. 1996;103:490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. The cholinergic neurotransmitter system in human memory and dementia: A review. The Quarterly Journal of Experimental Psychology. 1986;38A:535–573. doi: 10.1080/14640748608401614. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Corn TH. Cholinergic 'blockade' as a model for cholinergic depletion. A comparison of the memory deficits with those of alzheimer-type dementia and the alcoholic korsakoff syndrome. Brain. 1988;111:1079–1110. doi: 10.1093/brain/111.5.1079. [DOI] [PubMed] [Google Scholar]
- Koriat A. Monitoring one's own knowledge during study: a cue-utilization approach to judgments of learning. Journal of Experimental Psychology: General. 1997;126:349–370. [Google Scholar]
- Lichtenstien S, Fischhoff B, Phillips LD. Calibration of probabilities: The state of the art to 1980. In: Kahneman D, Slovic P, Tversky A, editors. Judgment under Uncertainty: Heuristics and Biases. Cambridge University Press; Cambridge: 1982. pp. 306–334. [Google Scholar]
- Mandler G. Recognizing: The judgement of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Massin-Krauss M, Bacon E, Danion J-M. Effects of the benzodiazepine lorazepam on monitoring and control processes in semantic memory. Consciousness and Cognition. 2002;11:123–137. doi: 10.1006/ccog.2001.0538. [DOI] [PubMed] [Google Scholar]
- Merritt P, Hirshman E, Hsu J, Berrigan M. Metamemory without the memory: Are people aware of midazolam-induced amnesia? Psychopharmacology. 2005;177:336–343. doi: 10.1007/s00213-004-1958-8. [DOI] [PubMed] [Google Scholar]
- Merritt P, Hirshman E, Zamani S, Hsu J, Berrigan M. Episodic representations support early semantic learning: Evidence from midazolam induced amnesia. Brain and Cognition. 2006;61:219–223. doi: 10.1016/j.bandc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Shimamura AP. Metacognition: Knowing about knowing. MIT Press; Cambridge: 1994. [Google Scholar]
- Mintzer MZ. Triazolam-induced amnesia and the word-frequency effect in recognition memory: Support for a dual process account. Journal of Memory and Language. 2003;48:596–602. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: A comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behavioural Pharmacology. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. False recognition in triazolam-induced amnesia. Journal of Memory and Language. 2001a;44:475–492. [Google Scholar]
- Mintzer MZ, Griffiths RR. Acute dose-effects of scopolamine on false recognition. Psychopharmacology. 2001b;153:425–433. doi: 10.1007/s002130000592. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: A single-dose comparison of effects on human memory and attentional processes. Experimental and Clinical Psychopharmacology. 2003;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Drugs, memory, and metamemory: A dose-effect study with lorazepam and scopolamine. Experimental and Clinical Psychopharmacology. 2005;13:336–347. doi: 10.1037/1064-1297.13.4.336. [DOI] [PubMed] [Google Scholar]
- Mohler H, Okada T. Benzodiazepine receptor: Demonstration in the central nervous system. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Research.Brain Research Reviews. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- Nelson TO. A comparison of current measures of the accuracy of feeling of knowing predictions. Psychological Bulletin. 1984;95:109–133. [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. The Psychology of Learning and Motivation. 1990;26:125–173. [Google Scholar]
- Nelson TO, Narens L. Why investigate metacognition? In: Metcalfe J, Shimamura AP, editors. Metacognition: Knowing about knowing. Massachusetts Institute of Technology; Cambridge: 1994. pp. 1–25. [Google Scholar]
- Oskamp S. The relationship of clinical experience and training methods to several criteria of clinical prediction. Psychological Monographs. 1962;76 28, Whole No. 547. [Google Scholar]
- Pocock SJ. Clinical trials: A practical approach. Johns Wiley & Sons; New York, NY: 1983. [Google Scholar]
- Polster MR. Drug-induced amnesia: Implications for cognitive neuropsychological investigations of memory. Psychological Review. 1993;114:477–493. doi: 10.1037/0033-2909.114.3.477. [DOI] [PubMed] [Google Scholar]
- Pompeia S, Rusted JM, Curran HV. Verbal fluency facilitated by the cholinergic blocker, scopolamine. Human Psychopharmacology. 2002;17:51–59. doi: 10.1002/hup.331. [DOI] [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: Performance impairment, subjective effects and abuse liability. Journal of Pharmacology and Experimental Therapeutics. 1985;234:120–133. [PubMed] [Google Scholar]
- Rhodes MG, Kelley CM. Executive processes, memory accuracy, and memory monitoring: An aging and individual difference analysis. Journal of Memory and Language. 2005;52:578–594. [Google Scholar]
- Squires RF, Braestrup C. Benzodiazepine receptors in rat brain. Nature. 1977;266:732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. Academic Press; New York: 1972. pp. 381–403. [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford University Press; New York: 1983. [Google Scholar]
- Wesnes K, Simpson P, Kidd A. An investigation of the range of cognitive impairments induced by scopolamine 0.6 mg s.c. Human Psychopharmacology. 1988;3:27–81. [Google Scholar]
- Yaniv I, Yates JF, Smith JEK. Measures of discrimination skill in probabilistic judgment. Psychological Bulletin. 1991;110:611–617. [Google Scholar]