Serum creatinine is ordered more than 281 million times annually in the United States (based on the 191,354,358 creatinine tests reported in 1996 and assuming annual growth rate in testing of 3%),1 and recent reports show that more than 70% of laboratories now report estimated glomerular filtration rate (GFR) using the Modification of Diet in Renal Disease (MDRD) Study equation.2 Recently, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) developed and validated a new equation, the CKD-EPI creatinine equation, which uses the same variables as the MDRD Study, but is more accurate.3,4 Accuracy of GFR estimating equations is evaluated in comparison to measured GFR. However, as for other diagnostic tests, other criteria are also important in clinical practice and public health, including detecting disease, predicting prognosis, and guiding therapy. In this issue of the American Journal of Kidney Diseases, 2 articles compare the CKD-EPI equation with the MDRD Study equation for estimating the prevalence of CKD and predicting the risk of subsequent events in the general population.5,6 In this editorial, we comment briefly on these articles and review the accuracy and applications of current GFR estimating equations (Table 1).
Table 1.
Comparison of 3 GFR Estimating Equations for Creatinine
| Cockcroft-Gault | MDRD Study | CKD-EPI | |
|---|---|---|---|
| General Information | |||
| Year of publication | 1973 | 1999 | 2009 |
| Reference standard for mGFR |
Urinary clearance of creatinine |
Urinary clearance of 125I-iothalamate |
Urinary clearance of 125I-iothalamate |
| Unit | mL/min | mL/min/1.73 m2 | mL/min/1.73 m2 |
| Variables included Creatinine Age Sex Race Weight |
Yes Yes No (coefficient added later) No Yes |
Yes Yes Yes Black vs white and other (Chinese and Japanese coefficients added later) No |
Yes Yes Yes Black vs white and other (Japanese coefficient added later) No |
| Standardized creatinine assay |
No (cannot be standardized) |
Yes (2006) | Yes |
| Characteristics of Development Dataset | |||
| No. of participants | 249 | 1,628 | 5,504 |
| Age (mean, y) | 57 | 51 | 47 |
| mGFR (mean) | 73 | 40 | 67 |
| Sex (% men) | 100 | 60 | 57 |
| Race (%) Black Asian Hispanic White and other |
NR NR NR 100 |
12 NR NR 88 |
32 1 5 63 |
| CKD (%) | NR | 100 | 73a |
| Diabetes (%) | NR | 6 | 29 |
| Transplant recipients (%) |
NR | 0 | 4 |
| Validation | |||
| Validation in same report |
No | No | Yes |
| Validation in separate reports |
Many | Many | Now appearing |
| Comparative Performanceb | |||
| Bias | Overestimation of mGFR |
Underestimation of mGFR at eGFR <60 mL/min/1.73 m2; generally lesser bias than Cockcroft- Gault equation |
Underestimation of mGFR at higher range; lesser bias than MDRD Study equation |
| Precision | Limited throughout eGFR range |
Greater precision than Cockcroft- Gault equation; still limited |
Greater precision than MDRD Study equation; still limited |
| Application | |||
| eGFR reporting | Limited | Common; applicable for eGFR <60 mL/min/1.73 m2 |
Proposed; applicable throughout eGFR range |
| Disease detection and prevalence estimates |
Much higher US prevalence compared with MDRD Study equation |
Approximately 13% US prevalence |
Lower US prevalence for age <70, women, and whites compared with MDRD Study equation |
| Prognosis | Mortality; CVD; kidney disease progression; difficult to relate to CKD stages |
Mortality; CVD; kidney disease progression; uncertain for CKD stage 3a |
More accurate estimate for CKD stage 3a compared with MDRD Study equation |
| Treatment | Drug dosing | KDOQI CKD clinical action plan; drug dosing (proposed) |
KDOQI CKD clinical action plan (proposed); drug dosing (proposed) |
Note: Chronic kidney disease in this table refers to stage 3a only (GFR 45-59 mL/min/1.73 m2 [0.75-0.98 mL/s/1.73 m2]).
Abbreviations: CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; KDQOI, Kidney Disease Quality Outcomes Initiative; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate; NR, not reported.
Percent of individuals from studies of CKD.
Comparisons using creatinine assays calibrated to standardized creatinine and separate validation database.
ACCURACY
GFR estimating equations are derived from regression analysis in which the level of measured GFR is related to the serum concentration of an endogenous filtration marker and to observed clinical and demographic variables that serve as surrogates for the non-GFR determinants of the serum concentration. Age, sex, race, and body weight are surrogates for creatinine generation from muscle, which affects serum creatinine concentration independently from GFR. In principle, GFR estimating equations provide a more accurate estimate of measured GFR than the serum level of the filtration marker alone. In addition, GFR estimates are provided in the same units as measured GFR, thereby simplifying clinical decisions based on the level of kidney function. Inaccuracy of GFR estimates may be due to bias, defined as systematic deviation of estimated GFR compared with measured GFR using the reference (or “gold”) standard, or may be due to imprecision, defined as random variation (or “spread”) of estimated GFR values centered around the measured values.7
Variation in creatinine assays is a major source of bias. More accurate creatinine assays, traceable to gold-standard creatinine measurements, have now replaced less accurate methods, and a creatinine standardization program has been implemented in all clinical laboratories throughout the United States.8 The effect of standardizing creatinine assays will vary among clinical laboratories, but on average will lead to lower values for serum creatinine and higher values for estimated GFR compared with measurements before standardization. The MDRD Study and CKD-EPI equations can be used with standardized creatinine.3,9 The Cockcroft-Gault equation cannot be re-expressed for standardized serum creatinine; thus, older studies that used nonstandardized creatinine assays to examine the performance of the Cockcroft-Gault equation are no longer relevant.
Bias may also reflect systematic differences between development datasets and populations in which the equation will be used. These differences likely reflect differences in non-GFR determinants that are not captured by the variables used in the estimating equations. The systematic underestimation of measured GFR at higher estimated GFR by the MDRD Study equation is well known,10–12 and may reflect higher creatinine generation in healthy individuals compared with individuals with CKD in whom the MDRD Study equation was derived. This bias is reduced substantially, but not completely, by the CKD-EPI equation, which was derived from studies including people without CKD.3,13,14 In addition, the variable of black versus white or other does not capture all of the variation in creatinine generation among all racial and ethnic groups. This may be overcome in part by modifications using other race-ethnicity variables, as has been reported for use of the MDRD Study equation.15–17 A forthcoming article in AJKD introduces a modification of the CKD-EPI equation for use in Japan.18 However, even with these modifications, no equation will be free of bias in all settings and populations. Thus, knowledge of the effect of clinical conditions on non-GFR determinants of filtration markers is essential for interpretation of GFR estimates.
Imprecision may reflect GFR measurement error or random variation in surrogates of non-GFR determinants. Accuracy may be improved by developing GFR estimating equations using more precise GFR measurement methods or multiple filtration markers with noncorrelated non-GFR determinants, such as cystatin C in addition to creatinine.19
DETECTING DISEASE
In principle, decreased GFR in acute and chronic kidney diseases is preceded by alterations in structure that can be detected by pathologic disturbances or markers of kidney damage. Biopsies are usually not obtained in clinical practice and markers of kidney damage are not sensitive; thus, in many patients decreased GFR is the earliest sign of kidney disease. Widespread reporting of estimated GFR using the MDRD Study equation simplifies the detection of CKD defined as GFR <60 mL/min/1.73 m2 [<1 mL/s/1.73 m2]. It is more difficult to use the Cockcroft-Gault equation for this purpose because of differences in the reference test, unit of measurement, and creatinine assay. In principle, the lesser bias of the CKD-EPI equation compared with the MDRD Study equation would lead to fewer false-positive diagnoses of CKD.
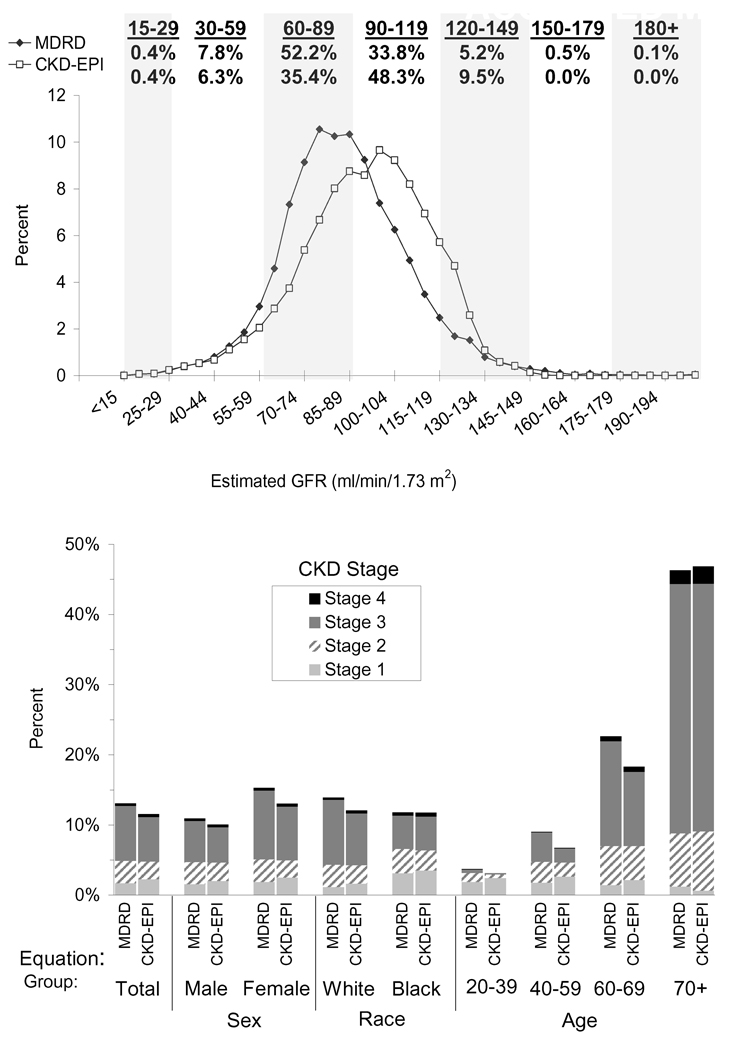
There are no studies of measured GFR in large representative populations. The MDRD Study equation has been used to estimate CKD prevalence in multiple countries, and results generally are in the range of 10%–15%.20–23 In 1 study that attempted to derive estimates from the Cockcroft-Gault equation, CKD prevalence in the United States was approximately 1.7 times higher compared with the estimated prevalence using the MDRD Study equation.24 The CKD-EPI investigators compared the estimated GFR distribution and CKD prevalence using the CKD-EPI and MDRD Study equations among 16,032 adult participants in the US National Health and Nutrition Examination Surveys (NHANES 1999–2006), a nationally representative survey of noninstitutionalized persons in the United States.3 Median estimated GFR was 94.5 versus 85.0 mL/min/1.73 m2, respectively [1.58 vs 1.42 mL/s/1.73 m2], and CKD prevalence was 11.6% versus 13.1%, respectively, due to a lower prevalence in people under 70 years of age, women, and whites (Fig 1).
Figure 1.
Comparison of distribution of estimated glomerular filtration rate (GFR) and chronic kidney disease (CKD) prevalence by sex, age, and race (NHANES 1999–2006, N=16,032). Upper panel: distribution of estimated GFR by 4-mL/min/1.73 m2 categories. Values are plotted at the mid point. Lower panel: prevalence of CKD by sex, race, and age. Abbreviation: NHANES, National Health and Nutrition Examination Survey. Reprinted with permission.3
The articles in this issue of AJKD confirm these observations. Matsushita and colleagues compared estimated GFR computed using both equations in 13,905 participants in the Atherosclerosis Research in Communities (ARIC) Study, a community-based cohort of African American and white individuals aged 45–64 years.5 In their study, 43.5% of participants with CKD stage 3a (estimated GFR 45–59 mL/min/1.73 m2 [0.75–0.98 mL/s/1.73 m2]) using the MDRD Study equation were reclassified to no CKD (defined as estimated GFR ≥60 mL/min/1.73 m2 [≥1 mL/s/1.73 m2]; albuminuria was not measured) using the CKD-EPI equation. White and colleagues compared estimated GFR using both equations in 11,247 participants in the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study, a representative sample of the adult population aged ≥25 years. In that study, 25.0% of participants with CKD Stage 3a were reclassified to no CKD using the CKD-EPI equation. In both studies, the individuals who were reclassified were more often younger, white, and women with higher estimated GFR.6
PREDICTING PROGNOSIS
Decreased GFR is now a well-established risk factor for cardiovascular disease and mortality, as well as for kidney failure. There has been much debate about whether increased risk is apparent for people with CKD stage 3a.25,26 The ARIC and AusDiab studies reported events during follow-up intervals of 16.9 and 7.5 years, respectively.5,6 In both studies, the individuals reclassified from CKD stage 3a using the MDRD Study equation to no CKD using the CKD-EPI equation had lower risk than those not reclassified, and similar risk to those classified as no CKD by both equations. Individuals not reclassified from CKD stage 3a had a higher risk for adverse outcomes compared with no CKD. Adjustment for other variables attenuated the higher relative risk of CKD Stage 3 for mortality and cardiovascular disease, but not for kidney failure.
GUIDING THERAPY
There have been few studies comparing the effect of estimating equations on clinical decisions regarding therapy. Since 1998, the US Food and Drug Administration (FDA) has recommended using the Cockcroft-Gault equation for pharmacokinetic studies during drug development to guide dosing in patients with decreased kidney function.27 Since then, the availability of more accurate creatinine assays and kidney function estimating equations has led to reassessment of clinical recommendations and FDA guidance.28,29 While there have been many studies showing differences between kidney function estimates using the Cockcroft-Gault and MDRD Study equations, few have included “gold standard” measures of GFR or clinical end points such as effectiveness or toxicity of therapy. One recent study simulated dosing recommendations for 15 medications cleared by the kidneys using the Cockcroft-Gault and MDRD Study equations as compared with measured GFR.30 Concordance of recommended drug dosages was 88% for the MDRD Study equation versus 85% for the Cockcroft-Gault equation compared with measured GFR. The investigators concluded that clinicians could use either equation for drug dosing. Presumably, even greater accuracy of the CKD-EPI equation would also be useful for drug dosing.
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) clinical action plan for CKD is based on stages defined by GFR, most commonly estimated using the MDRD Study equations. A recent study by Jain and colleagues studied referral of patients to nephrologists in Ontario, Canada before and after implementation of reporting of estimated GFR (computed using the MDRD Study equation) when serum creatinine is measured.31 The authors noted an increase in referrals, primarily in women. Presumably, using the CKD-EPI equation would lead to fewer false-negative diagnoses of CKD in low-risk patients and thus would allow more appropriate nephrology referral.
CONCLUSIONS
The CKD-EPI creatinine equation is currently the most accurate method for estimating GFR for diverse populations. The results of the ARIC and AusDiab studies in this issue of AJKD demonstrate some of the useful applications of more accurate GFR estimates. Compared with the MDRD Study equation, the CKD-EPI equation permits more accurate GFR estimation, fewer false-positive diagnoses of CKD, lower prevalence estimates for CKD, and more accurate risk prediction for adverse outcomes. This accumulating evidence supports the recommendations of the CKD-EPI investigators that the CKD-EPI equation could replace the MDRD Study equation for general use.3 In a previous editorial in AJKD, Becker and Vassalotti described implementation of the CKDEPI equation in activities of the National Kidney Foundation.32 There are few drawbacks to more widespread implementation of the CKD-EPI equation. Implementing a new GFR estimating equation requires an ongoing educational effort to promote an understanding of its strengths and limitations, as would be needed for advances in other diagnostic tests. Since the same 4 variables are used, the impact on information systems is minimal, and the differences observed by clinicians will be equivalent to reporting any analyte using a new assay.
Despite these improvements in GFR estimation, much uncertainty remains. More research is required to determine the usual levels of GFR and non-GFR determinants of creatinine and other filtration markers in representative populations, including the elderly and diverse racial and ethnic groups, and to determine the optimal application of GFR estimates in clinical medicine and public health. We have come a long way since serum creatinine alone was used for GFR estimation. We should now use the best available information we have for clinical practice, and we should continue our efforts to do better.
ACKNOWLEDGEMENTS
Aghogho Okparavero, MD, MPH assisted in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Levey was principal investigator of the Chronic Kidney Disease Epidemiology Collaboration, is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and is Editor-in-Chief of the American Journal of Kidney Diseases. Dr Stevens was coinvestigator for the Chronic Kidney Disease Epidemiology Collaboration.
REFERENCES
- 1.Steindel SJ, Rauch WJ, Simon MK, Handsfield J. National Inventory of Clinical Laboratory Testing Services (NICLTS). Development and test distribution for 1996. Arch Pathol Lab Med. 2000;124(8):1201–1208. doi: 10.5858/2000-124-1201-NIOCLT. [DOI] [PubMed] [Google Scholar]
- 2.Miller WG. Reporting estimated GFR: a laboratory perspective. Am J Kidney Dis. 2008;52(4):645–648. doi: 10.1053/j.ajkd.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens LA, Schmid CH, Zhang Y, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2009;25(2):449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Selvin E, Bash L, Astor B, Coresh J. Risk implications of the new CKD-EPI equations as compared to the MDRD Study equation for estimated GFR: the ARIC study. Am J Kidney Dis. 2010;55(4) doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White S, Polkinghorne K, Atkins R, Chadban S. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD-EPI and MDRD Study GFR estimating equations: the AusDiab study. Am J Kidney Dis. 2010;55(4) doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 8.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16(2):459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 12.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 13.White CA, Akbari A, Doucette S, Fergusson D, Knoll GA. Estimating glomerular filtration rate in kidney transplantation: is the new chronic kidney disease epidemiology collaboration equation any better? Clin Chem. doi: 10.1373/clinchem.2009.135111. [published online ahead of print Decmber 3, 2009] [DOI] [PubMed] [Google Scholar]
- 14.Tent H, Rook M, van Son W, et al. Performance of MDRD and CKD-EPI equations in higher ranges of GFR relates to GFR as such: paired data before and after kidney donation [ASN abstract F-FC165] J Am Soc Nephrol. 2008;19:37A. [Google Scholar]
- 15.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? Am J Kidney Dis. 2009;53(6):932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horio M. Modification of the CKD-EPI equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. doi: 10.1053/j.ajkd.2010.02.344. in press. [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 21.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 22.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14 suppl 2:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 23.Imai E, Matsuo S. Chronic kidney disease in Asia. Lancet. 2008;371(9631):2173–2182. doi: 10.1016/S0140-6736(08)60928-9. [DOI] [PubMed] [Google Scholar]
- 24.Snyder JJ, Foley RN, Collins AJ. Prevalence of CKD in the United States: a sensitivity analysis using the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2009;53(2):218–228. doi: 10.1053/j.ajkd.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75(10):1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53(6):915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services; Food and Drug Administration. [Accessed February 5, 2010];Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Gui dances/UCM072127.pdf.
- 28.Stevens LA, Levey AS. Use of the MDRD study equation to estimate kidney function for drug dosing. Clin Pharmacol Ther. 2009;86(5):465–467. doi: 10.1038/clpt.2009.124. [DOI] [PubMed] [Google Scholar]
- 29.National Kidney Disease Education Program. [Accessed February 5, 2010];Health professionals, CKD and drug dosing: information for providers. Available at: http://nkdep.nih.gov/professionals/drug-dosing-information.htm.
- 30.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain AK, McLeod I, Huo C, et al. When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney Int. 2009;76(3):318–323. doi: 10.1038/ki.2009.158. [DOI] [PubMed] [Google Scholar]
- 32.Becker BN, Vassalotti JA. A software upgrade: CKD testing in 2010. Am J Kidney Dis. 2010;55(1):8–10. doi: 10.1053/j.ajkd.2009.11.005. [DOI] [PubMed] [Google Scholar]