Abstract
Reading familiar words differs from reading unfamiliar non-words in two ways. First, word reading is faster and more accurate than reading of unfamiliar non-words. Second, effects of letter length are reduced for words, particularly when they are presented in the right visual field in familiar formats. Two experiments are reported in which right-handed participants read aloud non-words presented briefly in their left and right visual fields before and after training on those items. The non-words were interleaved with familiar words in the naming tests. Before training, naming was slow and error prone, with marked effects of length in both visual fields. After training, fewer errors were made, naming was faster, and the effect of length was much reduced in the right visual field compared with the left. We propose that word learning creates orthographic word forms in the mid-fusiform gyrus of the left cerebral hemisphere. Those word forms allow words to access their phonological and semantic representations on a lexical basis. But orthographic word forms also interact with more posterior letter recognition systems in the middle/inferior occipital gyri, inducing more parallel processing of right visual field words than is possible for any left visual field stimulus, or for unfamiliar non-words presented in the right visual field.
Keywords: word learning, reading, hemispheres, visual fields, word length, case alternation
1. Introduction
Every familiar word starts life as an unfamiliar non-word. Only through experience and repetition does it achieve the transition from unknown to known. That transition requires the language user to learn the meaning of a word, when and where it can be used, its grammatical (syntactic) properties, its pronunciation (phonology) and its written form (orthography). Our particular concern in this paper is with the acquisition of new written words. We will argue that there are multiple components to learning a new written word. One set of components involves the acquisition of the orthographic, semantic and phonological representations that support subsequent recognition of the word—what Leach & Samuel (2007) call the word's lexical configuration.
A different hypothesized component is more perceptual or pre-lexical in nature. If reading speeds for familiar words and unfamiliar non-words are compared, two differences are apparent. The first is that familiar words are read aloud more quickly than unfamiliar non-words, even when the words have regular, consistent spellings and could presumably, therefore, be read aloud using the same knowledge of letter-sound correspondences that is employed in reading the non-words (Salasoo et al. 1985; McCann & Besner, 1987; Rastle & Coltheart, 1999). The other difference between reading speeds for known words and unknown non-words is that reading times for non-words presented under normal reading conditions are strongly affected by the number of letters in the non-word, whereas reading speeds for familiar words show much less of an effect of word length (Weekes 1997; Juphard et al. 2004). This is often interpreted by proposing that the reading of non-words involves relatively serial processing in the identification of the component letters and/or the conversion of the letter string from print to sound. In contrast, the recognition of familiar words and their conversion into phonological forms is widely thought to involve more parallel processing, with the component letters being processed simultaneously and the word being converted from print to sound as a unit (e.g. Coltheart et al. 2001; Harm & Seidenberg 1999, 2004). We will argue that at least part of the observed differences between the processing of words and non-words is perceptual in nature, arising as a result of more effective parallel processing of the component letters of known words. One challenge is to explain how a pre-lexical component (letter identification) that operates before the stage at which words are recognized as familiar could nevertheless be influenced by whether a letter string forms a familiar word or an unfamiliar non-word.
We will further argue that efficient parallel processing of the component letters of known words is only possible when words are presented directly to the left (language dominant) cerebral hemisphere. If written words are presented initially to the right hemisphere, and hence require transfer across the corpus callosum to the left hemisphere, processing is more serial in nature, irrespective of whether the stimulus is a word or a non-word, and is therefore more sensitive to the number of letters needing to be identified (i.e. word length). Finally, we will consider the possibility that words presented in unfamiliar formats, such as MiXeD cAsE, are also processed in a relatively serial manner, regardless of whether they are presented to the left or the right hemisphere, resulting in length effects like those seen for unfamiliar non-words (cf. Cohen et al. 2008).
(a). Words, non-words, length and hemispheres
Before reporting the experiments on word learning and visual word recognition in the two cerebral hemispheres that form the basis of this paper, it is necessary to briefly review the literature on lateralized word recognition, paying particular attention to effects of word length, the factor we will employ as an indicator of the extent to which the processing of letters in word or non-words is serial or more parallelized.
The anatomy of the visual pathways connecting the two retinas to the brain ensures that stimuli presented to the left of a central fixation point (in the left visual field or LVF) are transmitted first to visual cortex in the right cerebral hemisphere. Conversely, stimuli presented to the right of a central fixation point (in the right visual field or RVF) are transmitted first to visual cortex in the left cerebral hemisphere (Hellige 1993; Bourne 2006). It is widely (though not universally) assumed that the process of recognizing a sequence of letters as forming a familiar word occurs within the language-dominant hemisphere, possibly within the left mid-fusiform gyrus at the site of the so-called ‘visual word form area’ (e.g. Cohen et al. 2002; McCandliss et al. 2003; Price & McCrory 2005; Simos et al. 2008). If that is the case, then words presented in the RVF which arrive in left visual cortex require only intra-hemispheric processing in order to access the visual word form area and be recognized as familiar. In contrast, words presented in the LVF, which arrive initially in right visual cortex, must be transferred across the corpus callosum to the left hemisphere if they are to be recognized (Whitney 2001; Cohen et al. 2002; Banich 2003; Ellis 2004).
Right-handed participants with left hemisphere language dominance recognize words presented in the RVF more quickly and more accurately than words presented in the LVF (Hellige 1993; Knecht et al. 2000; Hunter & Brysbaert 2008). But some words generate more of an RVF advantage than others. Word length is one factor that affects the observed size of the RVF advantage. Many studies have shown that longer words generate more of a difference between RVF and LVF recognition than shorter words. This is because RVF recognition speed and accuracy is relatively unaffected by word length, whereas in the LVF, shorter words are processed more quickly and more accurately than longer words. The greater impact of letter length on LVF than RVF word recognition means that the RVF advantage is greater for longer than for shorter words. This holds true for a range of tasks including word naming, lexical decision (deciding whether a letter string forms a familiar word or not) and semantic categorization (e.g. Bub & Lewine 1988; Ellis et al. 1988; Lavidor & Ellis 2001; Lavidor et al. 2002; Lindell et al. 2002; see Ellis 2004 for a review). The greater effect of letter length on responses to familiar words in the LVF than the RVF remains when words containing different numbers of letters are adjusted to the same physical length, and so appears to depend on the number of letters rather than, for example, differences in acuity owing to the fact that longer words may extend further away from the fovea than shorter words (Bruyer & Janlin 1989; Lavidor et al. 2001; Lavidor & Ellis 2002). The interaction between length and visual field has also been reported in Hebrew (Babkoff et al. 1997; Lavidor et al. 2002), a script that runs from right to left rather than from left to right, implying that the relative immunity of the RVF to effects of length when processing familiar words in English is not a reflection of factors such as habitual left-to-right scanning patterns or attentional biases generated by a lifetime's experience of reading text in a particular direction.
Stronger effects of length on the recognition of words projected directly to the right hemisphere are hard to reconcile with the notion that length effects arise exclusively in the process of converting a processed string of letters into phonological forms. For that to be true, one would have to argue that more use is made of letter-sound conversion when words are projected to the right hemisphere than when they are projected to the left hemisphere. Such a suggestion would run contrary to evidence from neuropsychological and neuroimaging studies indicating that letter–sound conversion processes, like other processes involving phonological representations, are strongly lateralized to the left hemisphere (Lambon Ralph & Patterson 1999; Halderman & Chiarello 2005; Hickok & Poeppel 2007). Bub & Lewine (1988) made the alternative proposal that a stronger effect of word length in the LVF than in the RVF could arise if all word recognition is achieved within the left hemisphere, and if the speed of transfer of information from the right hemisphere to the left across the corpus callosum is affected by the number of letters in the word. Two findings call this callosal transfer account of LVF length effects into question. First, presenting familiar words in unfamiliar formats (e.g. in mixed case or vertically) has been reported to induce length effects in the RVF comparable to those seen in the LVF for words in normal lower or upper case formats (e.g. Bub & Lewine 1988; Ellis et al. 1988; Lavidor & Ellis 2001; Lavidor et al. 2002; Young & Ellis 1985). If distorting the format of words induces a significant length effect in the RVF, despite the fact that RVF words do not need to undergo callosal transfer in order to access left hemisphere recognition processes, that effect must arise within the left hemisphere, not in the process of inter-hemispheric transfer.
The second finding that casts doubt upon a callosal transfer explanation of the LVF length effect is that non-words, like abnormally formatted words, have been reported to show similar length effects in both visual fields (though the only studies of which we are aware have used accuracy of report following brief presentations as the dependent variable, rather than reaction times (RTs) for correct responses in tasks such as naming or lexical decision). Young & Ellis (1985) and Bruyer & Janlin (1989) found an overall RVF advantage for the accuracy of reporting briefly presented non-words, but equal effects of length in the LVF and RVF. Once again, non-words presented in the RVF have no need of callosal transfer yet show length effects equal to those induced by non-words presented in the LVF. The length effects in reading non-words, like the length effects in reading familiar words seen in unfamiliar formats, must arise independently within each hemisphere. It is reasonable to think that the requirement for callosal transfer of LVF stimuli plays a part in generating the overall RVF advantage observed for both words and non-words in a variety of tasks (Zaidel et al. 1990), but an alternative explanation is required for the difference in length effects for familiar words presented to the left and right hemispheres in normal formats. The explanation we will propose here is that strong length effects arise when the early stage of letter identification operates independently before transferring the products of identification on to later stages involved with recognizing (or resolving) whole letter strings, whether words or non-words. Length effects are reduced when letter and word levels enter into a state of mutual interactive activation, with top-down influences from lexical representations helping to resolve the component letters of known words. That mutual facilitation of processing can only occur effectively when familiar words are presented in familiar formats in the RVF, projecting directly to the left hemisphere.
2. Experiment 1
These issues are addressed here through two experiments in which right-handed participants, who will be assumed to be left hemisphere dominant for language (Hellige 1993; Knecht et al. 2000), learned initially unfamiliar non-words containing either four or six letters. Training took place in two sessions separated by one day, allowing time for consolidation of representations acquired during the first training session (cf. Gaskell & Dumay 2003; Dumay & Gaskell 2007; Leach & Samuel 2007). The training involved reading the non-words aloud both as isolated letter strings and embedded in sentences which gave clues as to possible meanings; and also copying the non-words in their own handwriting. Each non-word was encountered a total of 26 times in the two training sessions. Before the first session, and after the second session, the participants read the unfamiliar non-words aloud as quickly and as accurately as possible from brief LVF and RVF presentations. The same task was repeated at the end of the second session, allowing the influence of learning to be investigated by comparing responses to the non-words before and after training. We shall refer to these experimental items as ‘non-words’ throughout, even though participants became familiar with them during the training. We will refer to the non-words encountered before training as ‘unfamiliar’ or ‘unknown’ non-words and to the non-words encountered after training as ‘familiar’, learned’ or ‘known’ non-words.
The non-words were interspersed during the tests with familiar four- and six-letter words, which were also to be read aloud as quickly and as accurately as possible. The same familiar words were presented in test 1 (before training on the non-words) and test 2 (after training on the non-words), but were not presented between the two tests. The purpose of including known words was to allow a direct comparison between the reading of non-words (unfamiliar and learned) and known words. Based on the literature reviewed above, the familiar words were expected to show faster and more accurate naming in the RVF than in the LVF, with more of a difference between four- and six-letter words in the LVF than in the RVF. Responses to unfamiliar non-words in test 1 (before any training) were expected to show an overall RVF advantage, with faster and more accurate responses to shorter than to longer items in both visual fields. The question at issue was whether a relatively modest amount of training on the non-words could result in a change of the pattern of responses from that characteristic of unfamiliar non-words (slow and relatively inaccurate responses with a RVF advantage and similar length effects in both visual fields) to that characteristic of familiar words (faster and more accurate responses, again with a RVF advantage, but this time with a reduced length effect in the RVF compared with the LVF).
(a). Method
(i). Participants
Thirty native speakers of English (14 male, 16 female) aged 19–22 (mean age 20.8; s.d. = 0.8) took part in Experiment 1. All were students at the University of York, UK, and had normal or corrected-to-normal vision with no history of reading problems. The participants were all right-handed, scoring at least 80 on the Edinburgh Handedness Inventory (Oldfield 1971).
(ii). Materials
The experimental stimuli were 40 familiar English content words (nouns, verbs and adjectives; 20 four-letter and 20 six-letter) and 40 orthographically regular, pronounceable non-words (20 four-letter and 20 six-letter). The four- and six-letter word sets were matched on initial phonemes, familiarity and imageability values from the MRC Psycholinguistic Database (Coltheart 1981; http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm), CELEX combined spoken and written frequency (Baayen et al. 1993) and bigram frequency from WordGen (Duyck et al. 2004). Examples of the word stimuli are camp, town, wish, common, travel and window. The sets of four- and six-letter non-words were matched to each other and to the word sets on initial phonemes and bigram frequency. Examples are comy, purb, wose, cucame, menfal and wanfon. Details of the matching are shown in table 1. To reduce problems with voice key activation, none of the words or non-words began with a voiceless fricative (‘f’, ‘s’, ‘sh’ or ‘th’). Each word and non-word was presented once in the LVF and once in the RVF in both tests, so that each item acted as its own control as far as the effects of visual field, initial phoneme and other variables were concerned. An additional six words and six non-words were selected for use in practice.
Table 1.
Familiarity, word frequency and imageability values for the four- and six-letter words, with bigram frequency values for the four- and six-letter words and non-words. (Note: words and non-words cannot be matched on familiarity, word frequency or imageability.)
| words |
non-words |
|||
|---|---|---|---|---|
| four letters | six letters | four letters | six letters | |
| familiarity | ||||
| mean | 568 | 576 | ||
| s.d. | 24.5 | 37.6 | ||
| word frequency | ||||
| mean | 2.35 | 2.30 | ||
| s.d. | 0.29 | 0.23 | ||
| imageability | ||||
| mean | 444 | 481 | ||
| s.d. | 88.9 | 103.5 | ||
| bigram frequency | ||||
| mean | 1883 | 2163 | 1910 | 2015 |
| s.d. | 577 | 433 | 1062 | 617 |
(iii). Design and procedure
There were two sessions, both of which involved testing and training. The sessions were separated by one day (e.g. Monday–Wednesday or Tuesday–Thursday). Session 1 began with participants completing a consent form and a handedness questionnaire. Participants then performed the first naming task (test 1) involving presentation of four- and six-letter words and non-words in the LVF and RVF.
For the naming tests, participants sat at a distance of 57 cm from a computer screen on which the stimuli were presented. A chin rest was used to keep the head at a fixed distance from the screen. Each trial began with the presentation of a central white fixation cross on a black background for 500 ms. The fixation cross was replaced by a word or non-word, also in white, which was presented in the LVF or RVF for 180 ms. The stimuli were displayed in lower case using 18 point Fixedsys font, a font in which letters are positioned at fixed distance from each other, so that all stimuli containing the same number of letters have the same physical length on the screen. The four-letter words and non-words were 22 mm long on the screen, extending from 1.5° to 3.7° to the left or right of the central fixation point. The six-letter words and non-words were 33 mm long, extending from 1.5° to 4.8° the left or right of the fixation point. At the offset of the word or non-word, the fixation cross reappeared on the screen for 820 ms, followed by a blank screen for 1500 ms.
Pilot work had indicated that the accuracy of reading unfamiliar non-words could be improved and brought within acceptable levels for the analysis of naming latencies if participants repeated each non-word once before the start of the experiment. Each of the experimental and practice words and non-words was therefore spoken by the experimenter and repeated by the participant at the beginning of the test phase in each session. If an item was repeated incorrectly, it was presented again. Twenty-four practice trials were then presented, involving six practice words (three × four-letter and three × six-letter) and six practice non-words (three × four-letter and three × six-letter) presented once each in the LVF and the RVF. The experimental words and non-words were then presented in a sequence of 160 trials. In the first 80 trials, each of the 40 words and 40 non-words was presented once, with half the items in each set being displayed in the LVF and half in the RVF. The words and non-words were presented again in the second 80 trials, which continued without a break. Items which appeared in the LVF in the first 80 trials were displayed in the RVF in the second 80 trials, and conversely. The presentation order was pseudo-random with the requirement that there were never more than three LVF or RVF trials in a row. Three different orderings of the stimuli were employed across the 30 participants. Instructions emphasized the importance of fixating carefully and maintaining fixation during presentation and the need to read the words and non-words aloud as quickly and as accurately as possible. Stimulus presentation and the collection of vocal RTs (via a voice key connected to a microphone attached to a head set) was controlled using E-Prime experiment generator software (Schneider et al. 2002). The experimenter noted on each trial whether the stimulus had been read correctly or not.
Following the first naming test, session 1 continued after a short break with training designed to familiarize the participants with the non-words. Four different training tasks were used. Participants began by copying each of the non-words once. For this exercise, the non-words were printed in three columns on a sheet of A4 paper using lower-case lettering in 14-point Courier new font. Participants copied each non-word alongside the original in their own handwriting. In the next exercise, the 40 experimental non-words were presented together on the screen in an array of eight rows and five columns. Participants read each non-word aloud. This was done six times using six different arrays. For the next familiarization task, each non-word was incorporated into a short sentence, with each non-word occupying a noun slot (e.g. The comy only eats fish; He picked the menfal off the shelf). The sentences were presented one at a time on the computer screen to be read aloud by the participant. The sentences were then presented again in a different order. Next the non-words were presented individually on the computer screen to be read aloud. This was done twice. The participants then copied the non-words twice more before reading the 40 sentences again once. All the computer-presented tasks involved non-words (and words for the sentences) being presented in white lower-case lettering on a black background. Each training task was self-paced by the participants. The training in session 1 lasted approximately 25 min in total. Each non-word was experienced 14 times in this first training session (copied three times, read aloud six times in arrays and twice as individual items, and read aloud three times in sentence contexts).
Participants returned for the second session after an interval of one day. The second session began with a slightly reduced version of the training procedure lasting approximately 15 min. There was one presentation of the copying task, then three arrays of non-words to be read aloud, one presentation of the sentences for reading aloud and one presentation of the task involving reading individual non-words off the screen. This was then repeated, with a further presentation of the copying task, three more arrays, one more set of sentences and one more presentation of the individual non-words for reading aloud. Each non-word was therefore experienced 12 times in training at the start of the second session (copied twice, read aloud six times in arrays and twice as individual items and read aloud twice in sentence contexts). By the end of the second training session, each non-word had been encountered a total of 26 times in training, in addition to having been repeated once before test 1 and seen twice in the course of test 1, once in the LVF and once in the RVF.
After a short break, participants completed the second session by repeating the experimental task of reading aloud the words and the non-words presented in the LVF or RVF (test 2). The procedure was the same as test 1 except that only 12 practice trials were given to re-familiarize participants with the task. Each participant received a different ordering of the trials in test 2 from the one they had received in test 1. Note that the words that accompanied the non-words in the naming tasks were not trained between the tests.
(b). Results
Only RTs for correct responses were analysed. Trials with RTs shorter than 200 ms or longer than 1200 ms were regarded as outliers and removed from the analyses of RTs. The means for the trimmed RTs and the error rates are shown in table 2. Initial analyses were performed separately on words (which were not trained between tests) and non-words. The full set of analyses of variance (ANOVAs) performed on the data for Experiment 1 are shown in table 3.
Table 2.
Mean RTs (with s.d.) and per cent errors for four- and six-letter words and non-words presented in the LVF and RVF in test 1 (before training on the non-words) and test 2 (after training on the non-words) in Experiment 1.
| LVF |
RVF |
|||
|---|---|---|---|---|
| four letters | six letters | four letters | six letters | |
| test 1 | ||||
| words | ||||
| mean RT | 534 | 569 | 503 | 519 |
| s.d. | 67.2 | 71.1 | 75.5 | 68.6 |
| % error | 3.2 | 5.3 | 2.8 | 2.8 |
| non-words | ||||
| mean RT | 591 | 673 | 569 | 640 |
| s.d. | 87.0 | 104.8 | 87.3 | 112.0 |
| % error | 9.2 | 24.5 | 7.2 | 20.8 |
| test 2 | ||||
| words | ||||
| mean RT | 495 | 522 | 472 | 486 |
| s.d. | 72.8 | 69.1 | 65.8 | 70.4 |
| % error | 1.7 | 3.0 | 2.2 | 1.3 |
| non-words | ||||
| mean RT | 505 | 552 | 493 | 499 |
| s.d. | 66.2 | 76.2 | 71.3 | 12.6 |
| % error | 2.2 | 4.7 | 1.3 | 2.3 |
Table 3.
Results of the ANOVAs for Experiment 1. m.s.e. = mean squared error, η2 = partial eta-squared (effect size).
| responses to words | |
| RTs (naming latencies) to words | |
| test 1: RTs to words before training on the non-words | |
| visual field (LVF versus RVF) | F1,29 = 95.45, m.s.e. = 516, p < 0.001, η2 = 0.767 |
| length (four versus six letters) | F1,29 = 33.99, m.s.e. = 599, p < 0.001, η2 = 0.540 |
| visual field × length | F1,29 = 6.27, m.s.e. = 449, p = 0.018, η2 = 0.178 |
| test 2: RTs to words after training on the non-words | |
| visual field | F1,29 = 40.68, m.s.e. = 640, p < 0.001, η2 = 0.584 |
| length | F1,29 = 36.57, m.s.e. = 355, p < 0.001, η2 = 0.558 |
| visual field × length | F1,29 = 4.82, m.s.e. = 272, p = 0.036, η2 = 0.142 |
| combined analysis of tests 1 and 2. RTs to words before and after training on the non-words | |
| test (1 versus 2) | F1,29 = 31.97, m.s.e. = 2668, p < 0.001, η2 = 0.524 |
| visual field | F1,29 = 92.74, m.s.e. = 792, p < 0.001, η2 = 0.762 |
| length | F1,29 = 73.93, m.s.e. = 445, p < 0.001, η2 = 0.718 |
| test × visual field | F1,29 = 5.05, m.s.e. = 364, p = 0.032, η2 = 0.148 |
| test × length | F1,29 = 0.82, m.s.e. = 508, p = 0.374, η2 = 0.027 |
| visual field × length | F1,29 = 12.96, m.s.e. = 307, p < 0.01, η2 = 0.309 |
| test × visual field × length | F1,29 = 0.35, m.s.e. = 413, p = 0.561, η2 = 0.012 |
| errors to words | |
| test 1: errors to words before training on the non-words | |
| visual field | F1,29 = 3.13, m.s.e. = 0.77, p = 0.088, η2 = 0.097 |
| length | F1,29 = 1.26, m.s.e. = 1.12, p = 0.270, η2 = 0.042 |
| visual field × length | F1,29 = 1.83, m.s.e. = 0.77, p = .187, η2 = 0.059 |
| test 2: errors to words after training on the non-words | |
| visual field | F1,29 = 0.41, m.s.e. = 0.99, p = 0.527, η2 = 0.003 |
| length | F1,29 = 0.09, m.s.e. = 0.80, p = 0.762, η2 = 0.003 |
| visual field × length | F1,29 = 1.42, m.s.e. = 0.99, p = 0.244, η2 = 0.047 |
| combined analysis of tests 1 and 2. Errors to words before and after training on the non-words | |
| test (1 versus 2) | F1,29 = 3.16, m.s.e. = 1.71, p = 0.086, η2 = 0.098 |
| visual field | F1,29 = 2.31, m.s.e. = 1.04, p = 0.139, η2 = 0.074 |
| length | F1,29 = 1.12, m.s.e. = 0.96, p = 0.301, η2 = 0.037 |
| test × visual field | F1,29 = 0.57, m.s.e. = 0.73, p = 0.455, η2 = 0.019 |
| test × length | F1,29 = 0.44, m.s.e. = 0.95, p = 0.513, η2 = 0.015 |
| visual field × length | F1,29 = 3.60, m.s.e. = 0.78, p = 0.068, η2 = 0.110 |
| test × visual field × length | F1,29 = 0.00, m.s.e. = 0.98, p = 1.00, η2 = 0.000 |
| responses to non-words | |
| RTs (naming latencies) to non-words | |
| test 1: RTs to non-words before training on the non-words | |
| visual field (LVF versus RVF) | F1,29 = 36.71, m.s.e. = 637, p < 0.001, η2 = 0.559 |
| length (four versus six letters) | F1,29 = 117.63, m.s.e. = 1477, p < 0.001, η2 = 0.802 |
| visual field × length | F1,29 = 2.25, m.s.e. = 436, p = 0.144, η2 = 0.072 |
| test 2: RTs to non-words after training on the non-words | |
| visual field | F1,29 = 56.41, m.s.e. = 561, p < 0.001, η2 = 0.660 |
| length | F1,29 = 39.40, m.s.e. = 536, p < 0.001, η2 = 0.576 |
| visual field × length | F1,29 = 38.60, m.s.e. = 328, p < 0.001, η2 = 0.571 |
| combined analysis of tests 1 and 2. RTs to non-words before and after training on the non-words | |
| test (1 versus 2) | F1,29 = 100.40, m.s.e. = 6674, p < 0.001, η2 = 0.776 |
| visual field | F1,29 = 65.03, m.s.e. = 842, p < 0.001, η2 = 0.692 |
| length | F1,29 = 109.38, m.s.e. = 1145, p < 0.001, η2 = 0.790 |
| test × visual field | F1,29 = 0.87, m.s.e. = 357, p = 0.358, η2 = 0.029 |
| test × length | F1,29 = 64.78, m.s.e. = 568, p < 0.001, η2 = 691 |
| visual field × length | F1,29 = 25.67, m.s.e. = 402, p < 0.001, η2 = 0.470 |
| test × visual field × length | F1,29 = 9.11, m.s.e. = 361, p < 0.01, η2 = 0.239 |
| errors to non-words | |
| test 1: errors to non-words before training on the non-words | |
| visual field | F1,29 = 3.46, m.s.e. = 2.79, p = 0.073, η2 = 0.106 |
| length | F1,29 = 57.98, m.s.e. = 4.35, p < 0.001, η2 = 0.667 |
| visual field × length | F1,29 = 0.46, m.s.e. = 1.82, p = 0.506, η2 = 0.016 |
| test 2: errors to non-words after training on the non-words | |
| visual field | F1,29 = 3.53, m.s.e. = 0.85, p = 0.070, η2 = 0.108 |
| length | F1,29 = 5.31, m.s.e. = 0.69, p = 0.029, η2 = 0.155 |
| visual field × length | F1,29 = 0.70, m.s.e. = 0.97, p = 0.411, η2 = 0.023 |
| combined analysis of tests 1 and 2. Errors to non-words before and after training on the non-words | |
| test (1 versus 2) | F1,29 = 52.32, m.s.e. = 7.51, p < 0.001, η2 = 0.643 |
| visual field | F1,29 = 5.89, m.s.e. = 1.99, p = 0.022, η2 = 0.169 |
| length | F1,29 = 47.89, m.s.e. = 3.31, p < 0.001, η2 = 0.623 |
| test × visual field | F1,29 = 0.57, m.s.e. = 1.65, p = .457, η2 = .019 |
| test × length | F1,29 = 56.19, m.s.e. = 1.74, p < 0.001, η2 = 0.660 |
| visual field × length | F1,29 = 0.83, m.s.e. = 1.81, p = 0.369, η2 = 0.028 |
| test × visual field × length | F1,29 = 0.004, m.s.e. = 0.98, p = 0.948, η2 = 0.000 |
(i). Reaction times to words
Separate ANOVAs were conducted on RTs for correct trimmed responses to words in tests 1 and 2 using factors of visual field (LVF versus RVF) and length (four versus six letters). In both tests there were significant main effects of visual field (faster overall responses in the RVF than in the LVF) and length (faster overall responses to four- than six-letter words) (table 3). Twenty-nine of the 30 participants showed an overall RVF advantage for word naming RTs in test 1.
Tests 1 and 2 showed significant interactions between visual field and length in naming RTs as a result of smaller effects of word length in the RVF than in the LVF. Bonferroni-corrected t-tests (α = 0.05) found that in test 1, the 35 ms difference between four- and six-letter words in the LVF was significant, t(29) = 8.36, while the 16 ms difference in the LVF was only marginally significant, t(29) = 2.28. There were significant RVF advantages for both four-letter, t(29) = 5.66, and six-letter words, t(29) = 8.53. In test 2, the 27 ms difference in RTs to four- and six-letter words in the LVF and the 14 ms effect in the RVF were both significant, LVF: t(29) = 6.16; RVF: t(29) = 3.03. There were again significant RVF advantages for both four-letter, t(29) = 3.72, and six-letter words, t(29) = 7.51.
The RT data for tests 1 and 2 were combined in an overall ANOVA with factors of test (1 versus 2), visual field and length. The overall main effects of visual field and length across the two tests were significant. There was also a significant main effect of test, with faster responses in test 2 than test 1, despite the fact that the words were not presented in training between the two tests, with grand means of 531 ms in test 1 and 494 ms in test 2. The interaction between test and visual field was significant, reflecting more speeding up between tests 1 and 2 of RTs to words in the LVF (difference = 43 ms) than in the RVF (difference = 32 ms), and more of an RVF advantage in test 1 (difference = 41 ms) than in test 2 (difference = 30 ms). Bonferroni-corrected t-tests (α = 0.05) found, however, that the differences between tests 1 and 2 were significant in both visual fields, LVF: t(29) = 5.75; RVF: t(29) = 4.73, and that there were significant RVF advantages in both tests, test 1: t(29) = 9.78; test 2: t(29) = 6.38.
The interaction between visual field and length was significant in the combined analysis. Importantly, that interaction was not modified by test (p = 0.561 for the three-way interaction between test, visual field and length). That is, although RTs to words were faster in test 2 than test 1, the form of the interaction between length and visual field did not change significantly across the two tests. As we have seen, the effect of length on RTs to words was greater in the LVF than the RVF in both tests.
(ii). Errors to words
Analysis of the errors to words in test 1 was performed using the same factors of visual field and length. The results need to be interpreted in the context of the generally low error rates for words (table 2) and the consequent issue of ceiling effects. In test 1, the main effect of visual field was marginally significant, reflecting a tendency for participants to make more errors in the LVF than in the RVF. The main effect of length and the visual field × length interaction were not significant. No effects were significant in the analysis of the low error rates for words in test 2.
A combined analysis of errors to words in tests 1 and 2 found a marginally significant effect of test, reflecting a tendency towards fewer errors in test 2 than in test 1. There was also a marginally significant interaction between visual field and length, reflecting a tendency towards more of a difference in errors to four- and six-letter words in the LVF than in the RVF across the two tests. No other effects approached significance.
(iii). Reaction times to non-words
Analysis of naming RTs to non-words encountered for the first time in test 1 found significant main effects of visual field (faster RVF than LVF responses) and length (faster responses to four- than six-letter non-words). Unlike the words in test 1, naming RTs for the unfamiliar non-words did not show a significant visual field × length interaction (p = 0.144). The effect of length was 82 ms in the LVF and 71 ms in the RVF.
In test 2, the main effects of visual field and length on naming learned non-words were also significant, but this time those factors combined in a highly significant interaction with a large effect size (p < 0.001, η2 = 0.571). Bonferroni-corrected t-tests (α = 0.05) found that the 47 ms difference between four- and six-letter learned non-words in the LVF was significant, t(29) = 7.41, but the difference of just 6 ms in the RVF was not, t(29) = 1.32.
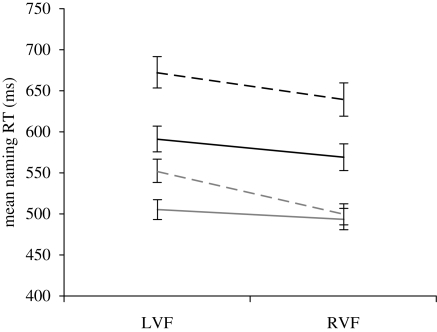
RTs to non-words in tests 1 and 2 were combined in a single analysis with test, visual field and length as factors. The main effect of test was significant, with faster overall responses in test 2 (grand mean = 513 ms) than test 1 (grand mean = 618 ms). There was a significant main effect of visual field, with faster overall responses in the RVF than in the LVF, and a significant main effect of length, with faster overall responses to four-letter than six-letter non-words. The two-way interactions between length and test, and length and visual field, were both significant. Those interactions were subsumed within a significant three-way interaction involving test, visual field and length. Figure 1 illustrates how, with training, the pattern of results for non-word naming RTs changed from one characterized by an overall RVF advantage with similar effects of length in both visual fields (test 1) to a pattern in which the difference between shorter and longer items was reduced in the RVF compared with the LVF (test 2).
Figure 1.
Mean naming latencies to four- and six-letter non-words in test 1 (before training) and in test 2 (after training) in Experiment 1. Black line, test 1 four-letter non-words; black dashed line, test 1 six-letter non-words; grey line, test 2 four-letter non-words; grey dashed line, test 2 six-letter non-words. Error bars show s.e. values.
(iv). Errors to non-words
Analysis of the errors to non-words in test 1 found a marginal effect of visual field, reflecting a tendency towards more errors in the LVF than in the RVF, and a highly significant effect of length, with more errors to longer than to shorter non-words. The interaction between visual field and length was not significant.
Errors rates to learned non-words in test 2 showed a marginally significant effect of visual field, with a tendency towards more errors in the LVF than the RVF, and a significant effect of length, with more errors to longer than to shorter non-words. The interaction between visual field and length was not significant.
A combined analysis of errors to non-words in tests 1 and 2 found significant main effects of test (fewer errors in test 2 than test 1), visual field (fewer errors in the RVF than the LVF) and length (fewer errors to four- than to six-letter non-words). The only significant interaction was between test and length, with more of an effect of length on the accuracy of naming unfamiliar non-words in test 1 than learned non-words in test 2. The separate analyses of tests 1 and 2 reported above showed, however, that the effect of length was significant in both tests.
(c). Discussion
The implications of the results of Experiment 1 will be discussed in detail after Experiment 2. For now, we would note the following features of the results:
Familiar words were included in tests 1 and 2, but not trained between those tests. Error rates to the words were generally low, and while some marginal trends were observed (e.g. towards a RVF advantage in test 1 and a reduction in errors between tests 1 and 2), no significant effects were observed. In the context of the low error rates, naming RTs to words showed the expected overall RVF advantage, with 29 of the 30 participants showing RVF advantages in test 1. That is consistent with the claim that the majority of right-handed people are left hemisphere language dominant and that such dominance is associated with RVF advantages in naming tasks (cf. Hunter et al. 2007; Knecht et al. 2000).
Word naming RTs were generally faster to four- than to six-letter words. In keeping with many other reports in the literature (Ellis 2004), the effect of length was greater in the RVF than in the LVF, resulting in significant visual field × length interactions. The post hoc analyses found significant effects of length in the LVF in both test 1 (a 35 ms effect) and test 2 (a 27 ms effect). Though reduced, the effect of length in the RVF was marginally significant in test 1 (16 ms) and significant in test 2 (14 ms).
Words were named more quickly and more accurately in test 2 than in test 1, despite not being trained between the two tests, a result which can be attributed to long-term repetition priming across the two presentations (cf. Scarborough et al. 1977; Forster & Davis 1984; Stark & McClelland 2000; Weems & Zaidel 2005). The interaction of length with visual field was not affected by the general speeding up of word naming RTs between tests 1 and 2.
Responses to unfamiliar non-words in test 1 showed an RVF advantage for both accuracy and RT. Both measures found similar effects of length in the two visual fields for unfamiliar non-words. The results for accuracy of report are commensurate with the findings of Young & Ellis (1985) and Bruyer & Janlin (1989). As far as we are aware, there have been no previous studies of the effects of length on naming speed for unfamiliar, lateralized non-words.
Responses to learned non-words in test 2 were significantly faster and more accurate than responses to the same (unlearned) items in test 1. Inspection of table 2 shows that 26 exposures to the non-words across two sessions was enough to reduce their naming RTs to a level similar to that for familiar words, such that the mean naming RT for learned non-words in test 2 was actually 19 ms faster than the mean naming RT for familiar words seen for the first time in test 1. Feustel et al. (1983) and Salasoo et al. (1985) likewise reported that relatively modest amounts of training on unfamiliar non-words can reduce their processing speeds to levels comparable to those normally observed for familiar words (at least temporarily).
Importantly, the pattern of naming RTs to non-words changed as a result of training from one characteristic of unfamiliar non-words (comparable length effects in the two visual fields) to one similar to the pattern observed for familiar words (greater effect of length in the LVF than in the RVF). Thus, in test 1, the effect of length on naming RTs for unfamiliar non-words was not significantly modulated by visual field (82 ms in the LVF and 71 ms in the RVF), while in test 2, the effect of length on the naming of learned non-words was significant in the LVF (47 ms) but not in the RVF (6 ms). Training on the non-words induced parallelized processing in the RVF while processing in the LVF retained a more serial (length-sensitive) character.
Experiment 1 therefore achieved its goals of transforming unfamiliar non-words into stimuli which behaved like familiar written words, both in terms of general naming speed and accuracy, and in terms of the more parallelized responding to items presented directly to the left hemisphere compared with items presented initially to the right hemisphere.
3. Experiment 2
In Experiment 1, both training and testing occurred in a familiar, lower-case format. We noted in §1 that moving from familiar to unfamiliar formats (e.g. MiXeD cAsE) has been reported to induce similar length effects for words in the LVF and RVF (Lavidor & Ellis 2001; Lavidor et al. 2002). Such a finding could imply that parallelized processing of familiar words in the RVF depends on their being presented in formats that the left hemisphere is accustomed to processing (Ellis 2004; Cohen et al. 2008). Experiment 2 explored the consequences of training non-words in lower case then testing their recognition in both lower-case and mixed-case formats. Experiment 2 used the same stimuli as Experiment 1 and followed the same procedure as far as the end of session 2 (i.e. participants were tested on recognition of lower-case words and unfamiliar non-words at the start of session 1 (test 1) then trained on the non-words in the remainder of session 1 and the start of session 2). After the training in session 2, the words and the trained non-words were tested twice, once using lower-case presentation (test 2A) and once using mixed-case presentation (test 2B). The order of tests 2A and 2B was counterbalanced across participants.
The first aim of Experiment 2 was to replicate the findings of Experiment 1 through a comparison of performance on lower-case words and non-words in tests 1 and 2A. The second aim was to discover whether signs of parallelized processing in the left hemisphere for learned non-words would be reduced by a switch to mixed-case format (test 2B), as has been reported to occur for familiar words.
(a). Method
(i). Participants
A new group of 28 right-handed native speakers of English (12 males and 16 females) aged 19–22 acted as participants in Experiment 2. All were students at the University of York, UK, and had normal or corrected-to-normal vision with no history of reading problems.
(ii). Materials
The 40 word and 40 non-word stimuli were the same as in Experiment 1. Case-alternated forms of each item were created for use in test 2B in session 2. Half the mixed-case words and non-words of each length began with a lower-case letter (e.g. cAmP and wAnFoN) and half with an upper-case letter (e.g. PuRb and WiNdOw).
(iii). Design and procedure
The design was the same as for Experiment 1, except that after initial training in the second session, each participant was given two tests, one with the stimuli in lower-case (normal) format (2A) and one with the stimuli in case-alternated format (2B). The order in which tests 2A and 2B were administered was counterbalanced across participants, with a short break between tests. As in Experiment 1, three different orders of presentation of the items were used within each test. Each test began with 12 practice stimuli presented in the appropriate format for that test.
(b). Results
Only RTs for correct responses were analysed. As in Experiment 1, trials with RTs shorter than 200 ms or longer than 1200 ms were regarded as outliers and removed from the analyses of RTs. The means for the trimmed RTs and the error rates are shown in table 4. The results of all statistical analyses (ANOVAs) are shown in table 5.
Table 4.
Mean RTs (with s.d.) and per cent errors for four- and six-letter words and non-words presented in the LVF and RVF in lower case before training on the non-words (test 1), in lower case after training on the non-words (test 2A) and in mixed case after training on the non-words (test 2B) in Experiment 2.
| LVF |
RVF |
|||
|---|---|---|---|---|
| four letters | six letters | four letters | six letters | |
| test 1: lower-case presentation before training on the non-words | ||||
| words | ||||
| mean RT | 550 | 585 | 530 | 543 |
| s.d. | 57.2 | 68.2 | 62.4 | 64.5 |
| % error | 3.2 | 3.8 | 2.5 | 2.2 |
| non-words | ||||
| mean RT | 606 | 702 | 582 | 656 |
| s.d. | 80.6 | 99.9 | 78.8 | 104.5 |
| % error | 6.8 | 21.9 | 4.5 | 16.9 |
| test 2A: lower-case presentation after training on the non-words | ||||
| words | ||||
| mean RT | 528 | 558 | 503 | 512 |
| s.d. | 61.9 | 72.1 | 67.1 | 73.4 |
| % error | 2.7 | 3.1 | 3.9 | 3.2 |
| non-words | ||||
| mean RT | 536 | 589 | 524 | 534 |
| s.d. | 62.1 | 70.5 | 61.8 | 68.2 |
| % error | 3.4 | 4.8 | 2.9 | 3.4 |
| test 2B: mixed-case presentation after training on the non-words | ||||
| words | ||||
| mean RT | 564 | 601 | 555 | 566 |
| s.d. | 73.8 | 81.5 | 63.2 | 85.7 |
| % error | 5.2 | 6.1 | 5.4 | 4.8 |
| non-words | ||||
| mean RT | 588 | 631 | 550 | 610 |
| s.d. | 68.4 | 71.8 | 60.2 | 87.5 |
| % error | 5.6 | 7.3 | 4.0 | 7.2 |
Table 5.
Results of the ANOVAs for Experiment 2. m.s.e. = mean squared error, η2 = partial eta squared (effect size).
| responses to words | |
| RTs (naming latencies) to words | |
| test 1: RTs to words before training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 44.15, m.s.e. = 580, p < 0.001, η2 = 0.621 |
| length (four versus six letters) | F1,27 = 33.72, m.s.e. = 474, p < 0.001, η2 = 0.555 |
| visual field × length | F1,27 = 6.22, m.s.e. = 533, p = 0.019, η2 = 0.187 |
| test 2A: RTs to lower case words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 40.94, m.s.e. = 869, p < 0.001, η2 = 0.603 |
| length (four versus six letters) | F1,27 = 14.77, m.s.e. = 678, p < 0.001, η2 = 0.354 |
| visual field × length | F1,27 = 5.90, m.s.e. = 500, p = .022, η2 = .179 |
| test 2B: RTs to mixed-case words after training on the non-words | |
| visual field | F1,27 = 10.00, m.s.e. = 1396, p = 0.004, η2 = 0.270 |
| length | F1,27 = 11.66, m.s.e. = 1383, p = 0.002, η2 = 0.302 |
| visual field × length | F1,27 = 7.59, m.s.e. = 621, p = 0.01, η2 = 0.219 |
| combined analysis of tests 1 and 2A. RTs to words before and after training on the non-words (lower-case presentation in both tests) | |
| Test | F1,27 = 13.16, m.s.e. = 3077, p < 0.01, η2 = 0.328 |
| visual field | F1,27 = 60.67, m.s.e. = 1002, p < 0.001, η2 = 0.692 |
| length | F1,27 = 32.56, m.s.e. = 788, p < 0.001, η2 = 0.547 |
| test × visual field | F1,27 = 0.91, m.s.e. = 447, p = 0.348, η2 = 0.033 |
| test × length | F1,27 = 0.96, m.s.e. = 364, p = 0.336, η2 = 0.034 |
| visual field × length | F1,27 = 11.29, m.s.e. = 554, p = 0.002, η2 = 0.295 |
| test × visual field × length | F1,27 = 0.01, m.s.e. = 479, p = 0.918, η2 = 0.000 |
| combined analysis of tests 2A and 2B. RTs to words after training on the non-words (lower-case presentation in test 2A, mixed case in test 2B) | |
| order (test 2A first or second) | F1,26 = 0.25, m.s.e. = 4574, p = 0.624, η2 = 0.009 |
| test (2A versus 2B) | F1,26 = 124.58, m.s.e. = 973, p < 0.001, η2 = 0.827 |
| visual field | F1,26 = 29.17, m.s.e. = 1614, p < 0.001, η2 = 0.529 |
| length | F1,26 = 16.19, m.s.e. = 1593, p < 0.001, η2 = 0.384 |
| order × test | F1,26 = 10.98, m.s.e. = 973, p = 0.003, η2 = 0.297 |
| order × visual field | F1,26 = 1.48, m.s.e. = 1614, p = 0.236, η2 = 0.054 |
| order × length | F1,26 = 0.18, m.s.e. = 1593, p = 0.787, η2 = 0.003 |
| test × visual field | F1,26 = 3.97, m.s.e. = 626, p = 0.057, η2 = 0.132 |
| test × length | F1,26 = 0.74, m.s.e. = 493, p = 0.398, η2 = 0.028 |
| visual field × length | F1,26 = 11.96, m.s.e. = 631, p = 0.002, η2 = 0.315 |
| order × test versus visual field | F1,26 = 0.92, m.s.e. = 626, p = .347, η2 = .034 |
| order × test × length | F1,26 = 2.66, m.s.e. = 493, p = 0.115, η2 = 0.093 |
| order × visual field × length | F1,26 = 0.19, m.s.e. = 631, p = 0.666, η2 = 0.007 |
| test × visual field × length | F1,26 = 0.20, m.s.e. = 521, p = 0.661, η2 = 0.007 |
| order versus test × visual field × length | F1,26 = 0.33, m.s.e. = 521, p = 0.571, η2 = 0.013 |
| errors to words | |
| test 1: errors to words before training on the non-words | |
| visual field | F1,27 = 4.18, m.s.e. = 0.36, p = 0.051, η2 = 0.134 |
| length | F1,27 = 0.01, m.s.e. = 1.05, p = 0.927, η2 = 0.000 |
| visual field × length | F1,27 = 0.46, m.s.e. = 0.48, p = 0.502, η2 = 0.017 |
| test 2A: errors to lower-case words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 1.00, m.s.e. = 0.57, p = 0.326, η2 = 0.036 |
| length (four versus six letters) | F1,27 = 0.07, m.s.e. = 0.52, p = 0.795, η2 = 0.003 |
| visual field × length | F1,27 = 0.59, m.s.e. = 0.54, p = 0.449, η2 = 0.021 |
| test 2B: errors to mixed-case words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 0.24, m.s.e. = 1.32, p = 0.626, η2 = 0.009 |
| length (four versus six letters) | F1,27 = 0.06, m.s.e. = 0.55, p = 0.802, η2 = 0.002 |
| visual field × length | F1,27 = 0.63, m.s.e. = 0.91, p = 0.434, η2 = 0.023 |
| combined analysis of tests 1 and 2A. Errors to words before and after training on the non-words (lower-case presentation in both tests) | |
| test | F1,27 = 0.14, m.s.e. = 1.54, p = 0.709, η2 = 0.005 |
| visual field | F1,27 = 0.23, m.s.e. = 0.49, p = 0.637, η2 = 0.008 |
| length | F1,27 = 0.01, m.s.e. = 0.79, p = 0.941, η2 = 0.000 |
| test × visual field | F1,27 = 4.47, m.s.e. = 0.44, p = 0.044, η2 = 0.142 |
| test × length | F1,27 = 0.05, m.s.e. = 0.77, p = 0.821, η2 = 0.002 |
| visual field × length | F1,27 = 0.92, m.s.e. = 0.59, p = 0.346, η2 = 0.033 |
| test × visual field × length | F1,27 = 0.01, m.s.e. = 0.44, p = 0.920, η2 = 0.000 |
| combined analysis of tests 2A and 2B. Errors to words after training on the non-words (lower-case presentation in test 2A, mixed case in test 2B) | |
| order | F1,26 = 3.80, m.s.e. = 5.43, p = 0.062, η2 = 0.127 |
| test | F1,26 = 5.54, m.s.e. = 1,89, p = 0.028, η2 = 0.173 |
| visual field | F1,26 = 0.03, m.s.e. = 0.69, p = 0.873, η2 = 0.001 |
| length | F1,26 = 0.00, m.s.e. = 0.55, p = 1.00, η2 = 0.000 |
| order × test | F1,26 = 0.61, m.s.e. = 1.89, p = 0.443, η2 = 0.023 |
| order × visual field | F1,26 = 3.15, m.s.e. = 0.69, p = 0.088, η2 = 0.108 |
| order × length | F1,26 = 3.27, m.s.e. = 0.55, p = 0.082, η2 = 0.112 |
| test × visual field | F1,26 = 0.77, m.s.e. = 1.14, p = 0.389, η2 = 0.029 |
| test × length | F1,26 = 0.15, m.s.e. = 0.47, p = 0.701, η2 = 0.006 |
| visual field × length | F1,26 = 0.97, m.s.e. = 0.90, p = 0.334, η2 = 0.036 |
| order × test versus visual field | F1,26 = 1.27, m.s.e. = 1.14, p = 0.271, η2 = 0.046 |
| order × test × length | F1,26 = 1.36, m.s.e. = 0.47, p = 0.254, η2 = 0.050 |
| order × visual field × length | F1,26 = 0.18, m.s.e. = 0.90, p = 0.676, η2 = 0.007 |
| test × visual field × length | F1,26 = 0.30, m.s.e. = 0.59, p = 0.863, η2 = 0.001 |
| order versus test × visual field × length | F1,26 = 0.27, m.s.e. = 0.59, p = 0.606, η2 = 0.010 |
| responses to non-words | |
| RTs (naming latencies) to non-words | |
| test 1: RTs to non-words before training on the non-words | |
| visual field | F1,27 = 33.25, m.s.e. = 1057, p < 0.001, η2 = 0.552 |
| length | F1,27 = 108.4, m.s.e. = 1853, p < 0.001, η2 = 0.801 |
| visual field × length | F1,27 = 2.97, m.s.e. = 1040, p = 0.096, η2 = 0.099 |
| test 2A: RTs to lower-case non-words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 36.27, m.s.e. = 863, p < 0.001, η2 = 0.573 |
| length (four versus six letters) | F1,27 = 53.74, m.s.e. = 521, p < 0.001, η2 = 0.666 |
| visual field × length | F1,27 = 23.91, m.s.e. = 534, p < 0.001, η2 = 0.470 |
| test 2B: RTs to mixed-case non-words after training on the non-words | |
| visual field | F1,27 = 22.13, m.s.e. = 1049, p < 0.001, η2 = 0.450 |
| length | F1,27 = 62.58, m.s.e. = 1190, p < 0.001, η2 = 0.699 |
| visual field × length | F1,27 = 2.38, m.s.e. = 879, p = 0.134, η2 = 0.081 |
| combined analysis of tests 1 and 2A. RTs to non-words before and after training on the non-words (lower-case presentation in both tests) | |
| test | F1,27 = 66.59, m.s.e. = 6858, p < 0.001, η2 = 0.712 |
| visual field | F1,27 = 49.94, m.s.e. = 1329, p < 0.001, η2 = 0.649 |
| length | F1,27 = 107.7, m.s.e. = 1759, p < 0.001, η2 = 0.800 |
| test × visual field | F1,27 = 0.09, m.s.e. = 590, p = 0.761, η2 = 0.003 |
| test × length | F1,27 = 64.20, m.s.e. = 615, p < 0.001, η2 = 0.704 |
| visual field × length | F1,27 = 14.36, m.s.e. = 990, p < 0.001, η2 = 0.347 |
| test × visual field × length | F1,27 = 2.82, m.s.e. = 584, p = 0.104, η2 = 0.095 |
| combined analysis of tests 2A and 2B. RTs to non-words after training on the non-words (lower-case presentation in test 2A, mixed case in test 2B) | |
| order | F1,27 = 0.67, m.s.e. = 4071, p = 0.420, η2 = 0.025 |
| test | F1,27 = 122.18, m.s.e. = 1085, p < 0.001, η2 = 0.825 |
| visual field | F1,27 = 43.14, m.s.e. = 1256, p < 0.001, η2 = 0.624 |
| length | F1,27 = 69.46, m.s.e. = 1395, p < 0.001, η2 = 0.728 |
| order × test | F1,27 = 4.01, m.s.e. = 1085, p = 0.056, η2 = 0.134 |
| order × visual field | F1,27 = 1.52, m.s.e. = 1256, p = 0.229, η2 = 0.055 |
| order × length | F1,27 = 0.90, m.s.e. = 1395, p = 0.351, η2 = 0.034 |
| test × visual field | F1,27 = 0.47, m.s.e. = 640, p = 0.498, η2 = 0.018 |
| test × length | F1,27 = 17.48, m.s.e. = 319, p < 0.001, η2 = 0.402 |
| visual field × length | F1,27 = 3.00, m.s.e. = 754, p = 0.095, η2 = 0.103 |
| order × test versus visual field | F1,27 = 0.62, m.s.e. = 640, p = 0.438, η2 = 0.023 |
| order × test × length | F1,27 = 1.16, m.s.e. = 319, p = 0.291, η2 = 0.043 |
| order × visual field × length | F1,27 = 0.66, m.s.e. = 754, p = 0.423, η2 = 0.025 |
| test × visual field × length | F1,27 = 18.23, m.s.e. = 691, p < 0.001, η2 = 0.412 |
| order versus test × visual field × length | F1,27 = 0.10, m.s.e. = 691, p = 0.758, η2 = 0.004 |
| errors to non-words | |
| test 1: errors to non-words before training on the non-words | |
| visual field | F1,27 = 8.48, m.s.e. = 1.77, p = 0.007, η2 = 0.239 |
| length | F1,27 = 39.88, m.s.e. = 5.38, p < 0.001, η2 = 0.596 |
| visual field × length | F1,27 = 1.27, m.s.e. = 1.58, p = 0.270, η2 = 0.045 |
| test 2A: errors to lower-case non-words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 1.75, m.s.e. = 0.62, p = 0.197, η2 = 0.061 |
| length (four versus six letters) | F1,27 = 1.23, m.s.e. = 0.88, p = 0.277, η2 = 0.044 |
| visual field × length | F1,27 = 0.29, m.s.e. = 0.76, p = 0.592, η2 = 0.011 |
| test 2B: errors to mixed-case non-words after training on the non-words | |
| visual field (LVF versus RVF) | F1,27 = 0.43, m.s.e. = 2.10, p = 0.520, η2 = 0.016 |
| length (four versus six letters) | F1,27 = 7.41, m.s.e. = 0.94, p = 0.011, η2 = 0.215 |
| visual field × length | F1,27 = 0.52, m.s.e. = 1.11, p = 0.479, η2 = 0.019 |
| combined analysis of tests 1 and 2A. Errors to non-words before and after training on the non-words (lower-case presentation in both tests) | |
| test | F1,27 = 28.88, m.s.e. = 6.18, p < 0.001, η2 = 0.517 |
| visual field | F1,27 = 9.33, m.s.e. = 1.29, p = 0.005, η2 = 0.257 |
| length | F1,27 = 36.51, m.s.e. = 3.37, p < 0.001, η2 = 0.575 |
| test × visual field | F1,27 = 3.68, m.s.e. = 1.09, p = 0.066, η2 = 0.120 |
| test × length | F1,27 = 32.07, m.s.e. = 2.89, p < 0.001, η2 = 0.543 |
| visual field × length | F1,27 = 1.37, m.s.e. = 1.30, p = 0.252, η2 = 0.048 |
| test × visual field × length | F1,27 = 0.43, m.s.e. = 1.04, p = 0.518, η2 = 0.016 |
| combined analysis of tests 2A and 2B. Errors to non-words after training on the non-words (lower-case presentation in test 2A, mixed case in test 2B) | |
| order | F1,26 = 4.06, m.s.e. = 5.24, p = 0.054, η2 = 0.135 |
| test | F1,26 = 4.76, m.s.e. = 2.63, p = 0.038, η2 = 0.155 |
| visual field | F1,26 = 1.25, m.s.e. = 1.58, p = 0.274, η2 = 0.046 |
| length | F1,26 = 6.46, m.s.e. = 1.05, p = 0.017, η2 = 0.199 |
| order × test | F1,26 = 0.90, m.s.e. = 2.63, p = 0.352, η2 = 0.033 |
| order × visual field | F1,26 = 1.50, m.s.e. = 1.58, p = 0.232, η2 = 0.054 |
| order × length | F1,26 = 0.72, m.s.e. = 1.05, p = 0.405, η2 = 0.027 |
| test × visual field | F1,26 = 0.00, m.s.e. = 1.46, p = 0.951, η2 = 0.000 |
| test × length | F1,26 = 1.59, m.s.e. = 0.81, p = 0.218, η2 = 0.058 |
| visual field × length | F1,26 = 0.05, m.s.e. = 0.82, p = 0.827, η2 = 0.002 |
| order × test versus visual field | F1,26 = 0.10, m.s.e. = 1.15, p = 0.757, η2 = 0.004 |
| order × test × length | F1,26 = 0.05, m.s.e. = 0.81, p = 0.825, η2 = 0.002 |
| order × visual field × length | F1,26 = 1.22, m.s.e. = 0.82, p = 0.279, η2 = 0.045 |
| test × visual field × length | F1,26 = 0.70, m.s.e. = 1.08, p = 0.411, η2 = 0.026 |
| order versus test × visual field × length | F1,26 = 0.00, m.s.e. = 1.08, p = 0.949, η2 = 0.000 |
(i). Reaction times to words
RTs for correct trimmed responses to words in tests 1 (lower case, before training on the non-words), 2A (lower case, after training on the non-words) and 2B (mixed case, after training on the non-words) were analysed using ANOVA with factors of visual field and length.
The results for test 1 replicated the results for the corresponding test in Experiment 1, showing significant main effects of visual field (faster overall responses in the RVF than in the LVF) and length (faster overall responses to four- than six-letter words), combined with a significant interaction between visual field and length. Bonferroni-corrected t-tests (α = 0.05) found that the 35 ms difference in RTs to four- and six-letter words in the LVF was significant, t(27) = 5.33, while the 13 ms effect of length in the RVF was only marginally significant, t(27) = 2.40. There were significant RVF advantages for both four-letter, t(27) = 3.82, and six-letter words, t(27) = 5.61. Twenty-six of the 28 participants showed an overall RVF advantage for word naming in test 1.
The analysis of RTs to words in test 2A found the same pattern of results as for test 2 of Experiment 1. The main effects of visual field and length were significant, as was the interaction between those factors. Bonferroni-corrected t-tests (α = 0.05) found that the difference of 30 ms in RTs to four- and six-letter words in the LVF was significant, t(27) = 4.49, but the 9 ms effect of length in the RVF was not, t(27) = 1.34. There were significant RVF advantages for both four-letter, t(27) = 3.40, and six-letter words, t(27) = 7.07.
A combined analysis of RTs to words in tests 1 and 2A (lower-case presentation before and after training on the non-words) found a significant main effect of test, faster responses to words in test 2A (grand mean = 525 ms) than test 1 (grand mean = 552 ms). There were also significant main effects of visual field (an overall RVF advantage) and length (faster responses to shorter words). As in Experiment 1, the interaction between visual field and length was significant in the combined analysis, but was not modulated by test (p = 0.918 for the three-way interaction between test, visual field and length). In contrast to Experiment 1, the interaction between test and visual field was not significant.
Analysis of RTs to mixed-case words in test 2B found significant effects of visual field and length. Contrary to expectations, there was a significant visual field × length interaction, with less of an effect of length in the RVF than the LVF. Bonferroni-corrected t-tests (α = 0.05) found that the 37 ms difference in RTs to four- and six-letter mixed-case words in the LVF was significant, t(27) = 4.73, while the 11 ms effect of length in the RVF was not, t(27) = 1.22. The RVF advantage was significant for six-letter words, t(27) = 3.80, but not for four-letter words, t(27) = 1.23.
A combined analysis of RTs to words in tests 2A and 2B (lower-case versus mixed-case presentation before and after training on the non-words) was carried out using within-subjects factors of test, visual field and length, plus a between-groups factor of order (whether test 2A was done before or after test 2B at the end of session 2). The main effects of visual field and length were significant across the two tests, reflecting an overall RVF advantage and faster RTs to shorter words. The main effect of test was significant, with faster naming of lower-case words in test 2A (grand mean = 525 ms) than mixed-case words in test 2B (grand mean = 572 ms). The main effect of the order in which tests 2A and 2B were taken was not significant, but a significant order × test interaction reflected the fact that overall RTs to lower-case words in test 2A were very similar whether that test preceded (grand mean = 526 ms) or followed (grand mean = 525 ms) test 2B. In contrast, overall RTs to mixed-case words in test 2B were slower when that test was presented first (grand mean = 585 ms) than when it followed the presentation of the same words in lower case in test 2A (grand mean = 558 ms). There was a marginally significant test × visual field interaction, reflecting a tendency for the overall RVF advantage to be greater for lower-case than mixed-case presentation. The visual field × length interaction was also significant in this combined analysis. As we have seen, effects of length were greater in the LVF than in the RVF for both lower-case words in test 2A and mixed-case words in test 2B. No other effects approached significance.
(ii). Errors to words
Analysis of the errors to words in test 1 was performed using the same factors of visual field and length. As with Experiment 1, the results should again be interpreted in the context of the low error rates for words and the consequent problems of ceiling effects. For test 1, the main effect of visual field approached significance, reflecting a tendency for participants to make more errors in the LVF than in the RVF. As in test 1 of Experiment 1, the main effect of length and the visual field × length interaction were not significant. Error rates to words in test 2A were low. Neither the main effects of visual field and length nor the interaction between those factors was significant. There were no significant effects in the analysis of errors to mixed-case words in test 2B.
In a combined analysis of errors to words in tests 1 and 2A (lower-case presentation before and after training on the non-words), the only significant finding was the interaction between test and visual field, reflecting a trend towards more of a difference between LVF and RVF errors in test 1 than test 2A, but in Bonferroni-corrected t-tests (α = 0.05) neither difference was significant, test 1: t(27) = 2.05; test 2A: t(27) = −1.00. The interaction between test and visual field was not significant in Experiment 1 and only narrowly attained significance in the present experiment.
The combined analysis of errors to words in tests 2A and 2B (lower-case versus mixed-case presentation before and after training on the non-words) found a marginally significant effect of order, with a tendency towards more errors when test 2B (mixed case) was performed first than when that test was performed after test 2A (lower case), and a marginally significant interaction between order and visual field, reflecting a tendency for there to be more errors in the RVF when test 2A was performed first but more errors in the LVF when test 2B was performed first.
(iii). Reaction times to non-words
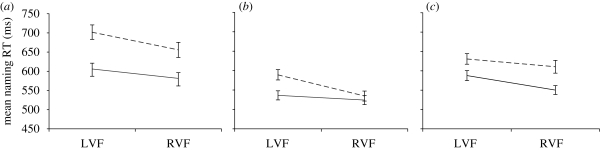
RTs to non-words in tests 1 (lower case, before training), 2A (lower case, after training) and 2B (mixed case, after training) were analysed with factors of visual field and length. Figure 2 shows the relationship between visual field and length for unfamiliar non-words in test 1 (figure 2a), learned non-words presented in lower case in test 2A (figure 2b) and learned non-words presented in mixed case (figure 2c).
Figure 2.
Mean naming latencies to non-words in (a) test 1 (before training), (b) test 2A (lower-case presentation after training) and (c) test 2B (mixed-case presentation after training) in Experiment 2. Solid lines, four-letter non-words; dashed lines, six-letter non-words. Error bars show s.e. values.
In test 1, RTs to unfamiliar non-words showed a significant RVF advantage and significantly faster responses to four- than six-letter items. The interaction between visual field and length was marginally significant (p = 0.096). Bonferroni-corrected t-tests (α = 0.05) found, however, that the 96 ms effect of length in the LVF was significant, t(27) = 10.18, as was the 74 ms effect in the RVF, t(27) = 6.80. The RVF advantage was marginally significant for four-letter unfamiliar non-words, t(27) = 3.23, and significant for six-letter items, t(27) = 4.84.
In test 2A, RTs to learned non-words presented in lower-case format showed significant effects of visual field and length, combined with a highly significant interaction between those two factors (p < 0.001, η2 = 0.470). Bonferroni-corrected t-tests (α = 0.05) found that the 63 ms effect of length in the LVF was significant, t(27) = 9.07, but the 10 ms effect in the RVF was not, t(27) = 1.60. The RVF advantage was significant for learned non-words of six letters, t(27) = 8.91, but not four letters, t(27) = 1.53.
RTs to non-words in tests 1 and 2A were incorporated into a combined analysis with factors of test (1 versus 2A), visual field and length. There was a significant main effect of test, with faster responses in test 2A (grand mean = 546 ms) than in test 1 (grand mean = 636 ms). There were also significant main effects of visual field (an overall RVF advantage) and length (faster responses to the shorter non-words). A significant test × length interaction reflected the fact that naming RTs for six-letter, lower-case non-words speeded up more between tests 1 and 2A than naming RTs for four-letter non-words. Bonferroni-corrected t-tests (α = 0.05) found, however, that both the overall 117 ms increase in naming speed for six-letter non-words and the 64 ms increase for four-letter non-words were significant, six-letter: t(27) = 9.11; four-letter, t(27) = 6.30. The interaction between visual field and length was significant, but the three-way interaction involving test, visual field and length was not. That is, although the separate analyses of tests 1 and 2A found a significant length × visual field interaction for test 2A (after training) than test 1 (before training), the three-way interaction that would have strengthened the claim that length and visual field interacted differently in two tests 1 and 2A was not significant (though the equivalent interaction was significant in Experiment 1).
In test 2B, RTs to learned non-words presented in mixed-case format showed a significant RVF advantage and faster responses to shorter non-words, but no interaction between length and visual field (p = 0.134). A combined analysis of non-word naming RTs in tests 2A and 2B with order of tests as a between-groups factor found faster overall responses to learned non-words presented in lower case (test 2A; grand mean = 546 ms) than in upper case (test 2B; grand mean = 595 ms). There was an overall RVF advantage and an overall effect of length. The main effect of order was not significant, but the interaction between order and test approached significance, reflecting a pattern similar to that for word RTs, with naming RTs for lower seven non-words in test 2A being little affected by whether that test was performed first or second at the end of session 1 (a difference of just 10 ms), while naming RTs for mixed-case non-words in test 2B were 29 ms faster when the same non-words had just been named in lower case in test 2A than when test 2B was administered first. A significant test × length interaction and a marginal visual field × length interaction were subsumed within a significant test × visual field × length interaction. This can be understood with reference to the separate analyses of RTs to learned non-words in tests 2A and 2B (above, and table 5) which found a smaller effect of length in the RVF than in the LVF for learned non-words presented in lower case (test 2A) but not mixed case (test 2B).
(iv). Errors to non-words
Errors to unfamiliar non-words in test 1 showed a significant RVF advantage and fewer errors to four- than to six-letter items. There was no significant interaction between visual field and length. Errors to learned non-words presented in lower case in test 2A found no significant effects. A combined analysis of errors in tests 1 and 2A found main effects of test (fewer errors after training in test 2A than before training in test 1), visual field (fewer RVF than LVF errors) and length (fewer errors to four- than six-letter non-words). A significant test × length interaction reflected the fact that length affected error rates to unlearned non-words in test 1 but not learned non-words in test 2A (where error rates were much lower). The test × visual field interaction approached significance, reflecting a tendency for training to reduce error rates more in the LVF (where the initial levels were higher) than in the RVF.
Errors to learned non-words presented in mixed-case format in test 2B showed significantly more errors to six- than to four-letter words but no effect of visual field and no visual field × length interaction. A combined analysis of errors in tests 2A and 2B, with order of tests as a between-groups factor, found significant effects of test (more errors in test 2B than test 2A) and length (more errors to longer non-words). The effect of task order was marginally significant, reflecting a tendency for error rates in test 2A (lower case) to be less affected by task order than in test 2B (mixed case). No other effects approached significance.
(c). Discussion
The main findings of Experiment 2 can be summarized as follows.
The results for words tested in lower case in both test 1 and test 2A replicated the findings of Experiment 1 and matched the results of many studies in the literature (Ellis 2004). There was an overall RVF advantage for word naming speed, with 26 of the 28 participants showing an overall RVF advantage for word naming RTS in test 1. The by-now familiar interaction of length with visual field was observed in the RT data. The effect of length was significant in the LVF in both tests 1 (35 ms) and 2A (30 ms). The 13 ms effect in the RVF in test 1 was marginally significant but the 9 ms effect in test 2A was not significant.
Responses to words were faster in test 2A than in test 1, despite the fact that the words were not trained between the two tests. The same result was observed in Experiment 1. We would attribute the facilitation of word naming RT in the absence of intervening practice to repetition priming (Scarborough et al. 1977; Forster & Davis 1984; Stark & McClelland 2000; Weems & Zaidel 2005). We note that, as in Experiment 1, the general speeding up of responses to words between tests 1 and 2A did not affect the length × visual field interaction. We note also that many studies of long-term repetition priming have shown that those effects are item-specific, so must be due to training on the items rather than the task more generally (Bowers 2000; Henson 2003).
As in Experiment 1, non-words presented in lower case showed a dramatic reduction in error rates and a speeding up of RTs between tests 1 and 2A as a result of training. RTs for trained non-words in test 2A were comparable to those for familiar words seen for the first time in test 1, demonstrating once again that modest amounts of training on unfamiliar non-words can generate levels of performance on those items comparable to those for familiar words experienced countless times over the course of a participant's life (cf. Feustel et al. 1983; Salasoo et al. 1985). How well the performance on newly learned non-words holds up over time is a matter for future research.
There was an RVF processing advantage for non-word naming RTs both before and after training. As in Experiment 1, the impact of word length on naming speed for lower-case non-words changed between tests 1 and 2A. Before training (test 1), there were significant effects of length in both the LVF (96 ms) and the RVF (74 ms), with no significant visual field × length interaction (figure 2a). After training (test 2A), the 53 ms effect of length in the LVF was significant, but the 10 ms effect in the RVF was not (figure 2b). Unfortunately, and unlike Experiment 1, when non-word naming RTs from tests 1 and 2A were entered into a combined analysis, the three-way interaction between test, visual field and length was not significant, meaning that the statistical support for the claim that the impact of length on naming RTs in the two visual fields changed as a result of training was not as strong as in Experiment 1.
-
The results for mixed-case presentations in test 2B were not as anticipated. Naming RTs for mixed-case words showed a length × visual field interaction similar to that for words in normal, lower-case formats, with a significant 37 ms length effect in the LVF and a non-significant 11 ms effect in the RVF. Lavidor & Ellis (2001) and Lavidor et al. (2002) used lexical decision rather than word naming. They found that RTs to both lower- and upper-case words showed reduced effects of length in the RVF compared with the LVF, but mixed-case words did not. Similar results were obtained in lexical decision by Ellis et al. (1988) using a ‘stepped’ format in which alternate letters were raised or lowered, by Bub & Lewine (1988) using a word naming task and vertical presentation of words, and by Young & Ellis (1985) for accuracy of report following brief presentation in both stepped and vertical formats. The results of modulation reported by Lavidor & Ellis (2001) and Lavidor et al. (2002) would seem to fit with a general pattern that disrupting the normal appearance of words limits the left hemisphere's ability to processing their component letters simultaneously and in parallel.
The predicted outcome was not obtained for word naming RTs in test 2B of Experiment 2 where mixed-case words showed a visual field × length interaction similar to that shown by lower-case words. In contrast, case mixing induced the anticipated modulation of the visual field × length interaction when the stimuli were learned non-words. Whereas lower-case learned non-words showed a reduced length effect in the RVF (test 2A), mixed-case non-words did not (figure 2c). These puzzling results require further investigation.
In the remainder of this paper, we will concentrate on the results obtained for words and non-words presented in standard, lower-case format.
4. General discussion
Our general discussion will focus on two broad aspects of the data from Experiments 1 and 2. The first is the large reduction in error rates and general speeding up of naming RTs that occurs as a result of word learning. The second is the more subtle change in the balance of length effects across the two visual fields/hemispheres which, in our view, tells us something about differences in the early processing of letters in unfamiliar non-words versus known words within and between the cerebral hemispheres. Consideration of those more subtle effects will lead us into a discussion of the possible neural basis of serial versus more parallel, unitized processing of letters in word and non-words. We will end with some thoughts on how the effects reported for stimuli presented in the LVF or RVF may apply to words seen in central vision, at fixation.
(a). Lexical versus sublexical conversion from print to sound and the establishment of orthographic representations
The general reduction in errors and the facilitation of naming speed that occur as a result of word learning can be seen by comparing performance on unfamiliar non-words versus familiar words in test 1 of the Experiments 1 and 2 or by comparing performance on non-words before and after training (tests 1 and 2 in Experiment 1 and tests 1 and 2A in Experiment 2). Error rates to unfamiliar non-words in test 1 of the two experiments were 12–15% compared with just 3–4% for known words seen for the first time in test 1 or for learned non-words in test 2/2A. Correct naming RTs for unfamiliar non-words in test 1 averaged around 625 ms compared with 530–540 ms for familiar words in test 1 or learned non-words in test 2/2A. The advantage for known words or learned non-words over unfamiliar non-words in both visual fields reflects the well-known lexicality advantage for words over unfamiliar non-words in reading (McCann & Besner 1987; Rastle & Coltheart 1999; Kinoshita et al. 2004). These lexicality and learning effects were of similar magnitudes in both visual fields, but were substantially greater for six- than four-letter stimuli in both fields. Thus, across the two experiments, if performance on words versus unfamiliar non-words in test 1 is compared, error rates were 18 per cent lower and 113 ms faster for the six-letter words compared with around 4 per cent lower and RTs 58 ms faster for the shorter words. Similarly, if performance on learned non-words in test 2/2A versus unfamiliar non-words in test 1 is compared, error rates were 17 per cent lower and 124 ms faster for six-letter learned non-words compared with around 5 per cent lower and RTs 72 ms faster for shorter, learned non-words.
Weekes (1997) found a substantial effect of length on naming RTs for centrally presented unfamiliar non-words (around 20 ms per letter) but no significant effect for high-frequency words, with the result that the advantage in naming speed for words over non-words increased with increasing word length. Weekes (1997) argued that differential effects of length on word and non-word reading can be explained in terms of a ‘dual route’ model of reading such as the DRC model of Coltheart et al. (2001) if it is assumed that familiar words are converted from orthography to phonology using a procedure that operates on whole strings, whereas non-words are converted from orthography to phonology using a procedure which operates serially on individual letters or groups of letters (graphemes). Juphard et al. (2004) obtained similar results for word naming in French and offered a similar explanation in terms of lexical conversion from orthography to phonology for familiar words and analytic, sublexical conversion for unfamiliar non-words. In the context of word learning, the process of learning a new word (or non-word in experimental tasks like ours) will allow the creation of orthographic, phonological and semantic representations for new items. Orthographic representations appear to be housed in the left mid-fusiform gyrus, while left posterior superior temporal cortex and the left inferior frontal gyrus appear crucial for representing phonological word forms (Cornelissen et al. 2009; Price & McCrory 2005). Semantic representations, for which more anterior regions of the temporal lobes seem crucial (Patterson et al. 2007), may play a role in reading aloud if the new words employ unusual (irregular) spelling-sound correspondences (McKay et al. 2008), but for novel items like the ones used here, whose pronunciations were regular and predictable from their spellings, reading aloud is probably mediated by direct mappings between newly formed orthographic and phonological representations, without significant involvement of semantics. In the absence of word-level mappings, conversion from orthography to phonology for unfamiliar non-words must be achieved at a finer scale, based on correspondences between letters or letter clusters and phonemes. On this view, the process of assembling phonology from print for unfamiliar words or non-words is more analytic and componential, and consequently more length-sensitive than the process of retrieving whole-word phonology for known words, which is more holistic and therefore less sensitive to the number of letters in the word.
If word learning involves (among other things) the creation of orthographic, semantic and phonological representations, and if the establishment of lexical orthographic and phonological representations allows words to be named more quickly, more accurately, and on a more holistic basis, the present results remind us of the fact that lexical representations, once established, are not fixed and immutable, but remain sensitive to experience. In both experiments, the real words interleaved with the non-words in the naming tests were named more quickly in test 2/2A than in test 1, despite not having been trained between those tests. We have attributed that observation to the phenomenon of long-term repetition priming by which recent exposure to familiar words facilitates recognition of those same stimuli hours, days or even weeks later (Scarborough et al. 1977; Forster & Davis 1984; Stark & McClelland 2000). Theories of repetition priming which fit comfortably with the present framework explain repetition priming in terms of strengthening the connections between different internal representations of familiar items (e.g. Rumelhart & McClelland 1985; Ellis et al. 1996; Stark & McClelland 2000). In the case of known words, those would be the associative connections between established orthographic, semantic and phonological representations. By this means, the reading system is constantly being modified in such a way as to prioritize the identification of words that have formed the content of recent experience. The same phenomenon is present in object and face recognition (Ellis et al. 1996; Vuilleumier et al. 2002), suggesting that the constant updating of the lexical system to reflect recent experience is inherited from evolutionarily older systems responsible for recognizing other types of familiar object. The point demonstrated by long-term repetition priming is that word learning is never finished: even after a word has been consolidated into the lexicon of an adult language user, its representation continues to be subject to modification through experience.
We note, however, that repetition priming did not modify the length × visual field interaction shown by familiar words. That pattern of results could be taken to imply that the post-lexical processes influenced by repetition (beyond the lexical orthographic representations) are distinct from the pre-lexical processing stages responsible for the modulation of the effects of length × the visual field in which words or learned non-words are presented, and therefore the cerebral hemisphere to which they were projected. Functional magnetic resonance imaging (fMRI) studies of repetition priming for written words have reported repetition-contingent modification of activity in the left fusiform gyrus and other parietal and frontal regions, but not in the more posterior visual areas where, we shall argue, perceptual effects of letter length originate (Henson 2001; Maccotta & Buckner 2004; Fiebach et al. 2005).
(b). Length, hemispheres and interactive connectivity in the brain
The present results indicate that there is more to the presence or absence of length effects than the switch from sublexical to lexical mappings from orthography and phonology. In both experiments, the impact of letter length on RTs to unfamiliar non-words in test 1 was substantial in both visual fields, with no significant interaction between length and visual field (figures 1 and 2a). Comparable effects of length in the two visual fields on the accuracy of identifying briefly presented, unfamiliar non-words were also reported by Young & Ellis (1985) and Bruyer & Janlin (1989). To the best of our knowledge, there have been no previous studies of the impact of letter length on non-word naming latencies in the LVF and RVF, but the results for naming speed accord with those for accuracy. The present experiments also replicated the well-attested finding that recognition of familiar words in familiar formats is more length-sensitive in the LVF than in the RVF (Ellis 2004) and showed that the same holds true for recently learned non-words (figures 1 and 2b). We considered in §1 the reasons for not believing that LVF length effects in the processing of familiar words occur either in the course of inter-hemispheric transfer of information from the right to the left hemisphere or as a result of greater application of sublexical orthography-to-phonology conversion for LVF than RVF words. The fact that the interaction of length with visual field changes as the same letter sequences pass from being unfamiliar to being familiar also argues against the notion that LVF length effects are a consequence of the way in which early, retinotopic representations of letter strings are transformed into more abstract representations which encode the identity and order of letters in the string, irrespective of whether that string forms a familiar word or an unfamiliar non-word (Whitney & Lavidor 2004). How then are we to explain the observation that recognition of familiar words, or learned non-words, is more parallel in the RVF than the LVF?
Ellis (2004) attempted to breathe fresh life into an idea originally mooted by Ellis et al. (1988) and Bub & Lewine (1988) that there may be two distinct ‘modes’ of visual perception of letter sequences. According to that view, the first mode of letter perception (‘mode A’) involves relatively parallel processing of the component letters within a string, resulting in small, and often non-significant, effects of length on speed and accuracy. According to the theory, mode A is only available for familiar words presented in the RVF in familiar formats (lower case or upper case). All other forms of letter sequence are processed in a more serial and therefore more length-sensitive manner (‘mode B’). That includes unfamiliar words and non-words wherever they appear in the visual world, familiar words presented in the LVF regardless of their format, and familiar words presented in the RVF in unusual formats such as mixed case, vertical or stepped presentations. The literature on familiar word recognition reviewed by Ellis (2004) suggested that LVF length effects for words were reasonably consistent in magnitude at around 20–25 ms per additional letter. The LVF length effects for lower-case words in the present experiments were somewhat smaller (around 16 ms per letter), but were also very consistent between tests 1 and 2/2A of Experiments 1 and 2. What the present results make clear, however, is that length effects are not either present or absent. The largest length effects were seen for unfamiliar non-words presented in either the LVF (around 45 ms per letter) or the RVF (around 36 ms per letter). Learned non-words presented in the LVF showed the next largest effect (25 ms per letter), followed by familiar words in the LVF (16 ms per letter). The smallest length effects were observed for RVF presentations of familiar words (7 ms per letter) and learned non-words (4 ms per letter). All the LVF length effects were significant. The RVF effects for familiar words, though smaller than the corresponding LVF effects, were also significant or marginally significant in three of four tests. Only the RVF effects for learned non-words in lower-case format did not approach significance.
These considerations suggest that we should be thinking of length effects as graded rather than all or nothing. It is not as simple as saying that one mode of processing operates under some circumstances while a different mode of processing operates under others: there are degrees of serialization or parallelization of perceptual processing that need to be explained. Our attempt at an explanation will draw upon recent neuroimaging studies of word and non-word processing in the brain. Cohen et al. (2000, 2002) showed that words and consonant strings presented in the RVF induce activity in the left middle/inferior occipital gyri of the left (contralateral) hemisphere. Other studies have associated that region with the processing of letter shape information (e.g. Cohen et al. 2002; Jobard et al. 2003; Tarkiainen et al. 1999; Vigneau et al. 2005). A popular view proposes that processing advances from those lateral occipital areas along the left fusiform gyrus where, according to Vinckier et al. (2007), progressively larger letter groups are resolved as processing moves in an anterior direction. As a result of this processing, both familiar words and legal, pronounceable non-words of the sort used in the present experiments cause an activation peak at the site of the so-called visual word form area in the mid region of the left fusiform gyrus (Jobard et al. 2003; McCandliss et al. 2003; Vinckier et al. 2007).
We propose that when the left middle/inferior occipital gyri are analysing the component letters of unfamiliar non-words displayed in the RVF, perceptual identification of letter identities is serial in nature for unfamiliar non-words, and therefore sensitive to the number of letters in the non-word. The products of letter identification are fed serially along the left fusiform gyrus as individual letters are resolved. The serial character of the letter recognition processes for unfamiliar RVF non-words, and the absence of whole-string orthographic representations, mean (i) that left hemisphere processing of such non-words is a largely feed-forward process involving flow of information from lateral occipital cortex along the fusiform gyrus and (ii) that orthography-to-phonology conversion for unfamiliar non-words is necessarily analytic and componential in nature.
The situation is different for familiar words and learned non-words. The existence of whole-string representations within the left mid-fusiform gyrus alters the nature of the relationship between lateral occipital and more anterior fusiform processing, introducing the possibility for the two regions to enter into a more interactive relationship. Computational models of visual word recognition have long incorporated the notion that letter and word recognition systems may exist in a state of mutual interactive activation when familiar words are being recognized (McClelland & Rumelhart 1981; Coltheart et al. 2001). Under such circumstances, the letter identification system receives external input first. It passes activation forward in a ‘bottom-up’ manner to a stage at which whole words are recognized as familiar. Crucially, as soon as the whole-word stage begins to process the input, it passes activation back to the earlier letter-processing stage in a ‘top-down’ manner. The two systems enter a temporary phase of interactive activation which persists until the component letters in the familiar word are resolved. The consequence of this mutual interaction is faster resolution of the stimulus at both earlier and later processing stages and a reduced impact of letter length. Interactive activation was employed in models of visual word recognition to explain superior processing of words in comparison to non-words in a range of perceptual tasks, of which the lexicality and learning effects observed here would be examples (McClelland & Rumelhart 1981; Paap et al. 1982; Rumelhart & McClelland 1982; Coltheart et al. 2001).
The existence of feedback as well as feed-forward connections within the brain has long been recognized, along with the possibility such connections allow for top-down modulation of bottom-up processing (Gilbert & Sigman 2007). Methods are being developed that allow researchers to determine whether the flow of information between areas is unidirectional or bidirectional, making interactive processing possible (Mechelli et al. 2005; Kujala et al. 2007). Unfortunately, these techniques have only been applied to information flow over relatively long time windows. Finer temporal resolution will be required to plot the changing evolution in the patterns of functional connectivity between lateral occipital and mid-fusiform regions suggested here. Martin et al. (2006) found that the greatest difference between the electrophysiological responses to centrally presented words and illegal non-words (consonant strings) occurred around 200 ms after stimulus onset, within the time window of the so-called N170 responses (or M170 in the case of magnetoencephalography (MEG); see Cornelissen et al. 2003; Tarkiainen et al. 1999). It may be that the N170/M170 response is the signature of the brief recruitment of left lateral occipital cortex and the left fusiform gyrus into a temporary reverberative network that facilitates the fast, parallel resolution of the component letters of known words (or recently learned non-words). If so, then when familiar words are being recognized, the flow of information along the fusiform gyrus from lateral occipital cortex should be unidirectional (bottom-up) for the first 100–150 ms, then bidirectional from around 150 to 200 ms as lateral occipital and mid-fusiform regions enter into a state of temporary interaction.
Thus far, we have only considered stimuli that are presented in the RVF and therefore project directly to the left (language dominant) hemisphere. Words and non-words presented in the LVF trigger activity within the right middle/inferior occipital gyri where we assume that letter shape analysis is begun (Cohen et al. 2002; Ben-Shachar et al. 2007a). The products of that analysis are then passed to the homologous region of the left hemisphere across large, fast-conducting fibres that course in the splenium of the corpus callosum (Binder & Mohr 1992; Molko et al. 2002; Ben-Shachar et al. 2007b). Processing of unfamiliar LVF non-words will be at least as serial as processing of RVF non-words, resulting in length effects that are at least as marked (figures 1 and 2a). Familiar words and learned non-words presented in the LVF will undergo some processing in right lateral occipital cortex before being transferred across to the left occipital letter shape area. If we assume with Cohen et al. (2002, 2008), Whitney (2001) and others that recognition of familiar words is primarily the responsibility of the left hemisphere, then the activation of left lateral occipital cortex by the component letters of LVF words being fed across from the right hemisphere should cause the left occipital region to enter into a state of interactive activation with the left mid-fusiform gyrus. Input from LVF words to the left hemisphere will, however, be delayed in comparison with RVF words, and activation of the left lateral occipital area may be weaker than for RVF presentations. Cohen et al. (2002) and Ben-Shachar et al. (2007a) reported that both RVF and LVF words activate the left mid-fusiform gyrus, but there are clear indications in both studies that RVF activation is stronger than LVF (see Cohen et al. 2002, fig. 5; Ben-Shachar et al. 2007a, Experiment 4 and fig. 5). That conclusion is reinforced by MEG analyses reported by Barca et al. (in preparation), which found stronger responses in the left mid-fusiform region to RVF than LVF words. The interaction of left lateral occipital cortex with the mid-fusiform gyrus may be correspondingly weaker for LVF than RVF words, with the result that letter processing is more serial than for RVF words and the length effect less attenuated (from 45 ms per letter for LVF unfamiliar non-words to 25 ms per letter for LVF learned non-words and 16 ms per letter for LVF words).
(c). Perception of words and non-words viewed centrally, at fixation
Many questions remain unanswered, not least of which is the question of what happens in the case of words viewed centrally, at the fovea. We have noted reports by Weekes (1997) and Juphard et al. (2004) that while naming speed for centrally presented non-words shows strong length effects, indicative or serial processing, naming times for familiar words show little effect of length. That claim may have its limitations. An analysis of lexical decision data by New et al. (2006) suggested that RTs to words decrease from three to five letters and are then relatively stable up to lengths of around nine letters, but increase beyond that. Parallel processing of the component letters of familiar words may have its limitations, although New et al. (2006) note additional factors such as possible loss of acuity for the end letters of very long words.
Event-related potential (ERP) and MEG studies of central word recognition have reported length effects with a posterior occipital locus at around 100 ms (Assadollahi & Pulvermüller 2003; Wydell et al. 2003; Hauk & Pulvermüller 2004), which may correspond to activity in our proposed lateral occipital letter shape area. Positron emission tomography (PET) studies have reported length effects in the left and right lingual gyri (Indefrey et al. 1997; Mechelli et al. 2000) and in left and right fusiform gyri (Mechelli et al. 2000; Mayall et al. 2001), but those length-sensitive activations occur at sites 3–4 cm posterior to the putative visual word form area, on the route from lateral occipital cortex to the mid-fusiform gyrus. It may be that the top-down, interactive engagement of the mid-fusiform with early letter-processing area serves to modulate processing as it proceeds along the posterior gyrus, allowing the component structure of longer words to be resolved with an efficiency similar to that for shorter words, so that the sensitivity of early processes to the number of letters in a string is lost for familiar words by the time they reach the left mid-fusiform gyrus.
What role do the left and right cerebral hemispheres play in processing centrally presented words and non-words? There are two competing views about the processes underlying the recognition of words that fall within the fovea (which is most words fixated in the course of normal reading). The bilateral projection theory proposes that the fovea (taken to be the central 2 to 3 degrees of visual space) projects directly to both cerebral hemispheres (Jordan & Paterson 2009). If that is the case, then words and non-words displayed at fixation that fall within the fovea should act like RVF stimuli because they will project in their entirety directly to the left hemisphere. In contrast, the more recent split fovea theory proposes that the fovea, like the rest of the visual world, is split, with everything that falls to the left of fixation projecting initially to the right hemisphere while everything that falls to the right of fixation projects initially to the left hemisphere (Shillcock et al. 2000; Whitney 2001; Lavidor & Walsh 2004; Ellis & Brysbaert in press). That split would extend to individual words whose initial letters would project first to the right hemisphere while the end letters would project directly to the left hemisphere. On this view, the left and right parts of a centrally fixated word would need to be reunited within the brain, probably by the transfer of initial letters across the corpus callosum to the left hemisphere where they can be brought together with the end letters.
If the split fovea theory is correct, and if the account of pre-lexical length effects that we have provided above is on the right lines, then for familiar words, the number of letters falling to the left of fixation should have more of an effect on word recognition than the number of letters falling to the right of fixation. In a study of lexical decision RTs, Lavidor et al. (2001) reported that recognition times for words were indeed more affected by variation in the number of letters falling to the left of fixation than to the right. Ellis (2004) identified a similar pattern in data from a word naming experiment reported by Brysbaert (1994), and Ellis & Brysbaert (in press) found the same pattern in a post hoc analysis of naming data from Hunter et al. (2007). This observation fits more comfortably with split fovea theory than with the bilateral projection theory, as do other reports of differential effects on processing of the letters in centrally presented words that fall to the left or right of fixation (Lavidor et al. 2004; Ellis et al. 2005). The notion of a split fovea and the evidence for and against it are the subject of ongoing debate (Ellis & Brysbaert in press; Jordan & Paterson 2009; Jordan et al. 2009), but if future work supports the claim that the number of letters in a fixated word falling to the left of fixation affects recognition more than the number of letters falling to the right, the implication would be that interactive engagement of left lateral occipital cortex with the mid-fusiform area is stronger as applied to the end letters of fixated words than to the initial letters.
(d). Summary and conclusions
We will end this paper with a brief summary of the main features of the theory we have developed in the course of this paper. References in support of the various statements that follow can be found earlier in the paper.
Participants in our experiments received training on initially unfamiliar non-words that allowed the participants to establish orthographic representations of their written word forms, phonological representations of their spoken word forms and semantic representations that provided at least some indication of their meaning. In line with the currently dominant view, we propose that the left mid-fusiform gyrus is where orthographic word forms are housed. Phonological word forms may be created as a result of interactions between the left posterior superior temporal region and the left inferior frontal gyrus. The anterior temporal poles appear important for the establishment of semantic representations.
Creating orthographic and phonological representations for new words (or learned non-words) allows those items to be converted rapidly from print to sound on a holistic (lexical) basis. Before orthographic and phonological word forms are established, unfamiliar non-words must be converted from print to sound on a piecemeal, sublexical basis. The transition from sublexical to lexical conversion of print to sound that occurs as a consequence of word learning reduces naming speeds in both visual fields and reduces the impact of the number of letters in the word on naming latencies. Although we have not focused on comprehension processes here, creating lexical representations will also mean that previously unfamiliar sequences of letters now activate meanings as well as sounds. The links between orthographic, semantic and phonological representations are not fixed and immutable, but are constantly modified by experience. The ongoing modification is revealed in the phenomenon of long-term repetition priming.
The holistic mapping of unitized letter strings onto sound and meaning can be categorized as post-lexical processing. We propose that orthographic word forms in the left mid-fusiform gyrus can, in addition, influence earlier stages of processing that are involved in identifying the component letters of words. Our suggestion is that orthographic word forms in the left mid-fusiform gyrus interact with letter recognition processes operating in the left middle/inferior occipital gyri in a manner similar to that proposed in interactive activation models of visual word recognition. Those influences reach back to pre-lexical processes and result in faster perceptual resolution of the component letters of known words (and learned non-words). These pre-lexical influences of orthographic word forms are responsible for word superiority effects of a perceptual nature and for further unitization of the visual processing of known words. Functional interaction between lateral occipital and mid-fusiform areas occurs some 150–200 ms after the presentation of a word and is at its maximum for familiar words presented in the RVF (because RVF words project directly to left visual cortex). The impact of letter length is smallest for such words. If words are viewed in the LVF, they must be transferred across the corpus callosum to left lateral occipital cortex before they can be fed along the fusiform gyrus. This reduces the ability of the lateral occipital and mid-fusiform areas to enter into an interactive state and results in larger length effects. When words are fixated centrally, as in normal reading, those letters that fall to the right of fixation project directly to the left hemisphere. They experience the benefits of interactive pre-lexical processing, so that the cost associated with increased numbers of word-final letters is reduced. Letters that fall to the left of fixation project first to the right hemisphere. They must be transferred across the corpus callosum to left lateral occipital cortex before they can be directed down the fusiform gyrus. They trigger interactive processing less successfully, so that reading times for central words depend more on the number of letters falling to the left of fixation than the number of letters falling to the right. Unfamiliar non-words cannot call upon dedicated orthographic word forms in the left mid-fusiform gyrus. Their processing is the most serial, and therefore most length sensitive, regardless of whether they appear in the RVF, the LVF or at the centre.
Word-learning paradigms offer a new and insightful way of investigating lexical processing in both behavioural and neural terms. Their potential is only just starting to be realized.
Acknowledgements
A.W.E. and L.B. were members of the EU Marie Curie Research Training Network on Language and Brain funded under Framework 6.
Footnotes
One contribution of 11 to a Theme Issue ‘Word learning and lexical development across the lifespan’.
References
- Assadollahi R., Pulvermüller F.2003Early influences of word length and frequency: a group study using MEG. NeuroReport 14, 1183–1187 (doi:10.1097/00001756-200306110-00016) [DOI] [PubMed] [Google Scholar]
- Baayen R. H., Piepenbroack R., Van Rijn H.1993. In The CELEX lexical database (CD-ROM) Philadelphia, PA: University of Pennsylvania, Linguistic Data Consortium [Google Scholar]
- Babkoff H., Faust M., Lavidor M.1997Lexical decision, visual hemifield and angle of orientation. Neuropsychologia 35, 487–495 (doi:10.1016/S0028-3932(96)00072-3) [DOI] [PubMed] [Google Scholar]
- Banich M. T.2003The divided visual field technique in laterality and interhemispheric integration. In Experimental methods in neuropsychology (ed. Hugdahl K.), pp. 47–63 Boston, MA: Kluwer [Google Scholar]
- Barca L., Cornelissen P., Simpson M., Urooj U., Woods W., Ellis A. W.In preparation Interhemispheric transfer and the right visual field advantage for word recognition: an MEG analysis. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R. F., Deutsch G. K., Wandell B. A.2007aDifferential sensitivity to words and shapes in ventral occipito-temporal cortex. Cereb. Cortex 17, 1604–1611 (doi:10.1093/cercor/bhl071) [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R. F., Wandell B. A.2007bWhite matter pathways in reading. Curr. Opin. Neurobiol. 17, 258–270 [DOI] [PubMed] [Google Scholar]
- Binder J. R., Mohr J. P.1992The topography of callosal reading pathways. Brain 115, 1807–1826 (doi:10.1093/brain/115.6.1807) [DOI] [PubMed] [Google Scholar]
- Bourne V. J.2006The divided visual field paradigm: methodological considerations. Laterality 11, 373–393 [DOI] [PubMed] [Google Scholar]
- Bowers J. S.2000In defense of abstractionist theories of word identification and repetition priming. Psychonom. Bull. Rev. 7, 83–99 [DOI] [PubMed] [Google Scholar]
- Bruyer R., Janlin D.1989Lateral differences in lexical access: word length vs stimulus length. Brain Lang. 37, 258–265 (doi:10.1016/0093-934X(89)90018-7) [DOI] [PubMed] [Google Scholar]
- Brysbaert M.1994Interhemispheric transfer and the processing of foveally presented stimuli. Behav. Brain Res. 64, 151–161 (doi:10.1016/0166-4328(94)90127-9) [DOI] [PubMed] [Google Scholar]
- Bub D., Lewine J.1988Different modes of word recognition in the left and right visual fields. Brain Lang. 33, 161–188 (doi:10.1016/0093-934X(88)90060-0) [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehéricy S., Dehaene-Lambertz G., Hénaff M. A.2000The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123, 291–307 (doi:10.1093/brain/123.2.291) [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Cochon F., Lemer C., Rivaud S., Dehaene S.2002Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125, 1054–1069 (doi:10.1093/brain/awf094) [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Vinckier F., Jobert A., Montavont A.2008Reading normal and degraded words: contribution of the dorsal and ventral visual pathways. Neuroimage 40, 353–366 (doi:10.1016/j.neuroimage.2007.11.036) [DOI] [PubMed] [Google Scholar]
- Coltheart M.1981The MRC psycholinguistic database. Q. J. Exp. Psychol. 33A, 497–505 [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., Ziegler J.2001DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 108, 204–256 (doi:10.1037/0033-295X.108.1.204) [DOI] [PubMed] [Google Scholar]
- Cornelissen P., Tarkiainen A., Helenius P., Salmelin R.2003Cortical effects of shifting letter position in letter strings of varying length. J. Cogn. Neurosci. 15, 731–746 (doi:10.1162/jocn.2003.15.5.731) [DOI] [PubMed] [Google Scholar]
- Cornelissen P. L., Kringelbach M. L., Ellis A. W., Whitney C., Holliday I. A., Hansen P. C.2009Activation of the left inferior frontal gyrus in the first 200 msec of reading: evidence from magnetoencephalography (MEG). Plos ONE 4, e5359, 1–13. www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0005359 (doi:10.1371/journal.pone.0005359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumay N., Gaskell M. G.2007Sleep-associated changes in the mental representation of spoken words. Psychol. Sci. 18, 35–39 (doi:10.1111/j.1467-9280.2007.01845.x) [DOI] [PubMed] [Google Scholar]
- Duyck W., Desmet T., Verbeke L. P. C., Brysbaert M.2004WordGen: A tool for word selection and nonword generation in Dutch, English, German, and French. Behav. Res. Methods Instrum. Comput. 36, 488–499 [DOI] [PubMed] [Google Scholar]
- Ellis A. W.2004Length, formats, neighbors, and the processing of words presented laterally or at fixation. Brain Lang. 88, 355–366 (doi:10.1016/S0093-934X(03)00166-4) [DOI] [PubMed] [Google Scholar]
- Ellis A. W., Brysbaert M.In press Split fovea theory and the role of the two cerebral hemispheres in visual word recognition: a review of the evidence. Neuropsychologia [DOI] [PubMed] [Google Scholar]
- Ellis A. W., Young A. W., Anderson C.1988Modes of visual word recognition in the left and right cerebral hemispheres. Brain Lang. 35, 254–273 (doi:10.1016/0093-934X(88)90111-3) [DOI] [PubMed] [Google Scholar]
- Ellis A. W., Flude B. M., Young A. W., Burton A. M.1996Two loci of repetition priming in the recognition of familiar faces. J. Exp. Psychol. Learn. Mem. Cogn. 22, 295–308 (doi:10.1037/0278-7393.22.2.295) [DOI] [PubMed] [Google Scholar]
- Ellis A. W., Brooks J., Lavidor M.2005Evaluating a split fovea model of visual word recognition: effects of case alternation in the two visual fields and in the left and right halves of words presented at the fovea. Neuropsychologia 43, 1128–1137 (doi:10.1016/j.neuropsychologia.2004.11.022) [DOI] [PubMed] [Google Scholar]
- Feustel T. C., Shiffrin R. M., Salasoo A.1983Episodic and lexical contributions to the repetition effect in word identification. J. Exp. Psychol. Gen. 112, 309–346 (doi:10.1037/0096-3445.112.3.309) [DOI] [PubMed] [Google Scholar]
- Fiebach C. J., Gruber T., Supp G. G.2005Neural mechanisms of repetition priming in occipitotemporal cortex: spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. J. Neurosci. 25, 3414–3422 (doi:10.1523/JNEUROSCI.4107-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster K. I., Davis C.1984Repetition and frequency attenuation in lexical access. J. Exp. Psychol. Learn. Mem. Cogn. 10, 680–698 (doi:10.1037/0278-7393.10.4.680) [Google Scholar]
- Gaskell M. G., Dumay N.2003Lexical competition and the acquisition of novel words. Cognition 89, 105–132 (doi:10.1016/S0010-0277(03)00070-2) [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Sigman M.2007Brain states: top-down influences on sensory processing. Neuron 54, 677–696 (doi:10.1016/j.neuron.2007.05.019) [DOI] [PubMed] [Google Scholar]
- Halderman L. K., Chiarello C.2005Cerebral asymmetries in early orthographic and phonological reading processes. Brain Lang. 95, 342–352 (doi:10.1016/j.bandl.2005.02.005) [DOI] [PubMed] [Google Scholar]
- Harm M. W., Seidenberg M. S.1999Phonology, reading and dyslexia: insights from connectionist models. Psychol. Rev. 106, 491–528 (doi:10.1037/0033-295X.106.3.491) [DOI] [PubMed] [Google Scholar]
- Harm M. W., Seidenberg M. S.2004Computing the meaning of words in reading: cooperative division of labor between visual and phonological processes. Psychol. Rev. 111, 662–720 (doi:10.1037/0033-295X.111.3.662) [DOI] [PubMed] [Google Scholar]
- Hauk O., Pulvermüller F.2004Effects of word length and frequency on the human event-related potential. Clin. Neurophysiol. 115, 1090–1103 (doi:10.1016/j.clinph.2003.12.020) [DOI] [PubMed] [Google Scholar]
- Hellige J. B.1993Hemispheric asymmetry? What's right and what's left? Cambridge, MA: Harvard University Press [Google Scholar]
- Henson R. N. A.2001Repetition effects for words and nonwords as indexed by event-related fMRI: a preliminary study. Scand. J. Psychol. 42, 179–186 (doi:10.1111/1467-9450.00229) [DOI] [PubMed] [Google Scholar]
- Henson R. N. A.2003Neuroimaging studies of priming. Prog. Neurobiol. 70, 53–81 (doi:10.1016/S0301-0082(03)00086-8) [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D.2007The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402 (doi:10.1038/nrn2113) [DOI] [PubMed] [Google Scholar]
- Hunter Z. R., Brysbaert M.2008Visual half field experiments are a good measure of cerebral language dominance if used properly. Neuropsychologia 46, 316–325 (doi:10.1016/j.neuropsychologia.2007.07.007) [DOI] [PubMed] [Google Scholar]
- Hunter Z. R., Brysbaert M., Knecht S.2007Foveal reading requires interhemispheric communication. J. Cogn. Neurosci. 19, 1373–1387 (doi:10.1162/jocn.2007.19.8.1373) [DOI] [PubMed] [Google Scholar]
- Indefrey P., Kleinschmidt A., Merboldt K.-D., Krüger G., Brown C., Hagoort P., Frahm J.1997Equivalent responses to lexical and nonlexical visual stimuli in occipital cortex: a functional magnetic resonance imaging study. Neuroimage 5, 78–81 (doi:10.1006/nimg.1996.0232) [DOI] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N.2003Evaluation of the dual-route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage 20, 693–712 (doi:10.1016/S1053-8119(03)00343-4) [DOI] [PubMed] [Google Scholar]
- Jordan T. R., Paterson K.2009Re-evaluating split-fovea processing in visual word recognition: a critical assessment of recent research. Neuropsychologia 47, 2341–2353 (doi:10.1016/j.neuropsychologia.2008.07.020) [DOI] [PubMed] [Google Scholar]
- Jordan T. R., Paterson K., Stachurski M.2009Re-evaluating split-fovea processing in word recognition: effects of word length. Cortex 45, 495–505 (doi:10.1016/j.cortex.2007.07.007) [DOI] [PubMed] [Google Scholar]
- Juphard A., Carbonnel S., Valdoid S.2004Length effects in reading and lexical decision: evidence from skilled readers and a developmental dyslexic participant. Brain Cogn. 55, 332–340 (doi:10.1016/j.bandc.2004.02.035) [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Lupker S. J., Rastle K.2004Modulation of regularity and lexicality effects in reading aloud. Mem. Cogn. 32, 1255–1264 [DOI] [PubMed] [Google Scholar]
- Knecht S., Dräger B., Deppe M., Bobe L., Lohmann H., Floel A., Ringelstein E.-B., Henningsen H.2000Handedness and hemispheric language dominance in healthy humans. Brain 123, 2512–2518 (doi:10.1093/brain/123.12.2512) [DOI] [PubMed] [Google Scholar]
- Kujala J., Pammer K., Cornelissen P. L., Roebroeck P., Formisano E., Salmelin R.2007Phase coupling in a cerebro-cerebellar network at 8–13 Hz during reading. Cereb. Cortex 17, 1476–1485 (doi:10.1093/cercor/bhl059) [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M. A., Patterson K. E.1999Selective disorders of reading? Curr. Opin. Neurobiol. 9, 235–239 [DOI] [PubMed] [Google Scholar]
- Lavidor M., Ellis A. W.2001Mixed-case effects in lateralized word recognition. Brain Cogn. 46, 192–195 (doi:10.1016/S0278-2626(01)80063-4) [DOI] [PubMed] [Google Scholar]
- Lavidor M., Ellis A. W.2002Word length and orthographic neighborhood size effects in the left and right cerebral hemispheres. Brain Lang. 80, 45–62 (doi:10.1006/brln.2001.2583) [DOI] [PubMed] [Google Scholar]
- Lavidor M., Walsh V.2004The nature of foveal representation. Nat. Rev. Neurosci. 5, 729–735 (doi:10.1038/nrn1498) [DOI] [PubMed] [Google Scholar]
- Lavidor M., Ellis A. W., Shillcock R., Bland T.2001Evaluating a split processing model of visual word recognition: effects of word length. Cogn. Brain Res. 12, 265–272 (doi:10.1016/S0926-6410(01)00056-8) [DOI] [PubMed] [Google Scholar]
- Lavidor M., Ellis A. W., Pansky A.2002Case alternation and length effects in the two cerebral hemispheres: a study of English and Hebrew. Brain Cogn. 50, 257–271 (doi:10.1016/S0278-2626(02)00508-0) [DOI] [PubMed] [Google Scholar]
- Lavidor M., Hayes A., Shillcock R., Ellis A. W.2004Evaluating a split processing model of visual word recognition: effects of orthographic neighborhood size. Brain Lang. 88, 312–320 (doi:10.1016/S0093-934X(03)00164-0) [DOI] [PubMed] [Google Scholar]
- Leach L., Samuel A. G.2007Lexical configuration and lexical engagement: when adults learn new words. Cogn. Psychol. 55, 306–353 (doi:10.1016/j.cogpsych.2007.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell A. K., Nicholls M. E. R., Castles A.2002The effect of word length on hemispheric word recognition: evidence from unilateral and bilateral-redundant presentations. Brain Cogn. 48, 447–452 [PubMed] [Google Scholar]
- Maccotta L., Buckner R. L.2004Evidence for neural effects of repetition that directly correlate with behavioral priming. J. Cogn. Neurosci. 16, 1625–1632 (doi:10.1162/0898929042568451) [DOI] [PubMed] [Google Scholar]
- Martin C., Nazir T., Thierry G., Paulignan Y., Démonet F.2006Perceptual and lexical effects in letter identification: an event-related potential study of the word superiority effect. Brain Res. 1098, 153–160 (doi:10.1016/j.brainres.2006.04.097) [DOI] [PubMed] [Google Scholar]
- Mayall K., Humphreys G. W., Mechelli A., Olson A., Price C. J.2001The effects of case mixing on word recognition: evidence from a PET study. J. Cogn. Neurosci. 13, 844–853 (doi:10.1162/08989290152541494) [DOI] [PubMed] [Google Scholar]
- McCandliss B. D., Cohen L., Dehaene S.2003The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 7, 293–299 (doi:10.1016/S1364-6613(03)00134-7) [DOI] [PubMed] [Google Scholar]
- McCann R. S., Besner D.1987Reading pseudohomophones: implications for models of pronunciation assembly and the locus of frequency effects in naming. J. Exp. Psychol. Hum. Percept. Perform. 13, 13–24 [Google Scholar]
- McClelland J. L., Rumelhart D. E.1981An interactive activation model of context effects in letter perception: part 1. An account of basic findings. Psychol. Rev. 88, 375–407 (doi:10.1037/0033-295X.88.5.375) [PubMed] [Google Scholar]
- McKay A., Davis C., Savage G., Castles A.2008Semantic involvement in reading aloud: evidence from a nonword reading study. J. Exp. Psychol. Learn. Mem. Cogn. 34, 1495–1517 (doi:10.1037/a0013357) [DOI] [PubMed] [Google Scholar]
- Mechelli A., Friston K. J., Price C. J.2000The effects of presentation rate during word and pseudoword reading: a comparison of PET and fMRI. J. Cogn. Neurosci. 12(Suppl. 2), 145–156 [DOI] [PubMed] [Google Scholar]
- Mechelli A., Crinion J. T., Long S., Friston K. J., Lambon Ralph M. A., Patterson K., McClelland J. L., Price C. J.2005Dissociating reading processes on the basis of neural interactions. J. Cogn. Neurosci. 17, 1753–1765 (doi:10.1162/089892905774589190) [DOI] [PubMed] [Google Scholar]
- Molko N., Cohen L., Mangin J. F., Chochon F., Lehéricy S., Le Bihan D., Dehaene S.2002Visualising the neural basis of a disconnection syndrome with diffusion tensor imaging. J. Cogn. Neurosci. 14, 629–636 (doi:10.1162/08989290260045864) [DOI] [PubMed] [Google Scholar]
- New B., Ferrand L., Pallier C., Brysbaert M.2006Reexamining the word length effect in visual word recognition: new evidence from the English Lexicon Project. Psychonom. Bull. Rev. 13, 45–52 [DOI] [PubMed] [Google Scholar]
- Oldfield R. C.1971The assessment and analysis of handedness. The Edinburgh inventory. Neuropsychologia 9, 93–113 [DOI] [PubMed] [Google Scholar]
- Paap K. R., Newsome S. L., McDonald J. E., Schvaneveldt R. W.1982An activation-verification model for letter and word recognition. Psychol. Rev. 89, 573–594 (doi:10.1037/0033-295X.89.5.573) [PubMed] [Google Scholar]
- Patterson K., Nestor P. J., Rogers T. T.2007Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987 (doi:10.1038/nrn2277) [DOI] [PubMed] [Google Scholar]
- Price C. J., McCrory E.2005Functional brain imaging studies of skilled reading and developmental dyslexia. In The science of reading: a handbook (eds Snowling M. J., Hulme C.), pp. 473–496 Oxford, UK: Blackwell [Google Scholar]
- Rastle K., Coltheart M.1999Serial and strategic effects in reading aloud. J. Exp. Psychol. Hum. Percept. Perform. 25, 482–503 (doi:10.1037/0096-1523.25.2.482) [Google Scholar]
- Rumelhart D. E., McClelland J. L.1982An interactive activation model of context effects in letter perception: Part 2. The contextual enhancement effect and some tests and extensions of the model. Psychol. Rev. 89, 60–94 (doi:10.1037/0033-295X.89.1.60) [PubMed] [Google Scholar]
- Rumelhart D. E., McClelland J. L.1985Distributed memory and the representation of general and specific information. J. Exp. Psychol. Gen. 114, 159–197 [DOI] [PubMed] [Google Scholar]
- Salasoo A., Shiffrin R. M., Feustel T. C.1985Building permanent memory codes: codification and repetition effects in word identifications. J. Exp. Psychol. Gen. 114, 50–77 (doi:10.1037/0096-3445.114.1.50) [DOI] [PubMed] [Google Scholar]
- Scarborough D. L., Cortese C., Scarborough H. S.1977Frequency and repetition effects in lexical memory. J. Exp. Psychol. Hum. Percept. Perform. 3, 1–17 (doi:10.1037/0096-1523.3.1.1) [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A.2002E-prime user's guide Pittsburgh, PA: Psychology Software Tools [Google Scholar]
- Shillcock R., Ellison T. M., Monaghan P.2000Eye-fixation behavior, lexical storage and visual word recognition in a split processing model. Psychol. Rev. 107, 824–851 (doi:10.1037/0033-295X.107.4.824) [DOI] [PubMed] [Google Scholar]
- Simos P. G., Billingsley-Marshall R., Sarkari S., Papanicolaou A. C.2008Single-word reading: perspectives from magnetic source imaging. In Single-word reading: behavioral and biological perspectives (eds Grigorenko E. L., Naples A. J.), pp. 211–232 New York, NY: Lawrence Erlbaum Associates [Google Scholar]
- Stark C. E. L., McClelland J. L.2000Repetition priming of words, pseudowords, and nonwords. J. Exp. Psychol. Learn. Mem. Cogn. 26, 945–972 (doi:10.1037/0278-7393.26.4.945) [DOI] [PubMed] [Google Scholar]
- Tarkiainen A., Helenius P., Hansen P. C., Cornelissen P. L., Salmelin R.1999Dynamics of letter string perception in the human occipitotemporal cortex. Brain 122, 2119–2131 (doi:10.1093/brain/122.11.2119) [DOI] [PubMed] [Google Scholar]
- Vigneau M., Jobard G., Mazoyer B., Tzourio-Mazoyer N.2005Word and non-word reading: what role for the visual word form area? NeuroImage 27, 694–705 (doi:10.1016/j.neuroimage.2005.04.038) [DOI] [PubMed] [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J. P., Sigman M., Cohen L.2007Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron 55, 143–156 (doi:10.1016/j.neuron.2007.05.031) [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Henson R. N., Driver J., Dolan R. J.2002Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat. Neurosci. 5, 491–499 (doi:10.1038/nn839) [DOI] [PubMed] [Google Scholar]
- Weekes B. S.1997Differential effects of number of letters on word and nonword naming latency. Q. J. Exp. Psychol. 50A, 439–456 [Google Scholar]
- Weems S. A., Zaidel E.2005Repetition priming within and between the two cerebral hemispheres. Brain Lang. 93, 298–307 (doi:10.1016/j.bandl.2004.10.009) [DOI] [PubMed] [Google Scholar]
- Whitney C.2001How the brain encodes the order of letters in a printed word: the SERIOL model and selective literature review. Psychonom. Bull. Rev. 8, 221–243 [DOI] [PubMed] [Google Scholar]
- Whitney C., Lavidor M.2004Why word length only matters in the left visual field. Neuropsychologia 42, 1680–1688 (doi:10.1016/j.neuropsychologia.2004.04.007) [DOI] [PubMed] [Google Scholar]
- Wydell T. N., Vuorinen T., Helenius P., Salmelin R.2003Neural correlates of letter-string length and lexicality during reading in a regular orthography. J. Cogn. Neurosci. 15, 1052–1062 (doi:10.1162/089892903770007434) [DOI] [PubMed] [Google Scholar]
- Young A. W., Ellis A. W.1985Different methods of lexical access for words presented in the left and right visual fields. Brain Lang. 24, 326–358 (doi:10.1016/0093-934X(85)90139-7) [DOI] [PubMed] [Google Scholar]
- Zaidel E., Clarke J. M., Suyenobu B.1990Hemispheric independence: a paradigm case for cognitive neuroscience. In Neurobiology of higher cognitive function (eds Schneibel A. B., Wechsler A. F.), pp. 297–355 New York, NY: Guildford Press [Google Scholar]