Abstract
The Acanthopanax koreanum fruit is a popular fruit in Jeju Island, but the byproducts of the alcoholic beverage prepared using this fruit are major agricultural wastes. The fermentability of this waste causes many economic and environmental problems. Therefore, we investigated the suitability of using A. koreanum fruit waste (AFW) as a source of antiinflammatory agents. AFWs were extracted with 80% EtOH. The ethanolic extract was then successively partitioned with hexane, CH2Cl2, EtOAc, BuOH, and water. The results indicate that the CH2Cl2 fraction (100 μg/mL) of AFW inhibited the LPS-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production in RAW 264.7 cells by 79.6% and 39.7%, respectively. These inhibitory effects of the CH2Cl2 fraction of AFWs were accompanied by decreases in the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) proteins and iNOS and COX-2 mRNA in a dose-dependent pattern. The CH2Cl2 fraction of AFWs also prevented degradation of IκB-α in a dose-dependent manner. Ursolic acid was identified as major compound present in AFW, and CH2Cl2 extracts by high performance liquid chromatography (HPLC). Furthermore using pure ursolic acid as standard and by HPLC, AFW and CH2Cl2 extracts was found to contain 1.58 mg/g and 1.75 mg/g, respectively. Moreover, we tested the potential application of AFW extracts as a cosmetic material by performing human skin primary irritation tests. In these tests, AFW extracts did not induce any adverse reactions. Based on these results, we suggest that AFW extracts be considered possible anti-inflammatory candidates for topical application.
1. Introduction
Food- and beverage-processing industries create large quantities of byproducts that are difficult to dispose because of their high biological oxygen demand. These plant-material wastes may contain high levels of biological compounds that can adversely affect the environment. However, these biological compounds may also show many beneficial activities in humans, including antioxidant, antityrosinase, and antiinflammatory activities [1, 2]. A. koreanum is an economically important fruit of Jeju Island. Because of its special functionality and flavor, the fruit is processed into alcoholic liquors. After extraction, the fruit pulp is mostly dumped as waste at large expense. This waste causes many economical and environmental problems due to its fermentability. Therefore, it is worthwhile to determine how to utilize A. koreanum waste.
Acanthopanax species (Araliaceae) are widely distributed throughout Korea, Japan, China, and the far-eastern region of Russia [3]. Approximately 15 species of the genus Acanthopanax grow wild on the Korean peninsula. Among them, A. koreanum Nakai (Araliaceae) is a native plant that grows on Jeju Island in the south of Korea. Even though the roots and stems of A. koreanum have been used traditionally in Korea as a medicine that increases strength, energy, and general well-being, and in the treatment of rheumatism, diabetes, and hepatitis [4–8], there is little information on the biological potential of the A. koreanum fruit and its beverage-extracted waste.
During inflammation, macrophages play a central role in managing many different immunopathological phenomena, including the overproduction of proinflammatory cytokines and inflammatory mediators such as interleukin (IL)-1β, IL-6, nitric oxide (NO), prostaglandin E2 (PGE2), and tumour necrosis factor α (TNF-α). Indeed, a number of inflammatory stimuli, such as LPS (lipopolysaccharide) and proinflammatory cytokines, activate immune cells to upregulate these inflammatory states; therefore, these stimuli are useful targets in the development of new anti-inflammatory drugs and in the studies on the molecular anti-inflammatory mechanisms of a potential drug [9, 10].
Therefore, the present study focused on whether A. koreanum fruit waste (AFW) inhibited the production of NO and PGE2 and expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages. We also performed primary skin irritation tests on human skin and assessed the high-performance liquid chromatography (HPLC) fingerprint.
2. Methods
2.1. Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone (Logan, UT, USA). LPS (E. coli 0111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used were analytical grade. The Enzyme-linked immunosorbent assay (ELISA) kit for PGE2 was obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Antibody against inducible NOS (iNOS) was purchased from Calbiochem (San Diego, CA, USA) and antibodies against COX-2 and IκB-α were from Cell Signaling Technology (Beverly, MA, USA).
2.2. Materials and Solvent Extraction
AFWs were collected from Sansaemi Agricultural Association, Jeju Island, in October 2006. The materials for extraction were freeze-dried and then ground into a fine powder using a blender. The dried powder (50 g) was extracted with 80% ethanol (EtOH; 2 L) at room temperature for 24 hours and then evaporated under vacuum. The evaporated EtOH extract (20 g) was suspended in water (1 L) and fractionated with four solvents: n-hexane (1 L), dichloromethane (CH2Cl2; 1 L), ethyl acetate (EtOAc; 1 L), and butanol (BuOH; 1 L). The yield and recovery of these five solvent fractions were as follows (Figure 1): n-hexane (0.5 g, 2.5%), CH2Cl2 (1.2 g, 6.0%), EtOAc (2.0 g, 10.0%), BuOH (4.0 g, 20.0%), and H2O (12.2 g, 60.1%).
Figure 1.
Fraction scheme of AFW ethanolic extract.
2.3. Cell Culture
The murine macrophage RAW 264.7 (1.0 × 106 cells/mL) cells were purchased from the Korean Cell Line Bank (Seoul, Korea) and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, streptomycin (100 μg/mL), and penicillin (100 U/mL) at 37°C in a 5% CO2 atmosphere.
2.4. MTT Assay for Cell Viability
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW 264.7 cells (1.0 × 104 cells/mL) were cultured in 96-well plates for 18 hours, followed by treatment with LPS (1 μg/mL) in the presence of various concentrations (12.5, 25, 50, 100 μg/mL) of A. koreanum extract. After 24-hour incubation, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) stock solution (50 μl; 2 mg/mL in PBS) was added to the medium, and the medium was incubated for 4 hours. Then, the supernatant was removed, and the obtained formazan crystals were dissolved in 200 μL of dimethylsulfoxide (DMSO). Absorbance was measured at 540 nm. Percent of cells showing cytotoxicity was determined relative to the control group.
2.5. Measurement of NO Production
Nitrite in culture medium was measured by adding 100 μl of Griess reagent (1% sulfanilamide and 0.1% N-[1-naphthyl]-ethylenediamine dihydrochloride in 5% phosphoric acid) to 100 μl aliquots of medium. The concentration of NO2− was calculated by comparison to a standard curve prepared using NaNO2.
2.6. Measurement of PGE2 Production
The inhibitory effect of the A. koreanum extract on PGE2 production in LPS-treated RAW 264.7 cells was determined as previously described [11–13]. Medium was then harvested and assayed by ELISA.
2.7. RNA Preparation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
The mRNA expression was measured by RT-PCR. Total RNA was isolated using Tri-Reagent (MRC, Cincinnati, OH, USA) according to the manufacturer's instructions. RNA isolation was carried out in an RNase-free environment. Then, 4 μg of RNA were reverse-transcribed (RT) using MuLV reverse transcriptase (Promega, WI, USA), oligo (dT)15 primer, dNTP (0.5 μM), and 1 U RNase inhibitor. PCR analyses were performed with a DNA gene cycler (BIO-RAD, HC, USA) with 30 cycles for amplification of β-actin, iNOS, and COX-2. The PCR products were electrophoresed on 1.0% agarose gel and visualized by ethidium bromide (EtBr) staining and a gel documentation system (Gel Doc 2000, Life Science Research, Hercules, CA).
2.8. Western Blot Analysis
RAW 264.7 cells were preincubated for 18 hours before being stimulated with LPS (1 μg/mL) in the presence of test materials for 24 hours. After incubation, the cells were washed twice with cold PBS. The cells were lysed in lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 2 mM EDTA, 1 mM EGTA, 1 mM NaVO3, 10 mM NaF, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and kept on ice for 30 minutes. The cell lysates were centrifuged at 15,000 rpm at 4°C for 15 minutes and the supernatants were stored at −70°C until use. Protein concentration was measured using the Bradford method [14]. The cell lysates (30 μg) were separated by 8~12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene fluoride (PVDF) membrane (BIO-RAD, HC, USA). The membrane was incubated for 2 hours with TTBS (Tris-buffered Saline with tween-20) containing 1% bovine serum albumin (BSA) and then incubated with specific primary antibody (mouse monoclonal antirabbit iNOS, antimouse COX-2, or antimouse IκB-α) at 4°C overnight. The membrane was washed 4 times with TTBS and incubated for 30 minutes with peroxidase-conjugated secondary antibody (1 : 5000) at room temperature. Finally, immunoreactive proteins were detected using the WEST-ZOL Western Blot Detection System (iNtRON, Gyeonggi, Korea).
2.9. Human Skin Primary Irritation Test
This study was conducted in accordance with the intent and purpose of the Good Clinical Practice regulations described in the Code of Federal Regulations (CFR) Title 21, Part 50, 56, 312, and/or the Declaration of Helsinki, as appropriate. The subjects were healthy, nonsmoking women of Korean origin. The other inclusion criteria were age >20 years and skin types II and III according to the Fitzpatrick classification system [15]. The exclusion criteria were skin types I or IV, allergies, skin diseases, photosensitivity, sunbed tanning, metabolic diseases, use of any drugs (except contraceptives), alcohol consumption, infections, pregnancy, breast feeding, and participation in other studies during the last 1 month. An IQ Ultra Chamber was secured to the back of each subject with micropore tape. The round border of the chamber was placed firmly against the skin, causing tight occlusion of the test materials. The 80% EtOH extract of AFW formulated with squalane was prepared as the negative control and applied at 1% concentrations. The patches (chambers) remained in place for 48 hours. During this time, the subjects abstained from showering or performing any work or exercise that might wet or loosen the patches. The sites were read 30 minutes and 24 hours after the patches were removed; the readings were scored according to the Cosmetic, Toiletry, and Fragrance Association (CTFA) guidelines [16].
2.10. HPLC Fingerprint of AFW Extract and CH2Cl2 Fraction
Since ursolic acids had been reported as effective anti-inflammatory components from plants, we searched for ursolic acids in the AFW extract and CH2Cl2 fraction. Chromatographic analysis of AFW ethanolic extracts was performed using a high-performance liquid chromatograph (HPLC) with an Alliance Waters 2695 separation module coupled to a Waters 2998 photodiode array detector, utilizing a Capcell pak 4.6 mm × 250 mm C18 column (Particle size 5 μ, Shiseido Chemicals, Tokyo) at a flow rate of 1.0 ml/min. The column was placed in a column oven at 25°C. Mobile phase loading was performed in the isocratic mode using methanol: 0.5% acetic acid in water (88 : 12, v/v); the injection volume was 10 μl, and UV detection was performed at 203 nm. Detection of the ursolic acid content in the AFW extract and the CH2Cl2 fraction was performed using the external standard method, and pure ursolic acid was used as the standard stock solution (10, 20, 50, and 100 μg/mL).
2.11. Statistical Analysis
Results are expressed as mean ± standard error of at least triplicate experiments. Student's t-test was used to assess the statistical significance of differences. P-values of less than .05 were considered statistically significant.
3. Results and Discussion
3.1. Inhibitory Effects of AFW on NO/iNOS and PGE2/COX-2 Inflammatory Pathways
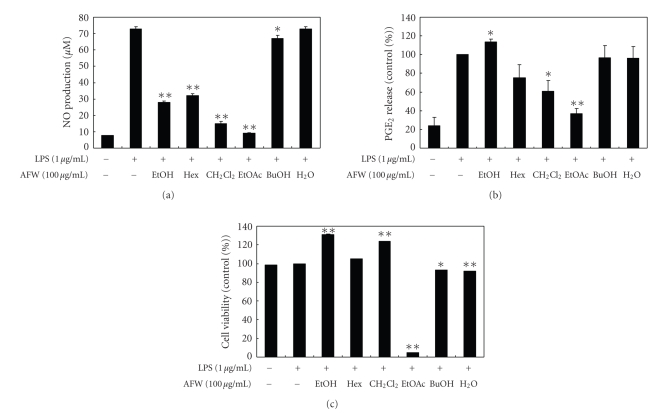
To investigate the effect of AFW on NO production, we measured the accumulation of nitrite, a stable oxidized product of NO, in culture media. NO production was examined in RAW 264.7 cells stimulated with LPS in the presence or absence of AFW extracts for 24 hours. Nitrite levels in LPS-stimulated cells increased significantly compared to levels in control cells. To evaluate whether AFW extracts could modulate NO production in activated macrophages, we examined the effects of the hexane, CH2Cl2, EtOAc, BuOH, and water fractions of AFW on NO production. As shown in Figure 2(a), the CH2Cl2 extract (100 μg/mL) inhibited the LPS-induced NO production in RAW 264.7 cells by 79.6%. The IC50 value of the CH2Cl2 extract, which was calculated from the graph, was 49.2 μg/mL. The ethanolic extract, hexane, and EtOAc fractions also inhibited LPS-induced production of NO by 61.4%, 55.8%, and 87.3%, respectively.
Figure 2.
Effect of crude extract and solvent fractions from A. koreanum fruit waste on nitric oxide (a) and PGE2 (b) production in RAW 264.7 cells. Effects of crude extract and solvent fractions from A. koreanum fruit waste on the nitric oxide production in RAW 264.7 cells were determined from the 24-hr culture of cells stimulated with LPS (1 μg/mL) in the presence of A. koreanum . Nitric oxide production was determined by the Griess reagent method. PGE2 released into the culture medium was assayed by ELISA. The data represent the mean ± SD of triplicate experiments. Values are the mean ± SEM of triplicate experiments. *P < .05; **P < .01.
The inducible enzyme COX-2 is expressed in the early stages of the inflammatory response and catalyzes the first step of the synthesis of PGE2, an important inflammatory mediator. In a variety of inflammatory cells, including macrophages, COX-2 is induced by cytokines and other activators, such as LPS, resulting in the release of a large amount of prostaglandin E2 at inflammatory sites [17, 18]. Therefore, we examined the effects of AFW on PGE2 production in LPS-stimulated RAW 264.7 macrophages. When macrophages were stimulated with LPS (1 μg/mL) for 24 hours, the levels of PGE2 increased in the culture medium. As shown in Figure 2(b), the EtOAc, CH2Cl2, and hexane fractions (100 μg/mL) suppressed LPS-induced PGE2 production by 63.1%, 39.0%, and 24.9%, respectively. As determined by MTT assays, the numbers of viable activated macrophages were not altered by the solvent fractions, indicating that the inhibition of NO synthesis by the ethanolic extract, hexane, and CH2Cl2 fractions was not simply due to cytotoxic effects. Although the EtOAc fraction also significantly inhibited NO synthesis at 100 μg/mL, this effect may have been caused by cytotoxicity (Figure 2(c)).
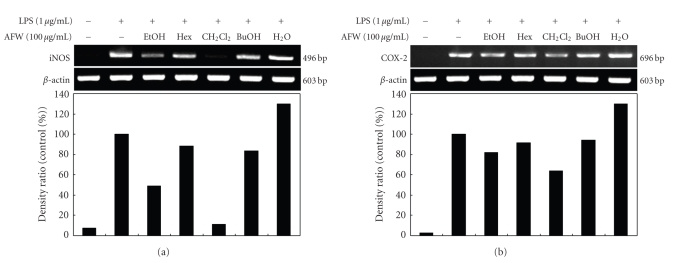
To further evaluate whether the inhibition of LPS-stimulated NO and PGE2 production by AFW was mediated by the regulation of iNOS and COX-2 gene expression, RT-PCR analyses were performed. As shown in Figure 3, the expressions of iNOS and COX-2 mRNA were significantly elevated in macrophages treated with LPS (1 μg/mL) compared to those in unstimulated cells (control). RT-PCR analyses indicated that AFW reduced iNOS and COX-2 mRNA without affecting the mRNA of β-actin, a house-keeping protein. Among the five AFW fractions, the CH2Cl2 fraction markedly reduced the gene expression of iNOS and COX-2. Therefore, the inhibitory effect of AFW on iNOS and COX-2 gene expression is one possible mechanism for the anti-inflammatory action of AFW. In conclusion, AFW actively suppressed the expression of genes implicated in inflammation.
Figure 3.
Inhibitory effects of AFW extracts and solvent fractions on iNOS (a) and COX-2 (b) mRNA expression in RAW 264.7 cells. RAW 264.7 cells (5.0 × 105 cells/mL) were pre-incubated for 18 hours, and the iNOS mRNA expression was determined in cells stimulated with LPS (1 μg/mL) for 24 hours in the presence of 80% EtOH extract and solvent fractions (100 μg/mL) of AFW.
3.2. Effects of the CH2Cl2 Fraction of AFW on LPS-Induced NO and PGE2 Production and Cell Viability
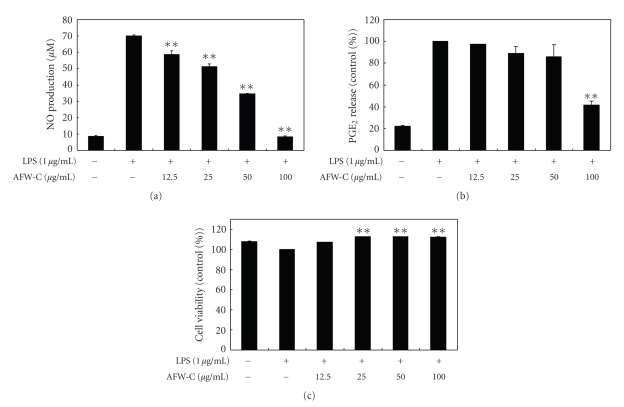
To analyze the potential anti-inflammatory properties of the CH2Cl2 fraction of AFW, cells were incubated with LPS (1 μg/mL) in the presence of various concentrations of the CH2Cl2 fraction (12.5, 25, 50, and 100 μg/mL). The cell culture media were collected; the nitrite and PGE2 levels were determined; and the CH2Cl2 fraction of AFW was found to reduce NO production in a dose-dependent manner. The IC50 value of the CH2Cl2 extract, which was calculated from the graph, was 54.7 μg/mL (Figure 4(a)). The CH2Cl2 fraction of AFW also dose-dependently inhibited PGE2 production (Figure 4(b)). The potential cytoxicity of CH2Cl2 fraction of AFW was evaluated by MTT assay after incubating cells for 24 hours in the absence or presence of LPS; cell viabilities were not affected at the concentrations used (12.5, 25, 50, 100 μg/mL) to inhibit NO and PGE2 (Figure 4(c)). Thus, the inhibitory effects were not attributable to cytotoxic effects.
Figure 4.
Effect of CH2Cl2 fractions from A. koreanum fruit waste on nitric oxide (a) and PGE2 (b) production in RAW 264.7 cells. Effects of CH2Cl2 fractions from A. koreanum fruit waste on nitric oxide production in RAW 264.7 cells were determined from the 24-hr culture of cells stimulated with LPS (1 μg/mL) in the presence of CH2Cl2 fractions. Nitric oxide production was determined by the Griess reagent method. PGE2 released into the culture medium was assayed by ELISA. The data represent the mean ± SD of triplicate experiments. Values are the mean ± SEM of triplicate experiments. *P < .05; **P < .01.
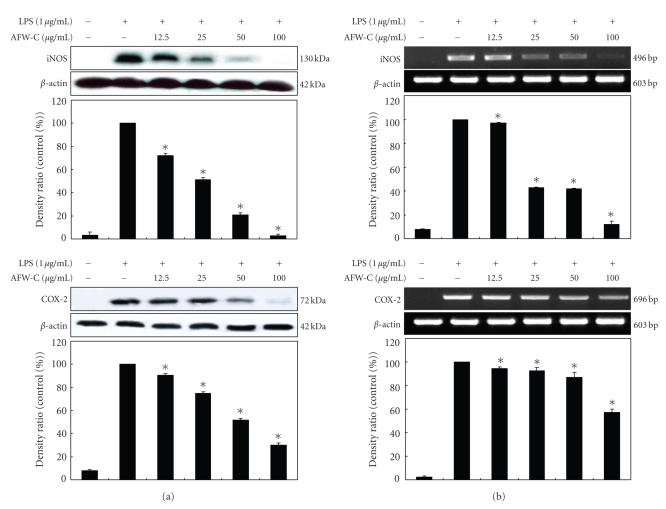
3.3. Effects of the CH2Cl2 Fraction of AFW on LPS-Induced iNOS and COX-2 Protein and mRNA Expressions
Western blot and RT-PCR analyses were performed to determine whether the inhibitory effects of the CH2Cl2 fraction of AFW on the proinflammatory mediators (NO and PGE2) were related to modulation of the expression of iNOS and COX-2. In unstimulated RAW 264.7 cells, iNOS and COX-2 protein and mRNA were not detected; however, LPS remarkably upregulated their protein levels, and pretreatment with the CH2Cl2 fraction of AFW inhibited these upregulations (Figure 5(a)). On the other hand, the CH2Cl2 fraction of AFW did not affect the expression of β-actin, a housekeeping gene. In general, these results indicate that the inhibitory effects of the CH2Cl2 fraction of AFW on LPS-induced NO and PGE2 production involve iNOS and COX-2 suppression. Furthermore, RT-PCR analysis showed that mRNA expression levels of iNOS and COX-2 correlated with their protein levels (Figure 5(b)).
Figure 5.
Effect of CH2Cl2 fraction of Acanthopanax koreanum fruit waste on the mRNA expression and protein levels of iNOS (a) and COX-2 (b) in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells (5.0 × 105 cell/mL) were stimulated with LPS (1 μg/mL) and the CH2Cl2 fraction of A. koreanum (12.5, 25, 50, 100 μg/mL) for 24 hours. The mRNA expression of iNOS and COX-2 was determined by RT-PCR. For the western blot analysis, RAW 264.7 cells (1.0 × 106 cell/mL) were stimulated with LPS (1 μg/mL) in the presence of A. koreanum (12.5, 25, 50, 100 μg/mL) for 24 hours. Whole-cell lysates (25 μg) were prepared and the protein was subjected to 10% SDS-PAGE; expression of iNOS, COX-2 and β-actin were determined by western blotting. β-Actin served as a loading control. The mRNA expression of iNOS and COX-2 was determined by RT-PCR.
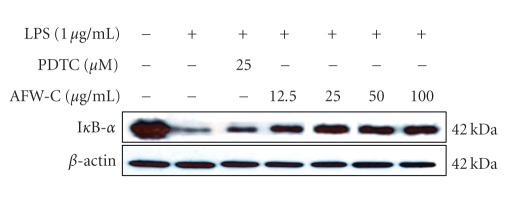
3.4. The CH2Cl2 Fraction of AFW Suppresses the Degradation of IκB-α in LPS-Activated Macrophages
Because activation of NF-κB is critical for induction of both COX-2 and iNOS by LPS or other inflammatory cytokines, we explored whether the CH2Cl2 fraction of AFW might be involved in the NF-κB pathway in LPS-activated macrophages. Since it has been well documented that activation of NF-κB correlates with rapid proteolytic degradation of IκB [19–22], prevention of IκB degradation was studied as an indication of inhibition of NF-κB activation by the CH2Cl2 fraction of AFW. As a control, we also applied pyrrolidine dithiocarbamate (PDTC), a specific NF-κB inhibitor. As shown in Figure 6, LPS induced transient degradation of IκB in RAW cells, whereas the CH2Cl2 fraction of AFW and PDTC prevented degradation of IκB in a dose-dependent manner. These results suggest that inhibition of COX-2 and iNOS expression by the CH2Cl2 fraction of AFW occurred via suppression of IκB degradation, thereby preventing NF-κB activation.
Figure 6.
Effects of CH2Cl2 fraction from A. koreanum on the degradation of IκB-α in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells (1.0 × 106 cells/mL) were stimulated with LPS (1 μg/mL) in the presence of A. koreanum (12.5, 25, 50, 100 μg/mL) for 15 minutes. As a control, we also applied PDTC (25 μM), a specific NF-κB inhibitor. Whole cell lysates (25 μg) were prepared and the protein was subjected to 12% SDS-PAGE. Expression of IκB-α and β-actin were determined by western blotting. β-Actin served as a loading control.
3.5. Human Skin Primary Irritation Test of AFW Ethanol Extract
To evaluate the irritation effect of AFW extracts for clinical applications to human skin, a patch test was performed. In our study, as shown in Table 1, none of the 32 subjects experienced a reaction based on the 48- and 72- hour readings. Specifically, we did not observe any adverse reactions such as erythema, burning, or pruritus in the study subjects that was related to the topical treatment of AFW extracts.
Table 1.
Results of human skin primary irritation tests (n = 32).
| Test materials | No. of responders | 48 hours | 72 hours | Reaction gradea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | 1+ | 2+ | 3+ | 48 hours | 72 hours | Mean | ||
| Squalane | 0 | b— | — | — | — | — | — | 0.0 | 0.0 | 0.0 |
| AFW extracts (1%) | 0 | — | — | — | — | — | — | 0.0 | 0.0 | 0.0 |
aReaction grade = Σ[{Grade × no. of responders}/{4 (maximum grade) × 32 (total subjects)}] × 100 × (1/2).
bNo reaction.
3.6. HPLC Fingerprint of AFW Ethanol Extract
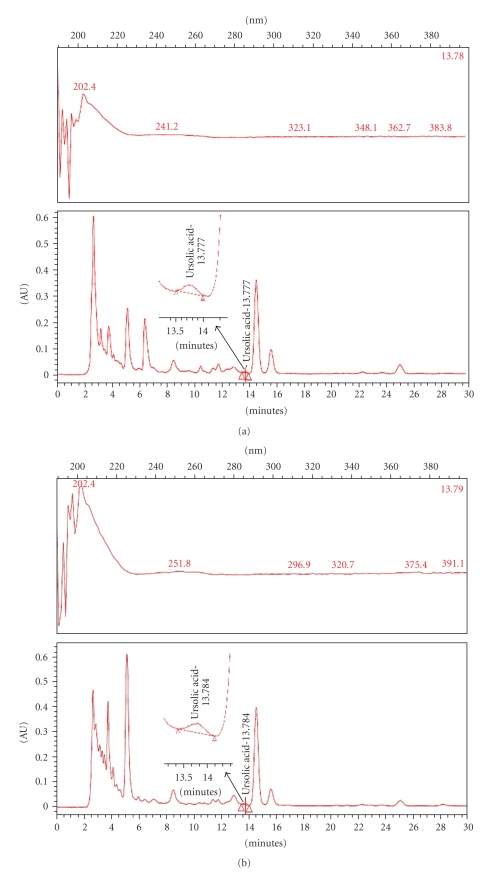
According to traditional oriental medicine, the therapeutic actions of herbal medicines are based on integral interaction of many ingredients combined. With the development of analytical technology, chromatographic methods can now be used to develop fingerprints of traditional oriental medicine and their raw materials. Thus, interest in HPLC fingerprint analysis has increased, not only in Asia but also around the world [23–25]. Therefore, a simple HPLC fingerprint was developed in this work. Since ursolic acids have been reported as effective anti-inflammatory ingredients in the Acanthopanax plant, they were used as standard substances. The conditions described in the experimental section yielded good resolution and well-defined peaks for ursolic acids in the AFW extract and the CH2Cl2 fraction. The ursolic acid content in the ethanol extract and CH2Cl2 fraction were 1.58 mg/g and 1.75 mg/g, respectively (Figure 7).
Figure 7.
HPLC (high performance liquid chromatography) fingerprint of ethanol extract and its CH2Cl2 fractions. A. koreanum ethanol extract (a) and its CH2Cl2 fractions (b) were analyzed by HPLC. The lower side (c) represents standard ursolic acid. Ursolic acid exhibits peak absorbance at 203-204 wavelength.
4. Conclusion
The present study was undertaken in order to better utilize A. koreanum fruit wastes (AFW) as functional materials. Since nitric oxide and prostaglandins, which are produced by iNOS and COX-2, respectively, have been implicated as important mediators in endotoxemia and inflammatory conditions, we first identified that AFW suppressed the production of NO and PGE2 in LPS-stimulated RAW 264.7 cells. This suppression correlated with downregulated gene expression of iNOS and COX-2. However, although other possible inhibitory mechanisms toward the proinflammatory cytokines remain to be evaluated in further studies, we determined that AFW prevented degradation of IκB in a dose-dependent manner. These results suggest that inhibition of COX-2 and iNOS expression by AFW partially occurred via suppression of IκB degradation, which thereby prevented NF-κB activation. To test the application of AFW extracts as topical materials, we performed primary skin irritation tests on human skin. AFW extracts did not induce any severe adverse reactions. Finally, the ursolic acids were also identified and quantified in AFW extracts. Based on our results, we suggest that AFW extracts be considered possible anti-inflammatory candidates for topical application.
Acknowledgment
The authors wish to acknowledge support from an MKE Grant (RTI04-02-07) and Research Grant of Jeju Special Self-Governing Province.
References
- 1.Kang HJ, Chawla SP, Jo C, Kwon JH, Byun MW. Studies on the development of functional powder from citrus peel. Bioresource Technology. 2006;97(4):614–620. doi: 10.1016/j.biortech.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Kim S-S, Lee J-A, Kim J-Y, Lee NH, Hyun C-G. Citrus peel wastes as functional materials for cosmeceuticals. Journal of Applied Biological Chemistry. 2008;51(1):7–12. [Google Scholar]
- 3.Phuong NT, Lee KA, Jeong SJ, et al. Capillary electrophoretic method for the determination of diterpenoid isomers in Acanthopanax species. Journal of Pharmaceutical and Biomedical Analysis. 2006;40(1):56–61. doi: 10.1016/j.jpba.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Na M, Oh WK, Kim YH, et al. Inhibition of protein tyrosine phosphatase 1B by diterpenoids isolated from Acanthopanax koreanum. Bioorganic and Medicinal Chemistry Letters. 2006;16(11):3061–3064. doi: 10.1016/j.bmcl.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Kang H-S, Kim Y-H, Lee C-S, Lee J-J, Choi I, Pyun K-H. Suppression of interleukin-1 and tumor necrosis factor-α production by acanthoic acid, (−)-pimara-9(11), 15-dien-19-oic acid, and its antifibrotic effects in vivo. Cellular Immunology. 1996;170(2):212–221. doi: 10.1006/cimm.1996.0154. [DOI] [PubMed] [Google Scholar]
- 6.Nan J-X, Jin X-J, Lian L-H, et al. A diterpenoid acanthoic acid from Acanthopanax koreanum protects against D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Biological and Pharmaceutical Bulletin. 2008;31(4):738–742. doi: 10.1248/bpb.31.738. [DOI] [PubMed] [Google Scholar]
- 7.Nan J-X, Park E-J, Nam JB, et al. Effect of Acanthopanax koreanum Nakai (Araliaceae) on D-galactosamine and lipopolysaccharide-induced fulminant hepatitis. Journal of Ethnopharmacology. 2004;92(1):71–77. doi: 10.1016/j.jep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Choi H-S, Kim HJ, Nam S-G, et al. Lupane glycosides from the leaves of Acanthopanax koreanum. Chemical and Pharmaceutical Bulletin. 2008;56(11):1613–1616. doi: 10.1248/cpb.56.1613. [DOI] [PubMed] [Google Scholar]
- 9.Jachak SM. PGE synthase inhibitors as an alternative to COX-2 inhibitors. Current Opinion in Investigational Drugs. 2007;8(5):411–415. [PubMed] [Google Scholar]
- 10.Zeilhofer HU, Brune K. Analgesic strategies beyond the inhibition of cyclooxygenases. Trends in Pharmacological Sciences. 2006;27(9):467–474. doi: 10.1016/j.tips.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Yoon WJ, Kim SS, Oh TH, Lee NH, Hyun C-G. Torreya nucifera essential oil inhibits skin pathogen growth and lipopolysaccharide-induced inflammatory effects. International Journal of Pharmacology. 2009;5(1):37–43. [Google Scholar]
- 12.Yoon W-J, Kim S-S, Oh T-H, Lee NH, Hyun C-G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids. 2009;44(5):471–476. doi: 10.1007/s11745-009-3297-3. [DOI] [PubMed] [Google Scholar]
- 13.Yoon W-J, Kim S-S, Oh T-H, Nam HL, Hyun C-G. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Polish Journal of Microbiology. 2009;58(1):61–68. [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 16.Casetti F, Jung W, Wölfle U, et al. Topical application of solubilized Reseda luteola extract reduces ultraviolet B-induced inflammation in vivo. Journal of Photochemistry and Photobiology B. 2009;96(3):260–265. doi: 10.1016/j.jphotobiol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Iezzi A, Ferri C, Mezzetti A, Cipollone F. COX-2: friend or foe? Current Pharmaceutical Design. 2007;13(16):1715–1721. doi: 10.2174/138161207780831293. [DOI] [PubMed] [Google Scholar]
- 18.Rainsford KD. Anti-inflammatory drugs in the 21st century. Sub-Cellular Biochemistry. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Mittal A. Current status of COX-2 inhibitors. Mini-Reviews in Medicinal Chemistry. 2008;8(1):73–90. doi: 10.2174/138955708783331577. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Jung KS, Jeong HG. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Letters. 2004;569(1–3):321–326. doi: 10.1016/j.febslet.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 21.Mauro C, Zazzeroni F, Papa S, Bubici C, Franzoso G. The NF-κB transcription factor pathway as a therapeutic target in cancer: methods for detection of NF-κB activity. Methods in Molecular Biology. 2009;512:169–207. doi: 10.1007/978-1-60327-530-9_10. [DOI] [PubMed] [Google Scholar]
- 22.Solt LA, May MJ. The IκB kinase complex: master regulator of NF-κB signaling. Immunologic Research. 2008;42(1–3):3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X-Y, Tao J-Y, Zhao L, et al. In vitro anti-inflammatory effects of different solution fractions of ethanol extract from Melilotus suaveolens Ledeb. Chinese Medical Journal. 2007;120(22):1992–1998. [PubMed] [Google Scholar]
- 24.Zhao L, Tao J-Y, Zhang S-L, et al. Inner anti-inflammatory mechanisms of petroleum ether extract from Melilotus suaveolens Ledeb. Inflammation. 2007;30(6):213–223. doi: 10.1007/s10753-007-9039-x. [DOI] [PubMed] [Google Scholar]
- 25.Yoon W-J, Ham YM, Yoo B-S, Moon J-Y, Koh J, Hyun C-G. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. Journal of Bioscience and Bioengineering. 2009;107(4):429–438. doi: 10.1016/j.jbiosc.2008.11.018. [DOI] [PubMed] [Google Scholar]