Abstract
CD8+ T cells have been shown to capture plasma membrane fragments from target cells expressing their cognate antigen, a process termed “trogocytosis”. Here, we report that human CD4, the Human Immunodeficiency Virus (HIV) receptor, can be found among the proteins transferred by trogocytosis. CD4 is expressed in a correct orientation after its capture by CD8+ T cells as shown by its detection using conformational antibodies and its ability to allow HIV binding on recipient CD8+ T cells. Although we could not find direct evidence for infection of CD8+ T cells having captured CD4 by HIV, CD4 was virologically functional on these cells as it conferred on them the ability to undergo syncytia formation induced by HIV-infected MOLT-4 cells. Our results show that acquisition of CD4 by CD8+ T cells via trogocytosis could play a previously unappreciated role for CD8+ T cells in HIV spreading possibly without leading to their infection.
1. Introduction
Trogocytosis refers to the process whereby lymphocytes capture plasma membrane fragments from target cells expressing their cognate antigen [1]. Among the components conveyed by membrane fragments from target cells, one finds the antigen itself but also additional molecules including costimulatory molecules, adhesion molecules, and various receptors [2] which can confer novel functions to acceptor lymphocytes. For instance, in the case of CD8+ CTL, capture of the antigen has been shown to result in their killing by CTL sharing the same antigen-specificity, a phenomenon known as fratricide and thought to be important for the contraction of the CTL response [3]. Another kind of consequence is that bystander captured molecules could help pathogens to spread through the organism. Natural killer cells (NK cells), which also perform trogocytosis, have recently been shown to capture virus receptors such as CD21, the receptor for Epstein-Barr virus (EBV) [4] and CD155, the receptor for poliovirus [5]. Whether these receptors are fully functional after their capture by NK cells remains unknown, but at least in the case of the EBV receptors, we have shown that its capture by NK cells confers them the ability to bind EBV [4]. The human CD4 antigen, the Human Immunodeficiency Virus (HIV) receptor, has previously been shown to be captured by CD8+ T cells in a rat [6] and in a mouse [7] model. Recently, by comparing the capture of a whole range of molecules associated to the plasma membrane, we found that CD4 was actually among the molecules that were most efficiently captured by murine T and B cells (manuscript submitted). Since trogocytosis had recently been implicated in HIV propagation between CD4+ T cells [8], we hypothesized that it could also play a role in the virus spreading to CD8+ T cells. Indeed, CD8+ CTL play an important role in the immune response against HIV infection [9], since, until the virus escapes the immune response, they are initially responsible for the elimination of most CD4+ infected cells until the virus escapes the immune response. Incidentally, it has been shown that during the late stages of HIV infection, a minute fraction of CD8+ T cells becomes infected [10] although the mechanism(s) leading to infection is(are) unknown [11, 12]. Among the possible mechanisms leading to CTL infection, it has been proposed that HIV could infect those CTL that express CD4 during their activation [13–20]. A recent report also suggests that CD8+CD4dim cells are enriched in anti-HIV lymphocytes [21]. It should be noted, however, that CD4 expression is not classically associated with the activation of CD8+ T cells and that not all HIV-infected CD8+ T cells are CD4+, suggesting that additional mechanisms may be involved. It has also been argued that double-positive CD4+CD8+ thymocytes, which could end up as CD8+ T cells at the end of their differentiation, could be targeted by HIV [22]. This hypothesis does, however, seem unlikely as a general explanation for the occurrence of HIV-infected CD8+ T cells because most of those are usually not naïve cells. Finally, some HIV strains have been shown to use CD8 as a receptor [23] but these particular virions were only isolated after many steps of enrichment, suggesting their very low frequency in natural viral stocks. Of interest, De Maria et al. reported that CD8+ T cells could be infected in culture, provided that CD4+ T cells were present, suggesting that direct contact between the two cells types is important for CTL infection [24, 25]. Combined with the notion that trogocytosis requires cell-to-cell contacts [2, 26], this last observation suggests that it is worth investigating if CD8+ T cells could capture CD4 from CD4-expressing cells in a coculture and, if so, whether this mechanism could result in CD8+ T cell infection.
2. Materials and Methods
2.1. Cell Lines and Mice
The T2 human lymphoblastoid cell line, which expresses HLA-A2.1 naturally, was used as a target cell in antigen (Ag)-mediated trogocytosis experiments. The mouse mastocytoma cell line P815, which naturally expresses FcγRII/III, and their stable transfectants expressing human CD4 fused or not to GFP were used as target cells in experiments involving redirected trogocytosis (i.e., trogocytosis triggered by antibodies (Ab)) [27]. The human embryonic kidney 293 (HEK) cell line and the stable transfectants of these cells expressing FcγRII/III and, for some of them, CD4 fused or not to GFP were used in the infection and syncytia formation assays. The human T-lymphoblastoid MOLT-4/CCR5 cell line uninfected or chronically infected with the NL4-3 HIV isolate (>90% producing HIV particles) has been previously described [28] and was used as a control in infection and in syncytia experiments. The human CD8+ T cell line specific for the CMV antigen N9V presented by HLA-A2.1 was a kind gift of Dr. Christian Davrinche (Toulouse). Peripheral blood cells from healthy blood donors were provided by the Banc de Sang i de Teixits (BST, Barcelona, Spain) and obtained after approval from the Ethical Committee of our research center. Peripheral blood mononuclear cells (PBMC) were purified by Ficoll density gradient, and immediately used to purify CD8+ T cells (>95%) by immunomagnetic negative selection (Miltenyi Biotec SL, Madrid, Spain). Purity of isolated populations (>95%) was assessed by flow cytometry after CD4 and CD8 staining (BD Biosciences). All cell lines were cultured in RPMI 1640 with 10% heat-inactivated fetal bovine serum (FBS), penicillin-streptomycin (100 U/mL), and 2 mM glutamine. Primary cells were cultured in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum (FBS, Invitrogen, Barcelona, Spain) in the absence of any other stimuli.
2.2. Reagents, Antibodies, and Molecular Biology
The N9V peptide (NLVPMVATV) was synthesized in our laboratory, HPLC-purified (>98%) and its identity confirmed by mass spectrometry. Monoclonal Abs (mAb) against human CD3 (OKT3) and human CD28 (kind gift from Dr. Pedro Romero) were obtained from hybridoma supernatants. The unlabelled mAb against the murine FcγRII/III (2.4G2) was produced in hybridoma supernatant. Fluorescent mAbs against CD4 (RPA-T4) or CD8 (RPA-T8) were from BD/Pharmingen. PE-labeled KC57 anti-HIV-p24 antigen mAb was from Beckman & Coulter. Vectors encoding human CD4 or CD4-GFP have been described previously [29]. The anti-CD4 mAb Leu3a was from Becton-Dickinson.
Trogocytosis assays were performed as previously described [27]. Target cells were cell surface biotinylated or not and incubated with effector CD8+ T cells (E : T = 1 : 5) for 1 hour at 37°C. For redirected trogocytosis experiments, T cells were previously incubated with 10% of the anti-CD3 and anti-CD28 mAbs supernatants for 15 minutes at 4°C before incubation with target cells (P815 or HEK-FcR) expressing human CD4 or CD4-GFP. In some assays, the blocking anti-FcγRII/III mAb 2.4G2 was preincubated with P815 cells before coculture with T cells. For Ag-dependent trogocytosis experiments, the corresponding N9V peptide (final, saturating concentration = 1 μM) was incubated with T2 target cells before T cells were added. At the end of the incubation period, T cells were then analyzed by flow cytometry on an FACSCalibur or LSRII (Becton Dickinson) using an anti-CD8 mAb to gate on the cells of interest and either fluorescent streptavidin (to detect biotin capture) or anti-CD4 RPA-T4 mAb (to detect CD4 capture). The efficiency of membrane capture was calculated as fold induction, that is, the median fluorescence intensity (MFI) of the molecule captured by acceptor cells that were cocultured with donor cells in the presence of the stimulus, divided by the MFI of acceptor cells cultured with donor cells in the absence of any stimulus.
2.3. Analysis of Sequential, Early Events of HIV Infection
2.3.1. HIV Binding to CD8+ T Cells
Chronically infected MOLT-4/CCR5 cells (200,000 cells/well) were cultured in 96-well plates either alone (control) or with 200,000 CD8+ T cells that had beforehand acquired CD4 or not by trogocytosis. Uninfected MOLT-4/CCR5 cells were used as negative control and in some assays, the anti-CD4 mAb Leu3a (0.25 μg/mL) that blocks HIV binding to CD4 was added. HIV particles associated to CD8+ T cells, which corresponds to incoming virions transferred during cellular contacts, were analyzed by flow cytometry after 24 hours of coculture. Fixed and permeabilized cells (Fix & Perm, Caltag) were stained with a PE-Cy-7-labeled anti-CD8 and the PE-labeled KC57 anti-HIV-p24 mAbs. P24 staining was analyzed in gated CD8+ T cells.
2.3.2. Analysis of Cell-to-Cell Fusion
In the cocultures described above, we monitored the formation of multinucleated giant cells (syncytia) resulting from the fusion of HIV infected cells with cells expressing viral receptors after 24 hours of coculture. Syncytium formation was scored by visualizing cultures in a Nikon Eclipse TE-200 microscope. Random fields were photographed and syncytia counted in at least four fields containing 500–600 cells.
2.3.3. Synthesis of Proviral DNA
In the same cocultures analyzed for HIV capture and syncytium formation, the newly synthesized proviral HIV DNA was quantified by real-time PCR as previously described [29, 30].
2.4. Analysis of Productive Infection
As an alternative approach to test the susceptibility of CD8+ T cells that had, or not, previously captured CD4 by trogocytosis to HIV infection, we performed classical cell-free virus infections. Briefly, 200,000 CD8+ T cells were incubated with a highly infectious NL4-3 virus stock produced in PBMCs of healthy donors (MOI = 1) in a final volume of 200 μl. All assays were performed in 96-well plates using RPMI1640 medium supplemented with 10% FBS. After 1, 3, and 5 days of culture at 37°C, supernatants were harvested and analyzed by ELISA (Innogenetics, Barcelona, Spain) for p24 content, as a measure of the production of HIV particles.
3. Results and Discussion
3.1. A Human CD8+ T Cell Line Captures CD4 by Trogocytosis from CD4+ Target Cells in the Presence of Their Cognate Antigen and Expresses it in a Correct Conformation
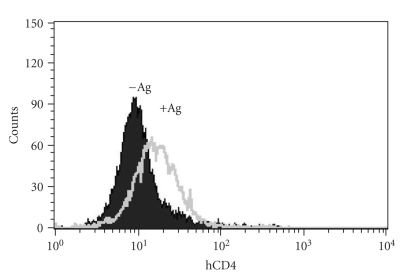
We and others have previously reported that CD4 was captured by CD8+ T cells upon antigen recognition on CD4-expressing target cells loaded with the cognate antigen recognized by CD8+ T cells in both a mouse and rat model [6, 7]. First we demonstrated that this was also true in a fully human system. CD4+ human T2 cells were incubated or not with the N9V antigen and washed thoroughly before incubation with the human CD8+CD4− T cell line specific for N9V. The cells were then analyzed by flow cytometry. As shown in Figure 1, after a 1-hour incubation period CD8+ T cells displayed CD4 expression when they were cocultured with T2 cells loaded with N9V but not in the absence of the antigen. Our results strongly suggest that in human cells as well, CD4 expression by CD8+ T cells can be due to capture from CD4-expressing T2 cells. Although it has occasionally been shown that activated CD8+ T cells can express CD4 as an activation marker [15, 18], whether this was due to de novo synthesis or capture was not determined in most studies where the expression of CD4 by CD8+ T cells was reported. This can be easily explained by the fact that the phenomenon of trogocytosis had not yet been described and characterized. In fact, in all the studies cited above [13, 14, 20, 31], the levels of CD4 expression reported were quite low compared with those detected on conventional CD4+ T cells, and would thus be compatible with the acquisition of CD4 by trogocytosis. Irrespective of the mechanism involved in CD4 expression by CD8+ T cells, it has been reported that CD8+CD4dim cells are enriched in anti-HIV lymphocytes [21], which would make these cells, which are by nature in frequent contacts with HIV-infected cells, possible targets of HIV infection.
Figure 1.
Human CD8+ CTL specific for the cytomegalovirus N9V Ag capture CD4 from T2 target cells. Human CD8+ T cells were incubated for 1 hour with T2 cells, which express CD4 and HLA-A2.1 naturally, that had been loaded (+Ag) or not (−Ag) with the N9V peptide. Cells were then analyzed by flow cytometry for CD4 and CD8 expression. Shown are overlapping histograms of CD4 expression on gated CD8+ T cells. Similar results were obtained in 3 experiments.
In our case, the fact that CD4 expression could be due to de novo synthesis is unlikely since, in our system, CD4 is expressed on CD8+ T cells after 1 hour of coincubation, or even sooner (not shown).
In addition, as the CMV-specific CTL used had been stimulated a few days earlier in the presence of PBMC and antigen (i.e., in conditions where trogocytosis occurs), this experiment measuring capture of CD4 shows a sequential gain and loss of the material acquired by trogocytosis. As the anti-CD4 mAb we used (clone RPA-T4) is conformational, our results also imply that the CD4 is exposed in a correct conformation to the extracellular milieu on the surface of the CD8+ T cells.
3.2. Redirected Trogocytosis Experiments Allow Nonantigen Specific CD8+ T Cells from Healthy Donors and HIV-Infected Patients to Capture CD4
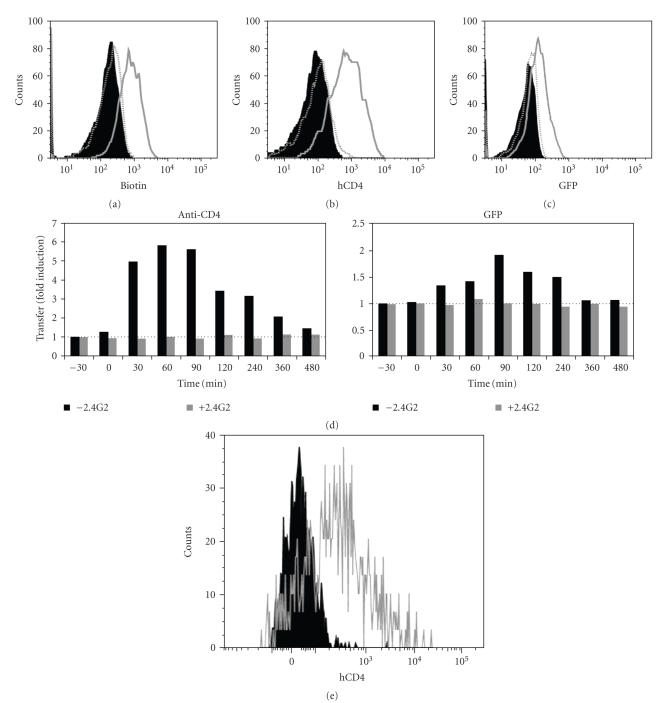
Because the experimental system presented above is not easily amenable to manipulation and is limited to one single antigen specificity, we exploited our recent observation that trogocytosis could be triggered by adding appropriate mAb in a coculture between T cells and FcR-expressing cells [27]. As target cells, we used the murine P815 cells naturally expressing FcR and generated stable transfectants expressing either the human CD4 molecule alone or fused to GFP. We have previously identified a set of mAbs that can trigger trogocytosis in human T cells (Daubeuf et al., manuscript submitted) and used the combination of anti-CD3 + anti-CD28 to trigger both efficient trogocytosis and T cell activation. As shown in Figure 2, coculture of activated CD8+CD4− T cells from healthy donors with P815-CD4 or P815-CD4-GFP in the presence of the anti-CD3 + anti-CD28 mAbs led to the capture of biotinylated plasma membrane proteins (Figure 2(a)), and in particular CD4-GFP (Figures 2(b) and 2(c)) by CD8+ T cells. Capture of CD4, and more generally of membrane fragments, was inhibited when P815 cells were preincubated with the blocking anti-FcγRII/III mAb 2.4G2. Furthermore, no capture was detected in cocultures performed without the anti-CD3 and anti-CD28 mAbs. The possibility that CD4 expression by CD8+ T cells could result from de novo expression was completely ruled out by the observation that, for CD4-GFP, capture could be documented both with the anti-CD4 mAb (Figure 2(b)) and based on GFP fluorescence (Figure 2(c)). The kinetics of CD4-GFP capture by trogocytosis (the efficiency of which is expressed as fold induction) show that CD8+ T cells have already acquired high level of CD4-GFP after 30 minutes of coculture and that they express transiently the captured molecules for a couple of hours, before internalizing them (Figure 2(d)). The observation that the curve of GFP detection is slightly shifted to the right compared to that obtained with CD4 staining is not surprising since the mAb used to detect CD4 can only stain this molecule when it is present at the cell surface whereas GFP will still be detectable after internalization. Similar results were obtained when we used HEK cells transfected with FcR and CD4 instead of P815 cells (not shown). In additional experiments not shown here, we also documented that CD8+ T cells could capture the HIV coreceptors, CXCR4 and CCR5, although CTL normally express both of these molecules. Since CD4 is captured rapidly by CTL during interactions with neighboring cells, and then lost relatively rapidly (in less than 24 hours), detection of captured CD4 on CTL would identify those having recently been in contact with CD4+ target cells. Although, for technical reasons, we did not check the sequential gain and loss of CD4 in the redirected-trogocytosis experimental setup, we suppose that it occurs as suggested for the experiments using CMV-specific CTL presented in Figure 1. This mechanism would allow CD8+ T cells to increase the capture of CD4 during multiple and occasional interactions with CD4+ target cells.
Figure 2.
CD8+ T cells from healthy donors and HIV+ patients capture CD4 and membrane fragments from P815-CD4-GFP cells during redirected trogocytosis. CD8+ T cells, immunomagnetically purified from human PBMC, were coated (grey open histogram) or not (black close histogram) with anti-CD3 + anti-CD28 mAb, then incubated with P815 cells expressing human CD4 fused to GFP, which had been previously cell surface biotinylated. (a) Cells were analyzed by flow cytometry for biotin capture using fluorescent streptavidin and CD8 expression. Shown are overlapping histograms of biotin detection on gated CD8+ T cells from healthy donor. Grey dotted open histogram shows biotin capture by anti-CD3 + anti-CD28-coated CD8+ T cells exposed to FcγRII/III-coated P815 cells previously coated by 2.4G2 mAb. (b) As in (a) except that CD4 rather than biotin capture was determined and is shown on gated CD8+ T cells from healthy donor. (c) As in (b) except that GFP rather than CD4 capture was determined and is shown on gated CD8+ T cells from healthy donor. (d) Kinetics of the transfer efficiency of CD4-GFP on CD8+ T cells co-incubated with P815-CD4-GFP. Transfer of CD4-GFP on CD8+ T cells was detected using anti-CD4 mAb (left panel) and GFP fluorescence (right panel). Results show the fold-induction, that is, the CD4 or GFP fluorescence on CD8+ T cells obtained from cocultures in the presence of stimulatory anti-CD3 + CD28 mAb divided by those obtained in the absence of stimulatory mAb. The experiments were performed in the presence (grey histograms) or absence (black histograms) of the blocking 2.4G2 mAb. (e) As in (b) except that CD8+ T cells were purified from PBMC of a-HIV-infected patient. Since we had only a few cells, we could not perform the control experiment with the blocking 2.4G2 mAb. Note that, in each case, similar results were obtained in at least three independent experiments.
As the HIV infection progresses, it severely alters the immune response, mainly by depleting the CD4+ T cells, but also by disturbing the CTL functions [9]. Thus we wanted to confirm that CD8+ T cells from infected patients were still able to perform trogocytosis. As shown in Figure 2(e), CD8+ T cells obtained from the blood of an HIV+ patient by negative selection captured CD4 from P815-CD4 cells when the coculture was performed in the presence of the anti-CD3 + anti-CD28 mAbs.
3.3. CD4 Captured by CD8+ T Cells Allows HIV Binding
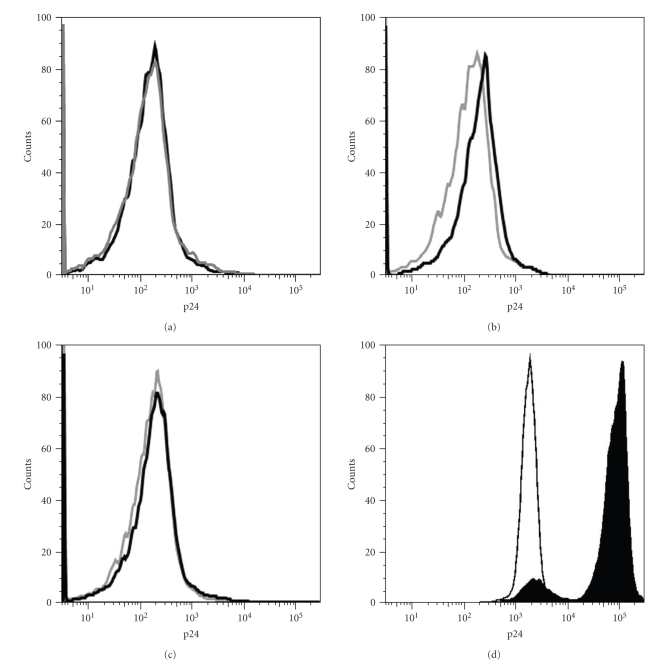
As CD4 is captured and expressed in an, a priori, correct conformation by CD8+ T cells, we next checked if this feature conferred to CD8+ T cells the ability to bind HIV. To collect CD8+ T cells after CD4 capture more easily, we used the adherent cell line HEK-FcR stably transfected with human CD4-GFP as CD4-donor cells. CD8+ T cells, preincubated or not with mAb triggering trogocytosis, were cocultured with HEK-FcR-CD4-GFP for 1 hour and were then harvested in the supernatant (CD8+ T cell purity > 95%, not shown). These cells were directly cocultured with MOLT-4 cells chronically infected or not with the NL4-3 HIV isolate. Note that, before coculturing the CD8+ T cells with MOLT-4 cells, we systematically confirmed that they had acquired levels of CD4 similar to what is shown on Figure 2 (not shown). We have previously shown that incubation of HIV-infected cells with uninfected CD4+ T cells induced a highly efficient binding of HIV to CD4, allowing the assessment of cell-to-cell fusion events and the rapid quantification of HIV entry by real-time PCR [8, 29, 30]. Taking advantage of this rapid and highly efficient mode of HIV spread, we determined, after 24 hours, the attachment of virions to CD8+ T cells by flow cytometry using an anti-p24 antibody. Although the levels of staining were very faint, we detected reproducible and significant staining for p24 on CD8+ T cells having acquired CD4 (open black histograms) when they had been cocultured with HIV-infected MOLT-4 cells (Figure 3(b)) but not with uninfected MOLT-4 cells (Figure 3(a)). No p24 staining could be detected on CD8+ T cells, which had been cocultured with HIV-infected MOLT-4 cells but had not acquired CD4 (open grey histograms). Interestingly, the presence of the Leu3a blocking mAb in the coculture fully inhibited the attachment of the virions (Figure 3(c)). Thus our results show that CD4 capture by CD8+ T cells confers on these cells the ability to bind HIV particles, and that this virion binding is inhibited when the CD4 is not available. This is reminiscent of our former observation that capture of the EBV receptor CD21 by NK cells allow them to bind the virus [4] and that more generally speaking, molecules captured by trogocytosis are functional in terms of ligand binding ([32] and references therein). Of note, although capture of molecules by activated T cells in the absence of stimulus (see [33] for instance) has been described, the possibility that CD8+ T cells could capture CD4 from HIV-infected MOLT-4 cells was ruled out, as these cells show complete downregulation of CD4 [34].
Figure 3.
CD8+ T having acquired CD4 bind HIV particles. (a) Expression of p24 on CD8+ T cells having captured (open black histogram) or not (closed grey histogram) CD4 in a previous coculture (as shown in Figure 2 but target cells are HEK-FcR stably transfected with human CD4) and incubated with uninfected MOLT-4 cells. Shown are overlaps of p24 staining on gated CD8+ T cells. (b) As in (a) except that infected MOLT-4 cells were used in the coculture with CD8+ T cells. (c) As in (b) except that the Leu3a neutralizing anti-CD4 mAb was present during the coculture. (d) Shown are overlap of p24 staining on uninfected MOLT-4 cells (as in condition shown in (a), open black histograms) and on infected MOLT-4 cells (as in conditions shown in (b) and (c), closed black histograms). Note that MOLT-4 cells were analyzed from the coculture with T cells and with the very same settings used for the analysis of T cells, which explains the high autofluorescence of MOLT-4 cells, which is related to their larger size. Similar results were obtained in a second independent experiment.
3.4. CD8+ T Cells Having Captured CD4 by Trogocytosis Induce Syncytia Formation in HIV-Infected MOLT-4 Cells
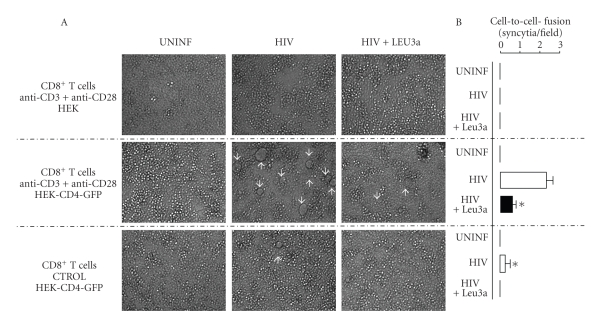
Having observed the ability of captured CD4 to bind HIV particles presented by MOLT-4 cells, we next evaluated whether subsequent membrane fusion events could also take place. Activated CD8+ T cells alone (not shown) or cocultured with HEK cells lacking CD4 expression (Figure 4 upper panels) do not form syncytia in cocultures with infected-MOLT-4. Interestingly, in the presence of CD8+ T cells having acquired CD4, syncytia were massively formed, a process that was abolished when the anti-CD4 Leu-3a neutralizing mAb was added to the coculture (Figure 4 middle panels). The extent of syncytia formation was dependent on trogocytic events, as it was strongly reduced when the CD8+ T cells had been cultured with HEK-CD4-GFP cells in the absence of mAbs triggering trogocytosis (Figure 4 lower panels). Syncytia formation was strictly dependent on the presence of HIV since none were observed whatever conditions used when uninfected MOLT-4 cells were employed (Figure 4, left panels). These results strongly suggest that CD4, once captured by CD8+ T cells is virologically functional and can induce syncytia formation with MOLT-4 cells.
Figure 4.
CD8+ T cells having acquired CD4 induced syncytia formation in infected MOLT-4 cultures. (A) Transmission microscopy analysis (low power fields) of cultures in which anti-CD3 + anti-CD28-activated CD8+ T cells that had been cocultured with HEK cells either expressing CD4-GFP (middle and lower panels) or not (upper panels), were then mixed with uninfected MOLT-4 cells (left panels) or HIV-infected MOLT-4 cells either in the absence (middle panels) or the presence (right panels) of the anti-CD4 mAb Leu3a. The controls shown on the bottom panels correspond to T cells that have been cocultured with HEK cells expressing CD4-GFP, but had not previously been activated with the anti-CD3 + anti-CD28 mAbs, (CTROL) white arrows indicate the positions of syncytia. (B) Quantification of syncytia in different randomly selected high power fields. Data are mean ± SD values of at least four different fields. Note that syncytia formation was never detected when uninfected MOLT-4 cells were used, whatever the condition tested. Asterisks denote statistically significant differences between activated and control CD8+ T cells cocultured with HEK-CD4-GFP cells (P = .002, T test) and significant inhibition was induced by Leu3a (P = .001, T test).
3.5. HIV Infection of CD8+ T Cells Having Acquired CD4 by Trogocytosis Remains Undetectable
To go one step further, we next investigated if CD8+ T cells having acquired CD4 could be infected by HIV. For this, we used two experimental approaches. First, we quantified the synthesis of proviral DNA in the cocultures of MOLT-4 cells with CD8+ T cells described above, and we found no specific increases in HIV proviral DNA associated with trogocytosis (not shown). This was not, however, a very sensitive approach because infected MOLT-4 already have a heavy load of proviral DNA, leading to relatively high levels of background. As an alternative, we evaluated whether CD8+ T cells, having acquired CD4 from HEK-FcR-CD4 target cells, may produce new HIV particles after infecting them with the cell-free viruses (HIV NL4-3 strain) and evaluating the level of HIV p24 Ag in the supernatant after 5 days of culture. Low levels of p24 were detected in all samples, and we found no correlation between the levels of p24 and the fact that CD8+ T cells had capture CD4 or not by trogocytosis. Furthermore, addition of inhibitors of the reverse transcriptase AZT or of the fusion C34, failed to reduce the low levels of p24 observed in control cultures (data not shown). These results suggest that, although the presence of captured CD4 on the surface of CD8+ T cells allows for binding of the virus, this may not be enough to allow for productive infection of these cells by HIV. Although these results were obviously disappointing to us, many different hypotheses can be proposed to explain this observation. One possibility would be that CD8+ T cells could replicate HIV but at levels too low to be detected. The inability of CD8+ T cells to replicate HIV could be directly related to their production of antiviral factors. For instance, CD8+ T cells are known to produce high amounts of antiviral cytokines such as IFN-γ and MCP-1 which could result in HIV being eliminated rather than replicated. Note that CD8+ T cells used in our assays came from healthy donors, whilst HIV+ CTL have been detected in patients having reached the late stages of HIV infection. It is thus possible that the altered status of CD8+ T cells observed among seropositive patients could facilitate the virus replication in these cells. Another possibility could be that CD8+ T cells are naturally refractory to HIV infection. Although productive HIV-infection of CD8+ T cell has been reported [10], our data suggest that CD8+ T cells might block HIV replication at some step between reverse transcription and expression of viral proteins. This apparent paradox may be explained by the reported requirement of specific envelope signals to infect primary cells [35]. Yet another possible explanation lies with the fact that, although the captured CD4 molecules are correctly oriented, only a small proportion of those may actually be fully inserted in the plasma membrane of the CD8+ T cells. In support of this view, we recently showed that FcR could be captured by T cells and could bind ligand but do not apparently transmit intracellular signals [32]. This result could be explained by our biochemical and functional observations that the majority, if not all FcR were not properly inserted in the plasma membrane of recipient cells, though being correctly oriented, and hence were not connected to the signaling machinery present in T cells. If a similar process applies for captured CD4, this could explain why CD8+ T cells having captured CD4 are able to bind HIV virions, but cannot be efficiently infected by the virus.
In summary, although one cannot conclude from our study that capture of CD4 by CTL via trogocytosis could result in their infection, our results unravel a previously unappreciated role for CTL during HIV infection that lies with their ability to acquire CD4 by trogocytosis from the cells they interact with. Furthermore, this mechanism could play a role during HIV infection by promoting syncytia formation, a mechanism postulated to contribute to CD4+ T cell loss [36].
4. Conclusions
Through acquisition of the CD4 receptor from their target cells, CD8+ T cells may play a previously unappreciated role in HIV infection in binding the virus and favoring syncytia formation. Remarkably, this can occur in the absence of detectable infection of the CD8+ T cells by HIV. At this stage, however, it remains unclear, whether our inability to detect infection in CD8+ T cells was due to technical limitations or if CD4 capture by CD8+ T cells never leads them to become susceptible to a productive HIV infection. If this was the case, explanation must therefore be sought to explain the infection of CD8+ T cells in HIV-infected patients, such as those published previously regarding transmission between CD4+ cells [37, 38] or from CD4+ cells to CD4− cells [39, 40]. Trogocytosis clearly occurs in vivo ([41] and references therein). In the case of CD4, it is however unclear if the fact that CD4 is sometimes found on CD8+ T cells can be due to trogocytosis solely as de novo CD4 expression has also been occasionally documented on activated CTL [13–20]. Then, another aspect to determine is if the role of CD4 transfer via trogocytosis in syncytia formation which we have documented in vitro is relevant during in vivo HIV infection. The answer to this question is, however, currently inaccessible, because we have no means to selectively block trogocytosis. Further studies will be warranted to explore whether additional roles could be played by trogocytic acquisition of virologically-relevant molecules by CD8+ T cells. Note that, as CD4 molecules are only transiently displayed by CTL after capture, one can speculate that functions due to trogocytosis could be controlled both spatially and temporally. This notion suggests a particular context in which CD4 captured by CTL could have functional consequences, which makes this process worth investigating.
Acknowledgments
The authors would like to sincerely thank Sidaction and its donators for financial support of their study (DH). They also thank the ATUPS program at the Université Paul Sabatier, Toulouse for a travel fellowship to A. Aucher. J. Blanco was supported by the HIVACAT Program, the FIS project PI08/1306, and the Spanish AIDS network “Red Temática Cooperativa de Investigación en SIDA (RD06/0006)”. J. Blanco is a researcher from Fundació Institut de Recerca en Ciències de la Salut Germans Trias i Pujol supported by the ISCIII and the Health Department of the Catalan Government (Generalitat de Catalunya). I. Puigdomènech is supported by a predoctoral grant from Generalitat de Catalunya and European Social Fund. The authors thank Christian Davrinche (Toulouse, France) for the kind gift of the human anti-CMV CTL, Gérald Gaibelet (INSERM U563, Toulouse, France) for the kind gift of the human CD4-GFP plasmid and Pedro Romero (LICR, Lausanne, Switzerland) for the kind gift of the anti-hCD28 mAb. D. Hudrisier and J. Blanco contributed equally to this work.
References
- 1.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nature Immunology. 2003;4(9):p. 815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 2.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Reviews Immunology. 2007;7(3):238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 3.Huang J-F, Yang Y, Sepulveda H, et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 4.Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournié J-J. Acquisition of viral receptor by NK cells through immunological synapse. The Journal of Immunology. 2003;170(12):5993–5998. doi: 10.4049/jimmunol.170.12.5993. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) Journal of Immunology. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 6.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. Journal of Immunology. 1999;163(10):5201–5210. [PubMed] [Google Scholar]
- 7.Hudrisier D, Riond J, Garidou L, Duthoit C, Joly E. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. European Journal of Immunology. 2005;35(8):2284–2294. doi: 10.1002/eji.200526266. [DOI] [PubMed] [Google Scholar]
- 8.Blanco J, Bosch B, Fernández-Figueras MT, Barretina J, Clotet B, Esté JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. The Journal of Biological Chemistry. 2004;279(49):51305–51314. doi: 10.1074/jbc.M408547200. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annual Review of Immunology. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Hill BJ, Ambrozak DR, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. Journal of Virology. 2004;78(3):1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stebbing J, Gazzard B, Douek DC. Where does HIV live? The New England Journal of Medicine. 2004;350(18):1872–1880. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 12.Livingstone WJ, Moore M, Innes D, Bell JE, Simmonds P. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. The Lancet. 1996;348(9028):649–654. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane A, Hughes GJ, Seaton RA, Simmonds P. First evidence of HIV infection of CD8 lymphocytes expressing CD4 during primary HIV-1 infection. AIDS. 2005;19(11):1237–1239. doi: 10.1097/01.aids.0000176229.86602.11. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane A, Imlach S, Leen C, Scott G, Kennedy D, Simmonds P. High levels of human immunodeficiency virus infection of CD8 lymphocytes expressing CD4 in vivo. Journal of Virology. 2004;78(18):9862–9871. doi: 10.1128/JVI.78.18.9862-9871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flamand L, Crowley RW, Lusso P, Colombini-Hatch S, Margolis DM, Gallo RC. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3111–3116. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imlach S, McBreen S, Shirafuji T, Leen C, Bell JE, Simmonds P. Activated peripheral CD8 lymphocytes express CD4 in vivo and are targets for infection by human immunodeficiency virus type 1. Journal of Virology. 2001;75(23):11555–11564. doi: 10.1128/JVI.75.23.11555-11564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchen SG, Jones NR, LaForge S, et al. CD4 on CD8+ T cells directly enhances effector function and is a target for HIV infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(23):8727–8732. doi: 10.1073/pnas.0401500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitchen SG, Korin YD, Roth MD, Landay A, Zack JA. Costimulation of naive CD8+ lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. Journal of Virology. 1998;72(11):9054–9060. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBreen S, Imlach S, Shirafuji T, et al. Infection of the CD45RA+ (Naive) subset of peripheral CD8+ lymphocytes by human immunodeficiency virus type 1 in vivo. Journal of Virology. 2001;75(9):4091–4102. doi: 10.1128/JVI.75.9.4091-4102.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4 dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood. 2003;102(6):2156–2164. doi: 10.1182/blood-2002-07-1972. [DOI] [PubMed] [Google Scholar]
- 21.Zloza A, Schenkel JM, Tenorio AR, Martinson JA, Jeziorczak PM, Al-Harthi L. Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that coexpresses CD4 on its surface. Blood. 2009;114(18):3841–3853. doi: 10.1182/blood-2009-02-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitchen SG, Uittenbogaart CH, Zack JA. Mechanism of human immunodeficiency virus type 1 localization in CD4- negative thymocytes: differentiation from a CD4-positive precursor allows productive infection. Journal of Virology. 1997;71(8):5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha K, Zhang J, Gupta A, Dave R, Yimen M, Zerhouni B. Isolation of primary HIV-1 that target CD8+ T lymphocytes using CD8 as a receptor. Nature Medicine. 2001;7(1):65–72. doi: 10.1038/83365. [DOI] [PubMed] [Google Scholar]
- 24.De Maria A, Colombini S, Schnittman SM, Moretta L. CD8+ cytolytic T lymphocytes become infected in vitro in the process of killing HIV-1-infected target cells. European Journal of Immunology. 1994;24(3):531–536. doi: 10.1002/eji.1830240306. [DOI] [PubMed] [Google Scholar]
- 25.De Maria A, Pantaleo G, Schnittman SM, et al. Infection of CD8+ T lymphocytes with HIV: requirement for interaction with infected CD4+ cells and induction of infectious virus from chronically infected CD8+ cells. Journal of Immunology. 1991;146(7):2220–2226. [PubMed] [Google Scholar]
- 26.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111(12):5621–5628. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudrisier D, Aucher A, Puaux A-L, Bordier C, Joly E. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. Journal of Immunology. 2007;178(6):3637–3647. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 28.Blanco J, Barretina J, Clotet B, Esté JA. R5 HIV gp120-mediated cellular contacts induce the death of single CCR5-expressing CD4 T cells by a gp41-dependent mechanism. Journal of Leukocyte Biology. 2004;76(4):804–811. doi: 10.1189/jlb.0204100. [DOI] [PubMed] [Google Scholar]
- 29.Massanella M, Puigdoménech I, Cabrera C, et al. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS. 2009;23(2):183–188. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 30.Puigdomènech I, Massanella M, Cabrera C, Clotet B, Blanco J. On the steps of cell-to-cell HIV transmission between CD4 T cells. Retrovirology. 2009;6, article 89 doi: 10.1186/1742-4690-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitchen SG, Whitmire JK, Jones NR, et al. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(10):3794–3799. doi: 10.1073/pnas.0406603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudrisier D, Clemenceau B, Balor S, et al. Ligand binding but undetected functional response of FcR after their capture by T cells via trogocytosis. The Journal of Immunology. 2009;183:6102–6113. doi: 10.4049/jimmunol.0900821. [DOI] [PubMed] [Google Scholar]
- 33.LeMaoult J, Caumartin J, Daouya M, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109(5):2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 34.Puigdomènech I, Massanella M, Izquierdo-Useros N, et al. HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology. 2008;5, article 32 doi: 10.1186/1742-4690-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu D, Wang W, Yoder A, Spear M, Wu Y. The HIV envelope but not VSV glycoprotein is capable of mediating HIV latent infection of resting CD4 T cells. PLoS Pathogens. 2009;5(10) doi: 10.1371/journal.ppat.1000633. Article ID e1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callahan L. HIV-1 virion-cell interactions: an electrostatic model of pathogenicity and syncytium formation. AIDS Research and Human Retroviruses. 1994;10(3):231–233. doi: 10.1089/aid.1994.10.231. [DOI] [PubMed] [Google Scholar]
- 37.Sowinski S, Jolly C, Berninghausen O, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature Cell Biology. 2008;10(2):211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 38.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. Journal of Experimental Medicine. 2004;199(2):283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muratori C, Sistigu A, Ruggiero E, et al. Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9. Journal of Virology. 2007;81(17):9078–9087. doi: 10.1128/JVI.00675-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkatachari NJ, Alber S, Watkins SC, Ayyavoo V. HIV-1 infection of DC: evidence for the acquisition of virus particles from infected T cells by antigen uptake mechanism. PLoS ONE. 2009;4(10, article e7470) doi: 10.1371/journal.pone.0007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riond J, Elhmouzi J, Hudrisier D, Gairin JE. Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8+ T cells. Scandinavian Journal of Immunology. 2007;66(4):441–450. doi: 10.1111/j.1365-3083.2007.01996.x. [DOI] [PubMed] [Google Scholar]