Abstract
Bone marrow-derived APC are critical for both priming effector/memory T cell responses to pathogens and inducing peripheral tolerance in self-reactive T cells. In particular, dendritic cells (DC) can acquire peripheral self-Ags under steady state conditions and are thought to present them to cognate T cells in a default tolerogenic manner, whereas exposure to pathogen-associated inflammatory mediators during the acquisition of pathogen-derived Ags appears to reprogram DCs to prime effector and memory T cell function. Recent studies have confirmed the critical role of DCs in priming CD8 cell effector responses to certain pathogens, although the necessity of steady state DCs in programming T cell tolerance to peripheral self-Ags has not been directly tested. In the current study, the role of steady state DCs in programming self-reactive CD4 cell peripheral tolerance was assessed by combining the CD11c-diphtheria toxin receptor transgenic system, in which DC can be depleted via treatment with diphtheria toxin, with a TCR-transgenic adoptive transfer system in which either naive or Th1 effector CD4 cells are induced to undergo tolerization after exposure to cognate parenchymally derived self-Ag. Although steady state DCs present parenchymal self-Ag and contribute to the tolerization of cognate naive and Th1 effector CD4 cells, they are not essential, indicating the involvement of a non-DC tolerogenic APC population(s). Tolerogenic APCs, however, do not require the cooperation of CD4+CD25+ regulatory T cells. Similarly, DC were required for maximal priming of naive CD4 cells to vaccinia viral-Ag, but priming could still occur in the absence of DC.
Bone marrow-derived APC play a critical role in both priming effector/memory T cell responses to pathogen-derived Ags (1–3) as well as inducing T cell tolerance to peripherally expressed self-Ags (4–6). Owing to their efficiency in capturing and presenting exogenous Ags as well as their abilities both to express critical costimulatory ligands and to interact with naive T cells in secondary lymphoid organs, a large body of literature has suggested that dendritic cells (DC)5 are the principal APC population that primes effector/memory T cell responses to pathogen-derived Ags (reviewed in Refs. 7 and 8). This possibility has been confirmed more recently using transgenic mice in which transient depletion of DC blocks priming of antipathogenic CD8 cells (9, 10).
It has also been suggested that DCs present parenchymally derived self-Ags to induce T cell tolerance. Thus, under steady state conditions, naive CD8 cells are able to recognize their cognate parenchymally derived self-Ag in transgenic mice where DC are the only APC population that is genetically capable of presenting the relevant class I-restricted epitope (11). Furthermore, when APC populations are fractionated from lymph nodes (LN) draining a particular self-Ag under steady state conditions, DCs are the only subset capable of stimulating cognate T cell lines or hybridomas in vitro (12, 13). Although the relevant DC subtype (i.e., CD8α− or CD8α+) might differ depending on the type or location of self-Ag (12, 13), the possibility that the same DC can induce either T cell tolerance or priming is supported by studies in which delivery of exogenous Ags directly to DCs under steady state conditions renders cognate naive T cells tolerant, whereas coadministration of either inflammatory cytokines (14) or costimulatory agonists (15) redirects these T cell responses toward immunogenic outcomes.
Even though the aforementioned studies demonstrate the tolerogenic potential of DCs, it has not been directly tested whether DCs are essential for T cell tolerance induction to parenchymally derived self-Ags. Because DCs appear to be the principal APC population that can present Ag to naive T cells in lymphoid organs (8), it would seem likely that they play an important role in the tolerization of naive self-reactive T cells. Given the differences in migratory patterns (16, 17) and requirements for activation (18, 19) between naive and effector T cells, however, tolerization of the latter might be more dependent on other APC populations such as macrophages (20) or B cells (21, 22).
In the current study, we assessed the role of DC in inducing tolerization and priming of naive and effector CD4 cells by combining our previously established system in which naive (5, 23) or Th1 effector (24, 25) TCR-transgenic hemagglutinin (HA)-specific clonotypic CD4 cells are tolerized or primed following adoptive transfer into recipients that express HA either as a parenchymal self-Ag or a recombinant vaccinia viral Ag, respectively, with the CD11c-diphtheria toxin receptor (DTR) transgenic system in which DC can be depleted via treatment with diphtheria toxin (DT; Ref. 9). Interestingly, naive self-reactive CD4 cells continue to recognize self-Ag and to undergo nonimmunogenic responses in the absence of DC (albeit this response was diminished), indicating the both DCs and other APC populations(s) can present parenchymal self-Ag. Similarly, Th1 effector CD4 cells encountering self-Ag in the absence of DC underwent partial tolerization. Furthermore, depletion of DCs caused naive CD4 cells encountering viral Ag to undergo impaired clonal expansion and effector differentiation when mice were infected with low viral titers, although effector differentiation did occur in response to infection with higher viral titers. Thus, DCs are required to achieve complete CD4 cell tolerization to parenchymal self-Ag and maximal priming to viral Ag but are not essential for either of these processes.
In addition to bone marrow-derived APC, CD4+CD25+ regulatory T cells (Tregs) have also been shown to play an important role in maintaining peripheral T cell tolerance in numerous models (reviewed in Refs. 26 and 27), although the precise mechanisms by which Tregs suppress autoreactive T cell responses in vivo have not been well defined. Given in vitro studies suggesting that Tregs are most active when DCs remain in nonactivated or steady state (28, 29), we hypothesized that Tregs might work in concert with steady state DC to program T cell tolerance to parenchymal self-Ags. To the contrary, neutralization of Tregs did not appreciably alter the response of CD4 cells encountering parenchymal self-Ag. Taken together, these data suggest that steady state bone marrow-derived APC (including but not limited to DCs), but not CD4+CD25+ Tregs, are critical for inducing tolerance in both naive and effector CD4 cells encountering parenchymal self-Ag.
Materials and Methods
Mice, adoptive transfer, and flow cytometry
C3-HAlow (5) and C3-HAhigh (30) transgenic mice that express influenza HA as a parenchymal self-Ag on both the B10.D2 (H-2d) and B6 (H-2b) Thy1.2+ backgrounds as well as 6.5-transgenic mice expressing a TCR specific for an I-Ed-restricted HA epitope (31) that have been backcrossed to the B10.D2 Thy1.1+ background have previously been described. CD11c-DTR-transgenic mice (9) were backcrossed from the B6 to the B10.D2 Thy1.2+ background. Adoptive transfers of 6.5 clonotypic naive and Th1 effector CD4 cells, vaccinia inoculations, and subsequent functional analyses were performed as previously described (23–25). Briefly, in experiments analyzing the response of naive clonotypic CD4 cells, single-cell suspensions prepared from pooled LN plus spleen dissected from naive 6.5 Thy1.1+-transgenic donors were depleted of CD8+ cells using magnetic beads and then labeled with the fluorescent dye CFSE to allow visualization of cell division after adoptive transfer. Thy1.2+ recipient mice included C3-HAlow and C3-HAhigh transgenic mice as well as nontransgenic (NT) mice that had been inoculated 1 day earlier with the indicated titer of a recombinant vaccinia virus that expresses HA (viral-HA). Single-cell suspensions were prepared from spleens of recipient mice 5 days after adoptive transfer, and the transferred clonotypic CD4 cells were identified in FACS analyses via expression of Thy1.1. Analysis of clonotypic CD4 cell frequency and CFSE dilution were performed directly ex vivo, whereas intracellular cytokine expression was measured on fixed and permeabilized cells after 5 h of in vitro restimulation with synthetic HA peptide in the presence of brefeldin A (23, 25). To analyze the response of Th1 effector clonotypic CD4 cells, naive 6.5 clonotypic CD4 cells were first transferred into multiple NT mice that had been infected with 106 PFU of viral-HA and recovered from spleens 6 days later, and then pooled and relabeled with CFSE and aliquots containing 2.5 × 106 clonotypic CD4 cells were retransferred into the indicated secondary recipients as previously described (24, 25). The University of Connecticut Health Center’s Institutional Animal Care and Use Committee approved all protocols used in this study.
Bone marrow chimeras and DC depletion
Bone marrow chimeras were generated as previously described (23). In short, NT, C3-HAlow, and C3-HAhigh hosts on the B6 (H-2b) Thy1.2+ background were depleted of NK cells by i.p. injection of 15 μl rabbit anti-asialo-GM1 γ-globulin (Wako Chemicals) 1 day before receiving 900–1000 rads of ionizing radiation followed by i.v. injection of 106 T cell-depleted bone marrow cells prepared from either NT or CD11c-DTR-transgenic donors on a B10.D2 (H-2d) Thy1.2+ background. Chimeras were allowed a minimum of 6 wk of recovery before experimentation. DC depletions were subsequently performed as previously described (32) by treating chimeric mice i.p. with DT (Sigma-Aldrich) at 4 ng/g body weight in PBS on days −4, −1, and +2 relative to adoptive transfer, and adoptively transferred clonotypic CD4 cells were recovered from spleens on day +5 for analysis. Verifying that splenic APCs from DT-treated chimeras reconstituted with DTR bone marrow could function equivalently to control chimeric APC in stimulating clonotypic CD4 cell intracellular cytokine expression in vitro, we found that splenocytes prepared from DTR→NT and NT→NT chimeras that had been treated with DT 3 days earlier elicited similar IFN-γ and TNF-α expression in cocultured clonotypic Th1 effectors that had themselves been depleted of MHC class II+ cells (data not shown).
Treg neutralization
In vivo neutralization of CD4+CD25+ Tregs was performed using the anti-CD25 mAb PC61 (eBioscience) as previously described (28, 33–35). In short, adoptive transfer recipients were treated i.v. with 100 μg of PC61 or control rat Ig 4 days before receiving adoptive transfers of clonotypic CD4 cells.
Results
Role of DC in presenting parenchymal self- and viral Ag to naive CD4 cells
To study peripheral tolerization of CD4 cells specific for parenchymal self-Ags, we previously generated C3-HA-transgenic mice that express influenza HA as a self-Ag in a variety of parenchymal tissues. When naive clonotypic HA-specific TCR-transgenic CD4 cells are adoptively transferred into C3-HA recipients expressing either high (C3-HAhigh) or low (C3-HAlow) levels of parenchymal HA, they undergo an initial proliferative response (albeit proliferation is more robust in C3-HAhigh recipients), followed by the development of a tolerant phenotype marked by an impaired ability to undergo further Ag-induced proliferation and to express cytokines such as IL-2, IFN-γ, and TNF-α (5, 23, 30, 36). Clonotypic CD4 cells that are initially primed by viral Ag to differentiate into Th1 effectors also develop impaired function after adoptive retransfer into C3-HA recipients (24), with a particularly rapid loss in their ability to express IFN-γ and TNF-α (25). Additionally, analysis of a series of bone marrow chimeric C3-HA mice in which the parenchymal and bone marrow compartments differentially express the relevant MHC-restricting element indicated that bone marrow-derived APC (rather than HA-expressing parenchyma) present parenchymally derived self-HA to induce the tolerization of both naive (5, 23) and Th1 effector (24) HA-specific CD4 cells.
To assess the role of DCs in presenting parenchymal self-Ag to induce CD4 cell tolerance in C3-HA mice, we used the CD11c-DTR transgenic system in which expression of a DTR-GFP fusion construct under the control of the DC-specific CD11c promoter simultaneously marks DCs by GFP expression and renders them susceptible to depletion after treatment with DT (9). Because DT treatment of CD11c-DTR mice is toxic (presumably due to transgene expression on unidentified parenchymal tissue), this issue was circumvented as previously described (32) by reconstituting lethally irradiated C3-HA hosts with bone marrow from CD11c-DTR (DTR) donors. To avoid the possibility that residual host-derived DCs (which cannot be depleted because they do not express DTR) would continue to present parenchymal HA after DT treatment, irradiated C3-HAhigh transgenic hosts backcrossed to the B6 background that expresses the HA-nonrestricting H-2b haplotype were reconstituted with bone marrow from DTR or control NT donors on the B10.D2 background that expresses the HA-restricting H-2d haplotype. Thus, in both DTR→C3-HA and NT→C3-HA chimeras, bone marrow-derived APC, but not HA-expressing parenchyma, are genetically capable of presenting the relevant I-Ed-restricted HA epitope. Additionally, in DTR→ C3-HA chimeras, all of the DC capable of presenting the relevant HA epitope should be susceptible to depletion via DT.
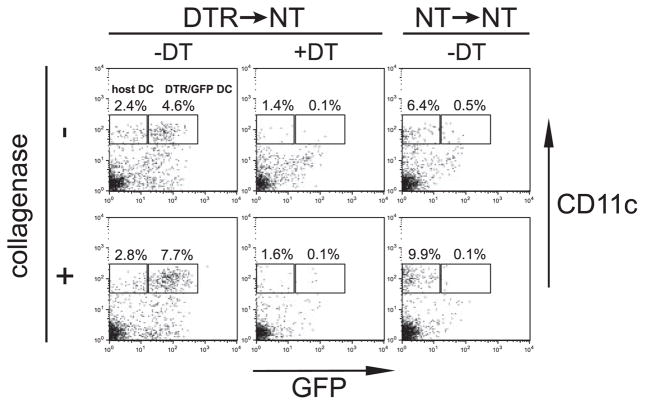
To confirm that DC could be efficiently depleted in chimeras reconstituted with DTR bone marrow, DTR→NT chimeras were treated twice with either DT or PBS 3 days apart, and spleens analyzed the day after the second treatment (Fig. 1). After mechanical disruption of a PBS-treated spleen, DTR-transgenic DCs (identified as MHC class II+CD11c+GFP+) constituted ~5% of splenocytes. When a portion of the same spleen was digested with collagenase to liberate more DCs, their frequency increased to ~8%. In DT-treated DTR→NT chimeras, the frequency of DTR/GFP-transgenic DCs decreased to 0.1% in both collagenase-digested and -nondigested spleen. As expected, a population of residual host DCs that did not express the DTR/GFP transgene (identified as MHC class II+CD11c+GFP−) persisted in DT-treated DTR→NT spleen. As mentioned earlier, these residual host DCs do not express H-2d, and therefore cannot complicate adoptive transfer experiments using TCR-transgenic HA-specific CD4 cells. There also seemed to be a residual population of CD11cintermediateGFP+ cells in DT-treated DTR→NT spleen, but in actuality they appear to represent background staining because a similar pattern was observed in NT→NT chimeras in which DCs do not express GFP. Taken together, these results confirm that DT can efficiently deplete donor-derived transgenic DCs in DTR chimeras.
FIGURE 1.
DT-induced depletion of DC in DTR chimeric mice. DTR→NT and NT→NT chimeras were treated with DT (4 ng/g body weight) or PBS on days −4 and −1, and on day 0 spleens were harvested. One half of each spleen was processed by mechanical disruption followed by digestion with collagenase D (1 mg/ml) for 1 h, whereas the other half was processed by mechanical disruption only. FACS plots show GFP vs CD11c expression on MHC class II+ gated cells, with the percentage of CD11c+GFP+ (DTR/GFP transgenic) and CD11c+GFP− (nontransgenic host-derived) DC indicated.
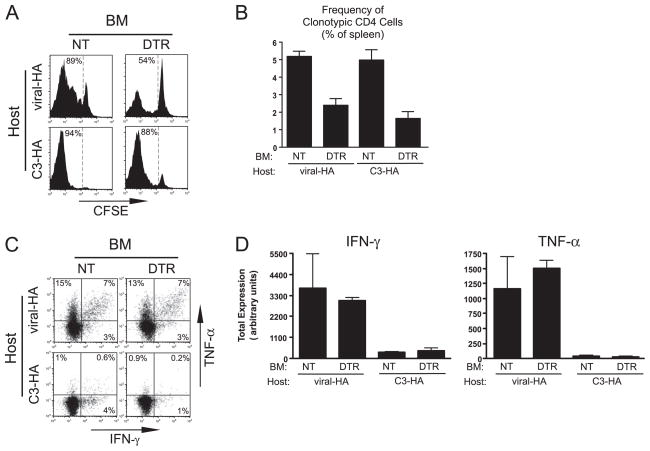
To investigate the role of steady state DC in the tolerization of naive CD4 cells specific for parenchymal self-Ag, naive clonotypic HA-specific CD4 cells were labeled with the fluorescent tracking dye CFSE, adoptively transferred into DT-treated DTR→C3-HAhigh and NT→C3-HAhigh (control) chimeras, and recovered from spleens 5 days posttransfer for analysis. DC begin to repopulate the spleen of CD11c-DTR mice 3 days after DT treatment but are not fully reconstituted until 6 days (9). To thus ensure that DC were depleted throughout the course of the adoptive transfer experiment, we used the previously described protocol (32) of treating chimeras with DT every third day (i.e., days −4, −1, and +2). As expected, in control DT-treated NT→C3-HAhigh chimeras, the transferred naive clonotypic CD4 cells underwent vigorous division as indicated by CFSE-dilution (Fig. 2A) and expanded to ~5% of splenocytes (Fig. 2B). At day +5, these clonotypic CD4 cells were no longer undergoing cell cycle progression as indicated by a lack of blastogenesis (data not shown) despite the continual presence of the transgenically expressed Ags that initiated the transient proliferative response, however, indicating that they had developed an anergic or nonresponsive phenotype (5, 24). This non-responsive state was further illustrated by the severely impaired ability of these self-Ag-exposed clonotypic CD4 cells to express intracellular TNF-α (a sensitive indicator of tolerization; see Refs. 25 and 36) and IFN-γ (indicating a lack of Th1 effector function) after in vitro restimulation with HA peptide-pulsed APCs relative to control CD4 cells recovered from NT recipients that had been infected with viral-HA (Fig. 2, C and D). In DT-treated DTR→C3-HAhigh recipients CFSE-dilution was only slightly reduced (Fig. 2A), although clonal expansion was reduced ~3-fold (p = 0.0005, unpaired two-tailed t test) (Fig. 2B) relative to control chimeras. Nevertheless, those clonotypic CD4 cells that encountered parenchymal self-HA in the absence of DC developed a similar inability to express IFN-γ and TNF-α (Fig. 2, C and D) and to undergo blastogenesis (data not shown) as did DC nondepleted counterparts. A comparable effect of DC depletion was also observed in chimeras generated from C3-HAlow hosts (data not shown). Taken together, these data suggest that although steady state DC do present parenchymal self-Ag, they are not essential for inducing the tolerization of cognate naive CD4 cells.
FIGURE 2.
Role of DC in presenting parenchymal self- and vaccinia viral-Ag to naive CD4 cells. Naive Thy1.1+ CFSE-labeled clonotypic CD4 cells were adoptively transferred into Thy1.2+ NT→C3-HAhigh, DTR→C3-HAhigh, NT→viral-HA, and DTR→viral-HA chimeric recipients that were treated with DT on days −4, −1, and +2 posttransfer and recovered from spleens on day +5 for analysis. Viral-HA chimeric recipients were infected with 106 PFU of viral-HA on day −1. A, Representative CFSE-dilution histograms of clonotypic CD4 cells (identified as CD4+Thy1.1+) with the percentage of CFSE-diluted cells indicated. B, Frequency of clonotypic CD4 cells within total splenocytes. C, Representative FACS plots of intracellular IFN-γ vs TNF-α expression on CFSE-diluted clonotypic CD4 cells after in vitro restimulation with HA peptide-pulsed APCs. D, Quantitative analysis corresponding to C. Total IFN-γ and TNF-α expression is expressed in arbitrary units (calculated as the product of the percent of cytokine-positive clonotypic CD4 cells multiplied by the mean fluorescence intensity of cytokine staining per positively expressing cell) as previously described (23). All quantitative data are expressed as the mean ± SEM, and n = 5–7 for each group. BM, Bone marrow.
To assess the role of DC in programming naive anti-viral CD4 cells to differentiate into Th1 effectors, similar transfers were also performed in DT-treated DTR→NT and NT→NT (control) chimeras that had been infected with viral-HA. DC depletion resulted in a reduction of ~2-fold in both CFSE-dilution (Fig. 2A) and clonal expansion (p < 0.0001; Fig. 2B). Thus, similar to parenchymal self-Ag, DC also present vaccinia viral-Ag to naive CD4 cells but are not the only APC population that can present. Those clonotypic CD4 cells that did proliferate in response to viral-Ag in the absence of DC were able to express equivalent levels of IFN-γ and TNF-α compared with counterparts primed in the presence of DC (Fig. 2, C and D).
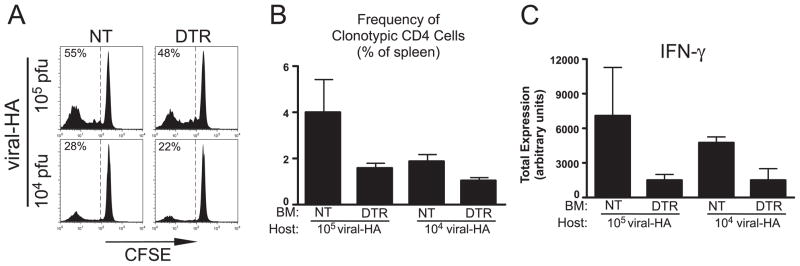
The ability of naive anti-viral CD4 cells to develop effector function (albeit with reduced clonal expansion) in the absence of DC was somewhat surprising given the superiority of DC relative to other APCs in priming naive T cells in vivo (37) as well as the previously observed defects in pathogen-induced CD8 cell priming in the CD11-cDTR system (9, 10). Because a relatively high titer of recombinant vaccinia virus (106 PFU) was used in the preceding experiment, we next analyzed whether DCs might be more critical in programming effector function when viral titers are more limiting (Fig. 3). When naive clonotypic CD4 cells were adoptively transferred into control DT-treated NT→viral-HA chimeras that had been infected with 105 PFU of virus, the percentage of cells that displayed diluted CFSE was reduced to 55% (Fig. 3A) compared with 89% elicited by 106 PFU (Fig. 2A) and was further reduced to 28% with 104 PFU (Fig. 3A). Likewise, accumulation was reduced from 5% of total splenocytes with 106 PFU (Fig. 2B) to 4% with 105 PFU and 2% with 104 PFU (Fig. 3B). Despite the reduced clonotypic CD4 cell proliferation elicited by lower viral titers, those cells that did divide developed a similar capacity to express the Th1 effector cytokine IFN-γ (compare Fig. 2D to Fig. 3C). Similar to the result using the 106 PFU (Fig. 2B), depletion of DC in DTR→viral-HA chimeras infected with 105 and 104 PFU of virus reduced clonotypic CD4 cell accumulation ~2-fold (Fig. 3B). Additionally, at the lower viral titers, DC depletion resulted in reduced development of IFN-γ expression potential in the divided clonotypic CD4 cells, in particular at the 104 PFU dose, DC depletion resulted in a 3-fold reduction in IFN-γ expression (p = 0.04; Fig. 3C. Thus, with a limiting viral load, DCs are critical for priming effector function in naive CD4 cells, and although another APC population(s) can prime effector function when the viral load is high, DCs are still required to achieve maximal clonal expansion.
FIGURE 3.
Role of DCs in priming naive antiviral CD4 cells in response to low viral titers. Adoptive transfers of naive CFSE-labeled Thy1.1+ clonotypic CD4 cells into Thy1.2+ NT→viral-HA and DTR→viral-HA chimeric recipients were performed as in Fig. 2, except that viral inoculations were given at 105 and 104 PFU as indicated. Representative CFSE histograms (A) and quantification of clonal expansion (B) and total IFN-γ expression (C) are also presented as in Fig. 2. n = 3 for each group. BM, Bone marrow.
The role of DCs in presenting parenchymal self- and viral-Ag to Th1 effector CD4 cells
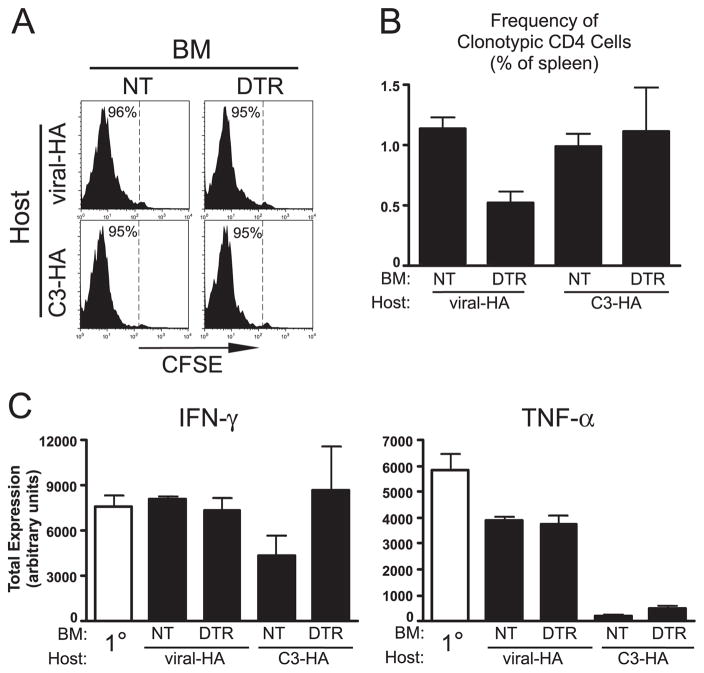
Similar to naive CD4 cells (5), tolerization of Th1 effector CD4 cells to parenchymal self-Ag can be mediated by bone marrow-derived APC (24). Given the many functional differences between naive and effector CD4 cells, steady state DCs might play different roles in their tolerization to parenchymal self-Ag. Thus, to assess the role of DCs in Th1 effector CD4 cell tolerization, we utilized our previously established adoptive retransfer protocol in which naive clonotypic CD4 cells are initially transferred into viral-HA recipients, recovered 6 days later from spleens after they have differentiated into resting Th1 effectors, relabeled with CFSE, and retransferred into secondary recipients expressing either parenchymal self-HA or viral-HA (24). In the current experiment (Fig. 4), the secondary recipients consisted of DT-treated NT→C3-HAhigh, DTR→C3-HAhigh, NT→viral-HA (106 PFU) and DTR→viral-HA (106 PFU) chimeras. Consistent with our previous results using nonchimeric recipients (24, 25), clonotypic effectors retransferred into control NT→C3-HAhigh and NT→viral-HA secondary recipients underwent robust CFSE-dilution (Fig. 4A). Additionally, these CD4 cells either maintained their ability to express IFN-γ and TNF-α in NT→viral-HA secondary recipients or became impaired in both their ability to express IFN-γ (2-fold reduction, p = 0.06) and TNF-α (18-fold reduction, p < 0.0001; Fig. 4C) and to undergo blastogenesis (data not shown) after retransfer into NT→C3-HAhigh chimeras. DC depletion in DTR→viral-HA secondary recipients reduced clonal expansion ~2-fold (p = 0.005; Fig. 4B), although IFN-γ and TNF-α expression potentials were not altered (Fig. 4C), indicating that DC are required for maximal clonal expansion but not maintenance of effector function after viral rechallenge. DC depletion in DTR→C3-HAhigh secondary recipients did not impact clonal expansion (Fig. 4B), but tolerization was partially mitigated. Thus, IFN-γ expression potential was no longer impaired, although TNF-α expression potential was not significantly rescued (Fig. 4C).
FIGURE 4.
Role of DC in presenting parenchymal self- and vaccinia viral-Ag to Th1 effector CD4 cells. CFSE-labeled Thy1.1+ Th1 effector clonotypic CD4 cells were adoptively retransferred into Thy1.2+ NT→C3-HAhigh, DTR→C3-HAhigh, NT→viral-HA, and DTR→viral-HA chimeric secondary recipients and recovered from spleens 5 days later. Viral inoculations were given at 106 PFU, and representative CFSE-dilution histograms (A) and clonotypic CD4 cell frequencies (B) are presented as in Fig. 3 except that intracellular cytokine staining data are also shown for the clonotypic effectors before retransfer (1° effector) in C. n = 4–7 for each group, except for the control NT→C3-HA group in which n = 2. For the quantitative analyses of the latter group, two additional nonchimeric C3-HA recipients on the B10.D2 background were included, which displayed comparable responses to the NT→C3-HA recipients (data not shown). BM, Bone marrow.
CD4+CD25+ regulatory T cells are not required for CD4 cell tolerization to parenchymal self-Ag
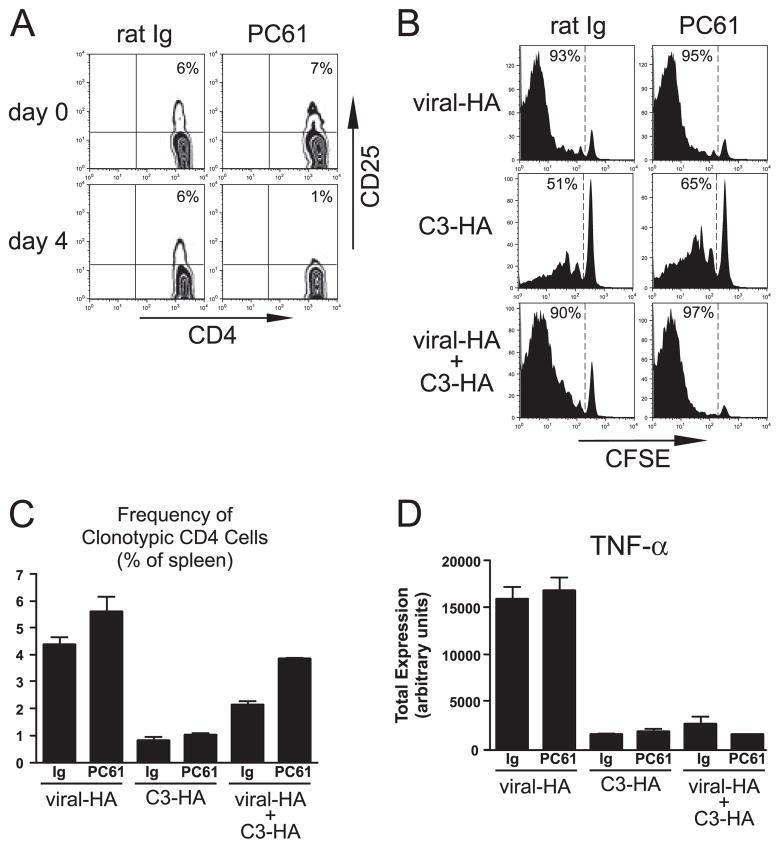
Given that in vitro studies have suggested that CD4+CD25+ Tregs are most active when DC remain in a nonactivated or steady state (28, 29), as well as the preceding data demonstrating the involvement of steady state DC in presenting parenchymal self-Ag and programming the tolerization of cognate CD4 cells, we hypothesized that Tregs might work in concert with steady state DC to program CD4 cell tolerance. To test this possibility, Treg function was neutralized using the established protocol of treatment with the anti-CD25 mAb PC61 (33–35, 38). Naive clonotypic CD4 cells were adoptively transferred into C3-HAlow and viral-HA (control) recipients that had been treated with either PC61 or rat Ig (control) 4 days earlier, and were recovered from spleens 5 days posttransfer for analysis. PC61 treatment reduced the frequency of CD4+ CD25+ cells from ~7% of the total CD4+ T cell population to background as measured on both the day after treatment (data not shown) and the day of adoptive transfer (Fig. 5A). Neutralizing levels of the PC61 mAb did not appear to be present at the time of clonotypic CD4 cell adoptive transfer, however, because the clonotypic CD4 cell response was not diminished in PC61-treated viral-HA recipients compared with rat Ig controls. Thus, because naive clonotypic CD4 cells transferred into viral-HA recipients transiently express high levels of CD25 (23, 39), they should have been susceptible to neutralization if the PC61 mAb remained at high levels. To the contrary, PC61 treatment did not influence the ability viral-HA-primed clonotypic CD4 cells to divide (Fig. 5B), to expand (Fig. 5C), or to express either IFN-γ (data not shown) or TNF-α (Fig. 5D). PC61 treatment, however, did not markedly rescue the ability of naive clonotypic CD4 cells encountering parenchymal self-Ag in C3-HAlow recipients to either express TNF-α (which is the most reliable indicator for tolerance in this system; Refs. 25 and 36) and Figs. 2 and Fig. 5D) or to undergo blastogenesis (data not shown). Similarly, PC61 treatment did not alter the response of either naive clonotypic CD4 cells transferred into C3-HAhigh recipients, or Th1 effector clonotypic CD4 cells transferred into either C3-HAlow or C3-HAhigh secondary recipients (data not shown). Furthermore, pretreatment of the TCR-transgenic clonotypic CD4 cell donor mice with PC61 did not alter the tolerogenic outcome (data not shown), arguing against a potential role of Tregs in the transferred T cell population.
FIGURE 5.
CD4+CD25+ Tregs are not required for CD4 cell tolerization to parenchymal self-Ag. Naive Thy1.1+ CFSE-labeled clonotypic CD4 cells were adoptively transferred into NT recipients infected with 106 PFU of viral-HA (viral-HA), C3-HAlow (C3-HA), and C3-HAlow recipients infected with 106 PFU of viral-HA (viral-HA + C3-HA) that had been treated 4 days earlier with either anti-CD25 (PC61) or control rat Ig, and were recovered from spleens for analysis 5 days posttransfer. A, Representative plots showing the frequency of CD4+CD25+ cells in peripheral blood both before and 4 days after Ab treatment. CD25 staining was performed using the 7D4 mAb, which does not cross-react with PC61. Representative CFSE-dilution histograms (B) and quantification of clonal expansion (C) and total TNF-α expression (D) are presented as in previous figures. n = 3 for each group.
To further explore the relationship between Treg function and CD4 cell response to parenchymal self- and viral-Ags, naive clonotypic CD4 cells were transferred into PC61-treated C3-HAlow recipients that had been infected with viral-HA. Similar to our previous results using non-Ab-treated mice (24), clonal expansion (Fig. 5C) in control rat Ig-treated viral-HA + C3-HAlow recipients was intermediate to that observed in viral-HA and C3-HAlow recipients, perhaps because the clonotypic CD4 cells that were primed by viral-HA were immediately subject to self-HA-mediated tolerization (24). Similarly, TNF-α expression potential in control viral-HA + C3-HAlow recipients was at best only marginally elevated relative to C3-HAlow recipients (Fig. 5D). PC61 treatment of viral-HA + C3-HAlow recipients did augment clonal expansion ~2-fold relative to control rat Ig-treated counterparts (p = 0.0002; Fig. 5C) but did not rescue TNF-α expression potential (Fig. 5D) or increase blastogenesis (data not shown). Taken together, these data suggest that although CD4+CD25+ Tregs can limit the clonal expansion of virally primed CD4 cells, they are not required to program tolerogenic CD4 cell differentiation to parenchymal self-Ag.
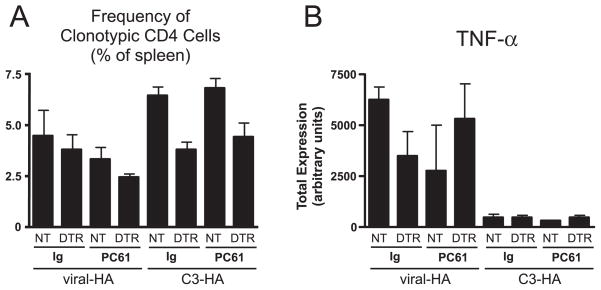
Although the previous experiment suggested that CD4+CD25+ Tregs are not required to program CD4 cell peripheral tolerance to parenchymal self-Ag in the presence of steady state DCs, given that a non-DC APC population(s) also appears to be capable of presenting parenchymal self-Ag to induce tolerization (Figs. 2 and 4), it might have been possible that Tregs act in concert with this other cell type(s) to program tolerance. Thus, we next analyzed the effect of Treg neutralization in DT-treated chimeric recipients that either contained (reconstituted with control NT bone marrow) or lacked (reconstituted with DTR bone marrow) HA-presenting DC (Fig. 6). Thus, DT-treated NT→C3-HAhigh, DTR→C3-HAhigh, NT→viral-HA (106 PFU) and DTR→viral-HA (106 PFU) chimeras were treated with PC61 or rat Ig 4 days before adoptive transfer of naive clonotypic CD4 cells, and spleens were analyzed 5 days posttransfer. In the previous DC depletion experiments (Figs. 2–4), the chimeric recipients were treated with DT every 3 days based on the previously reported rate of DC replenishment in the spleen of CD11c-DTR transgenics after DT injection (9). Because we could not exclude the possibility that tolerance-inducing DC could reside in other organs where replenishment might occur more rapidly, however, in the current experiment (Fig. 6) DT was administered daily after adoptive transfer to more rigorously prevent the potential for the clonotypic CD4 cells to encounter DCs presenting the relevant HA epitope. This daily DT treatment regimen does not appear to nonspecifically influence in vivo APC or T cell function because adoptively transferred clonotypic CD4 cells responded comparably in untreated and daily DT-treated non-chimeric viral-HA and C3-HAhigh recipients (data not shown). Similar to the results presented in Fig. 2, clonal expansion of the transferred HA-specific CD4 cells was reduced ~2-fold in control rat Ig-treated DTR→C3-HAhigh relative to NT→C3-HAhigh recipients (p = 0.002), although this effect was less pronounced in viral-HA chimeras (Fig. 6A). Additionally, DC depletion did not rescue impaired TNF-α expression potential (Fig. 6B) or increase blastogenesis (data not shown) in control rat Ig-treated C3-HAhigh chimeric recipients. Importantly, PC61 treatment in DTR→C3-HAhigh recipients had no effect on clonal expansion (Fig. 6A) and blastogenesis (data not shown), and only weakly rescued TNF-α expression (<2-fold, p = 0.03; Fig. 6B). Taken together, regardless of whether DC are present or absent, PC61 treatment does not appreciably influence the tolerogenic outcome of CD4 cells encountering cognate parenchymal self-Ag. Thus, CD4+CD25+ Tregs do not appear to work in concert with either steady state DC or other APC(s) to program this tolerogenic response.
FIGURE 6.
CD4+CD25+ Tregs do not act in concert with non-DC-tolerogenic APCs to program CD4 cell tolerization to parenchymal self-Ag. Naive Thy1.1+ CFSE-labeled clonotypic CD4 cells were adoptively transferred into Thy1.2+ NT→C3-HAhigh, DTR→C3-HAhigh, NT→viral-HA, and DTR→viral-HA chimeric recipients as in Fig. 2, except that DT injections were administered daily and recipients were treated 4 days before adoptive transfer with either rat Ig or PC61 mAb as indicated. Clonal expansion (A) and total TNF-α expression (B) are presented as in previous figures. n = 3–10, except for the rat Ig NT→viral-HA group where n = 2.
Discussion
Our current study indicates that although steady state DCs clearly present parenchymally derived self-Ag and contribute in mediating the tolerization of cognate naive and Th1 effector CD4 cells, they are not essential for these processes. Earlier work has described the ability of other APC population such as macrophages (20) and B cells (21, 22) to induce T cell tolerance; however, more recent attention has focused on DCs as being the most likely APC population that is responsible for inducing T cell tolerance to parenchymal self-Ags. Thus, delivery of exogenous Ags to DCs under steady state conditions renders cognate naive T cells tolerant, whereas coadministration of either inflammatory cytokines (14) or costimulatory agonists (15) programs effector function. Additionally, under steady state conditions, naive CD8 cells are able to recognize their cognate parenchymally derived self-Ag in transgenic mice where DCs are the only APC population that is capable of presenting the relevant class I-restricted peptide (11). Although these studies demonstrating the potential of steady state DCs to induce T cell tolerance, they did not rule out the involvement of other APC populations. Nevertheless, the possibility that DCs represent the only APC population that induces T cell tolerance to self-Ags under steady state conditions was supported by studies in which DCs were the only APC population that could be fractionated from LNs draining a particular self-Ag that could activate cognate T cell lines or hybridomas in vitro (12, 13). Our current observation that both naive and Th1 effector CD4 cells continue to undergo tolerization (albeit at reduced efficiency) after depletion of DCs suggests that other bone marrow-derived APC populations can also present parenchymal self-Ag to induce tolerance, and further suggests that in vitro T cell stimulation assays might not be sufficiently sensitive to detect Ag presentation by non-DC APC populations that might be presenting lower (but biologically significant) levels of parenchymal self-Ag. Consistent with this possibility, we have had difficulty activating the HA-specific clonotypic CD4 cells in vitro using fractionated APC from C3-HAhigh spleens (data not shown), despite the robust ability of these APC to induce the same T cells to proliferate in vivo. Alternatively, the ability of non-DC APC populations to present parenchymal self-Ag and induce tolerance in cognate T cells might depend on factors such as the level, location, and conditions of Ag expression. In this regard, it is interesting that in contrast to the C3-HA transgenic system examined in the current study in which parenchymal self-HA is expressed in a variety of organs, we have found that DC depletion completely abrogates Ag presentation to clonotypic CD4 cells in a model in which HA expression is limited to prostate epithelia and tumors (40). Nevertheless, our current data do suggest that DCs are not the exclusive mediators of T cell tolerance for parenchymal self-Ags.
Analogous to our results with parenchymal self-Ag, we also found that DC depletion did not abrogate priming of naive vaccinia virus-specific CD4 cells. Although the absence of DCs reduced clonal expansion and impaired the development of effector functions when viral titers were low, this result was nevertheless surprising given previous studies using the CD11c-DTR system in which naive CD8 cell priming to Listeria monocytogenes, Plasmodium yoelii (9), and lymphocytic choriomeningitis virus (10) was completely abrogated after DC depletion. One possibility to explain these results is that DCs are more critical for priming naive CD8 than naive CD4 cells in vivo, although it might also be possible that only certain pathogens can enable non-DC APC populations with the ability to prime naive T cells. Although this will be an interesting question to address in future studies, our current data do indicate that DCs are not the only APC population that can program naive T cells to develop effector functions in response to pathogen challenge.
It has recently been shown that in addition to DCs, DT treatment of CD11c-DTR-transgenic mice also induces the depletion of marginal zone and metallophilic macrophages (41). This does not alter our current conclusion that a non-DC APC population(s) can both tolerize and prime CD4 cells to parenchymal self- and vaccinia viral-Ags, respectively, but rather narrows the possible identity of this APC(s) to either macrophages (e.g., red pulp macrophage) or B cells (which are capable of priming CD4 cells under certain conditions (Refs. 42 and 43).
Given that CD4+CD25+ Tregs appear to be most active when DC remain in a nonactivated or steady state (28, 29), it was surprising that their neutralization did not alter the functional response of CD4 cells undergoing tolerization in response to cognate parenchymal self-Ag in our system. It was recently shown that CD4+CD25+ Tregs prevent autoimmune gastritis in lymphopenic mice by limiting the ability of adoptively transferred naive gastric parietal cell-specific CD4 cells to differentiate into IFN-γ-expressing Th1 effectors, even when an excess of nonregulatory T cells are added to reduce lymphopenia-induced proliferation (44). It has also been reported that Tregs facilitate the development of CD4 cell anergy during the recovery from lymphopenia (45). Tregs might therefore be more critical for programming CD4 cell tolerization under lymphopenic or partially lymphopenic conditions than during steady state conditions. Alternatively, because the gastric environment appears to contain irritants or inflammatory mediators that induce weak activation of gastric DC (13), Tregs may play a more critical role in programming CD4 cell tolerance when steady state APCs have been partially activated. Thus, in our C3-HA system, Treg function might be redundant because the relevant steady state APCs remain fully nonactivated.
Footnotes
This work was supported by National Institutes of Health Grants AI057441 and CA109339 (to A.J.A.).
Abbreviations used in this paper: DC, dendritic cells; DT, diphtheria toxin; DTR, diphtheria toxin receptor; HA, hemagglutinin; LN, lymph node; NT, nontransgenic; viral-HA, recombinant vaccinia virus expressing HA; Treg, CD4+CD25+ regulatory T cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 2.Lenz LL, Butz EA, Bevan MJ. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intra-cellular pathogens. J Exp Med. 2000;192:1135–1142. doi: 10.1084/jem.192.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad SA, Norbury CC, Chen W, Bennink JR, Yewdell JW. Cutting edge: recombinant adenoviruses induce CD8 T cell responses to an inserted protein whose expression is limited to nonimmune cells. J Immunol. 2001;166:4809–4812. doi: 10.4049/jimmunol.166.8.4809. [DOI] [PubMed] [Google Scholar]
- 4.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 7.Bannchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 11.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166:1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 12.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 15.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 18.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 19.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Suzuki G, Yamamura K. The role of macrophages in antigen presentation and T cell tolerance. Int Immunol. 1993;5:1023–1033. doi: 10.1093/intimm/5.9.1023. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 22.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 24.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169:3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-α and IFN-γ expression potentials. Cell Immunol. 2003;224:114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 28.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 30.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 31.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 39.Long M, Adler AJ. Cutting edge: paracrine, but not autocrine, IL-2 signaling is sustained during early antiviral CD4 T cell response. J Immunol. 2006;177:4257–4261. doi: 10.4049/jimmunol.177.7.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67:536–546. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453–1461. [PubMed] [Google Scholar]
- 43.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 44.DiPaolo RJ, Glass DD, Bijwaard KE, Shevach EM. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 45.Vanasek TL, Nandiwada SL, Jenkins MK, Mueller DL. CD25+Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J Immunol. 2006;176:5880–5889. doi: 10.4049/jimmunol.176.10.5880. [DOI] [PubMed] [Google Scholar]