Abstract
BACKGROUND
Prostate cancer promotes the development of T cell tolerance towards prostatic antigens, potentially limiting the efficacy of prostate cancer vaccines targeting these antigens. Here, we sought to determine the stage of disease progression when T cell tolerance develops, as well as the role of steady state dendritic cells (DC) and CD4+CD25+ T regulatory cells (Tregs) in programming tolerance.
METHODS
The response of naïve HA-specific CD4+ T cells were analyzed following adoptive transfer into Pro-HA × TRAMP transgenic mice harboring variably-staged HA-expressing prostate tumors on two genetic backgrounds that display different patterns and kinetics of tumorigenesis. The role of DC and Tregs in programming HA-specific CD4 cell responses were assessed via depletion.
RESULTS
HA-specific CD4 cells underwent non-immunogenic responses at all stages of tumorigenesis in both genetic backgrounds. These responses were completely dependent on DC, but not appreciably influenced by Tregs.
CONCLUSIONS
These results suggest that tolerogenicity is an early and general property of prostate tumors.
Keywords: prostate cancer, T cells, transgenic mice, dendritic cells, T regulatory cells
INTRODUCTION
The potential to focus T cell cytotoxicity on tumors has stimulated a considerable effort into developing T cell-based therapies for treating cancer. Nevertheless, despite the identification of numerous antigens expressed either specifically or preferentially on tumors, as well as improved vaccination strategies that can prime robust effector and memory T cell responses, results from recent clinical trials have only demonstrated partial successes [1,2]. This is likely to be at least partially due to the ability of tumors to dampen cognate T cell responses. Thus, certain tumors develop an immunosuppressive microenvironment that limits the ability of primed tumor-reactive T cells to express their effector functions locally, while others induce T cell tolerance at the systemic level [3].
Prostate cancer (the most common malignancy in American men [4]) has become an attractive target for T cell-based therapies, in part because prostate tumors can continue to express defined prostatic antigens that can serve as targets, and since the prostate is a non-vital organ, the potential autoimmune damage directed towards non-malignant prostatic tissue should not cause prohibitive side effects [5]. To understand the basic immunological properties of prostate tumors, and thus how T cell-based therapies can be tailored to treat prostate cancer, we recently developed a transgenic mouse model system in which the response of prostate-specific T cells could be examined under both normal conditions as well as following the development of prostate cancer [6]. In healthy individuals, prostate epithelial-specific T cells are ignorant of their cognate antigen (i.e., they are neither activated nor tolerized), apparently because the antigen is secreted into the prostatic lumen rather than the draining lymphatics. In animals with advanced prostate cancer, however, prostatic antigen reaches the draining lymph nodes (LN) where it is presented to cognate T cells. Rather than developing effector function, these prostate-specific T cells undergo an initial phase of proliferation followed by the development of tolerance as defined by impaired responsiveness to subsequent vaccination [6].
In the current study, we further examined the relationship between prostate tumorigenesis and the functional state of prostate-specific T cells by first characterizing the stage of prostate tumor progression during which prostatic antigen begins to be presented in the draining LN, and also assessing whether T cells encountering prostatic antigen in mice that display a more aggressive pattern of tumorigenesis develop effector function. Interestingly, prostatic antigen is presented in the draining LN at relatively early stages of disease progression regardless of the pattern of tumorigenesis. Although the more aggressive pattern of tumorigenesis was associated with a more robust prostate-specific T cell proliferative response, these T cells still failed to develop effector function, suggesting that non-immunogenicity/tolerogenicity is a general property of prostate tumors. Additionally, during prostate tumorigenesis steady state dendritic cells (DC), but not CD4+CD25+ T regulatory cells (Tregs), play a critical role in programming non-immunogenic prostate-specific T cell responses in the draining LN.
METHODS
Transgenic Mice, Adoptive Transfer and FACS Analysis
CD11c-DTR [8], 6.5 [7], TRAMP [9], C3-HA (line 142) [10], and Pro-HA [6] transgenic mice were all initially backcrossed to the B10.D2 (H-2d) genetic background. In experiments using pure B10.D2 mice, naïve CFSE-labeled 6.5 TCR transgenic clonotypic CD4 cells expressing the Thy1.1 congenic marker were adoptively transferred into Thy1.2-expressing recipients and recovered 5 days later from the prostate-draining periaortic and non-draining cervical LN to assess proliferative response (directly ex vivo) and cytokine expression potential following in vitro restimulation with peptide-pulsed APCs as previously described [6,11,12]. F1 donor and recipient mice were generated by crossing non-transgenic (NT), 6.5, Pro-HA, and Pro-HA × TRAMP transgenic mice on the B10.D2 background to NT FVB (H-2q, Thy1.1+) mice purchased from The Jackson Laboratory (Bar Harbor, ME). The F1 clonotypic CD4 cells (Thy1.1+/Thy1.1+) were tracked following adoptive transfer into F1 Thy1.1+/Thy1.2+ recipients via the absence of Thy1.2.
DC and Treg Neutralization
CD11c-DTR bone marrow chimeras were generated as per our standard protocol [11], and DC were depleted in conjunction with adoptive T cell transfer via i.p. treatment with diptheria toxin (DT) 4 ηg/g body weight (DT, Sigma-Aldrich, St. Louis, MO) as previously described [13]. CD4+CD25+ Tregs were neutralized in vivo using the anti-CD25 mAb PC61 (eBioscience, San Diego, CA) as previously described [14–17]. In short, adoptive transfer recipients were treated i.v. with 100 μg PC61 or control rat Ig 4 days prior to receiving clonotypic CD4 cells.
Disease Staging
Prostates were dissected and weighed following removal of the seminal vesicles, bladder, and urethra, and subsequently fixed in 10% formalin followed by 70% EtOH prior to embedding in paraffin, sectioning, and staining with hematoxylin and eosin (embedding, sectioning, and staining were performed by the University of Connecticut Health Center’s Research Histology Core Facility). Pathological staging of prostate tumors was performed blindly using the criterion previously established for TRAMP mice, with a score of 1 corresponding to normal prostate, 2 corresponding to low grade PIN, 3 corresponding to high grade PIN, 4 corresponding to well differentiated carcinoma, 5 corresponding to moderately differentiated carcinoma, and 6 corresponding to poorly differentiated carcinoma [18]. Prostates containing regions that differed morphologically were assigned a score corresponding to the worst (i.e., most transformed) region.
RESULTS
Prostatic Antigen Is Presented for T Cell Recognition in Secondary Lymphoid Organs at Early Stages of Prostate Tumor Development
To study how the development of prostate cancer impacts the function of prostate-specific T cells, we previously developed a TCR transgenic adoptive transfer system in which naïve clonotypic influenza hemagglutinin (HA)-specific CD4 cells were tracked following transfer into either Pro-HA transgenic mice that express a secreted form of HA under the control of the prostate epithelial-specific minimal rat Probasin promoter or double transgenic mice generated by crossing Pro-HA to TRAMP transgenic mice that develop spontaneous prostate tumors from the expression of SV40 large T antigen also driven by the Probasin promoter [6]. When transferred into healthy single transgenic Pro-HA recipients, the clonotypic CD4 cells retain their naïve phenotype and potential to respond to subsequent vaccination. In contrast, when transferred into 6 month-old double transgenic Pro-HA × TRAMP mice that have developed advanced prostate cancer, the naïve clonotypic CD4 cells undergo an abortive proliferative response in the prostate draining LN that does not lead to the development of effector function, but rather results in systemic tolerance as defined by the loss of responsiveness to subsequent vaccination. The lack of T cell recognition of prostatic antigen in healthy mice might have resulted from the secretion of HA antigen by prostate epithelia into the ejaculate rather than into the draining lymphatics, while the development of advanced prostate cancer might have allowed HA to reach the draining LN through one or more of the following mechanisms: (1) increasing the number of HA-expressing cells, (2) altering the prostatic architecture so that secreted HA becomes redirected into the draining lymphatics, or (3) delivering HA directly to the draining LN via metastases. To glean some insight into the relative contributions of these potential mechanisms, we compared the response of naïve clonotypic CD4 cells transferred into double transgenic Pro-HA × TRAMP recipients of different ages representing different stages of disease progression. Determining the stage of disease progression when prostatic antigen reaches the draining LN for T cell recognition might also provide a useful clinical correlate for predicting when tolerance might develop during the course of disease progression.
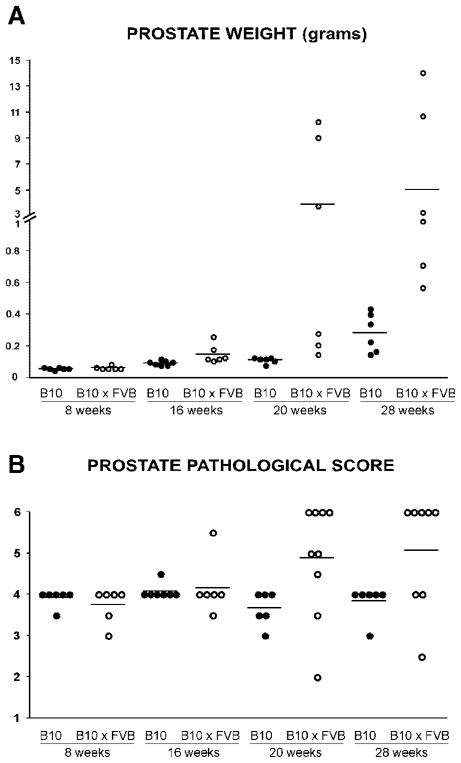
In addition to correlating T cell recognition of prostatic antigen with disease progression in Pro-HA × TRAMP mice on the B10.D2 (H-2d) genetic background that we had used in our previous study [6], we performed a similar analysis using Pro-HA × TRAMP mice on a F1 FVB (H-2q) × B10.D2 background. TRAMP mice on a F1 FVB × C57BL/6 background develop a more aggressive pattern of tumorigenesis compared to a pure C57BL/6 background [19], and similarly F1 Pro-HA × TRAMP mice generated from crossing FVB to B10.D2 mice (which are comparable to C57BL/6 excepting the MHC locus) also develop a more aggressive form of prostate cancer compared to pure B10.D2 Pro-HA × TRAMP mice. This is evidenced by increased prostate weights beginning at 16 weeks and becoming considerably more pronounced at 20 and 28 weeks (Fig. 1A) as well as increased pathological scores at the 20 and 28-week time points (Fig. 1B). Thus, this comparison could indicate whether the aggressiveness of prostate tumorigenesis influences the pattern of prostatic antigen presentation or the nature of the cognate T cell response.
Fig. 1.
The course of disease progression in Pro-HA × TRAMP mice on the B10.D2 (B10) versus F1 B10.D2 × FVB (B10 × FVB) genetic backgrounds. Scatter plots showing total prostate weights (A) and pathological scores of prostate tumors (B) for individual mice, with horizontal lines designating mean values. Note that the prostate weights of 8 week-old double transgenic Pro-HA × TRAMP mice are similar to that of single transgenic Pro-HA mice (data not shown).
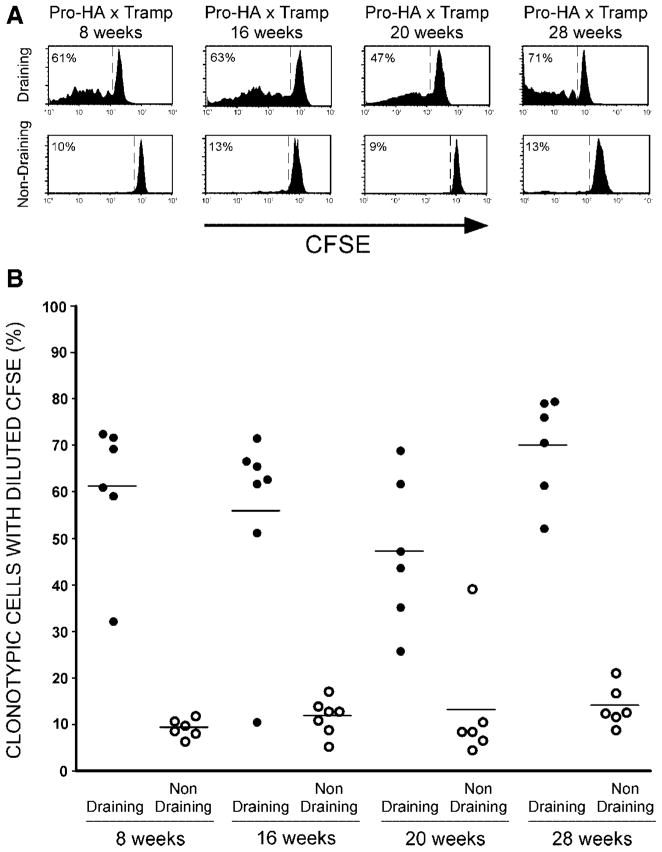
Similar to our previous results [6], when CFSE-labeled naïve clonotypic HA-specific CD4 cells were adoptively transferred into pure B10.D2 double transgenic Pro-HA × TRAMP mice that were 28 weeks-old (a time point associated with advanced disease) and recovered 5 days later, they were found to have undergone a proliferative response (as indicated by the dilution of CFSE on the majority of clonotypic cells) in the prostate-draining periaortic LN (Fig. 2). This CFSE-dilution pattern did not exhibit an accumulation of clonotypic cells that had undergone multiple divisions, and was therefore, suggestive of an abortive proliferative response that might lead to deletion. Furthermore, CFSE-dilution was not observed in the prostate-non-draining cervical LN, indicating that prostatic antigen presentation was limited to the prostate-draining LN. Interestingly, a similar pattern of clonotypic CD4 cell CFSE-dilution was observed in younger mice, even 8 week-old mice that had only recently initiated tumorigenesis and not yet developed enlarged tumors. This suggests that release of prostatic antigen into the draining lymphatics does neither require the development of a large or poorly differentiated primary tumor, nor in all likelihood metastases.
Fig. 2.
Time course of T cell prostatic antigen recognitionin B10.D2 Pro-HA × TRAMP mice. A: Representative CFSE-dilution histograms of adoptively transferred clonotypic CD4 cells recovered 5 days post-transfer from the prostate-draining periaortic and prostate-non-draining cervical LN of B10.D2 Pro-HA × TRAMP mice aged 8,16, 20, or 28 weeks. A dashed line separates divided from undivided clonotypic CD4 cells, and the percentage of clonotypic CD4 cells with diluted CFSE is indicated. B: Scatter plot showing the percentage of clonotypic CD4 cells with diluted CFSE for individual mice, with horizontal lines designating mean values.
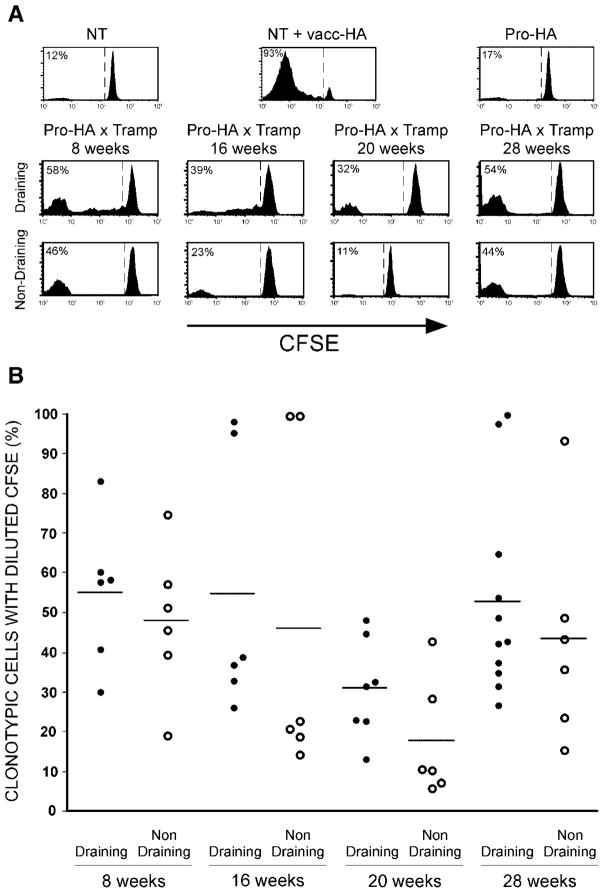
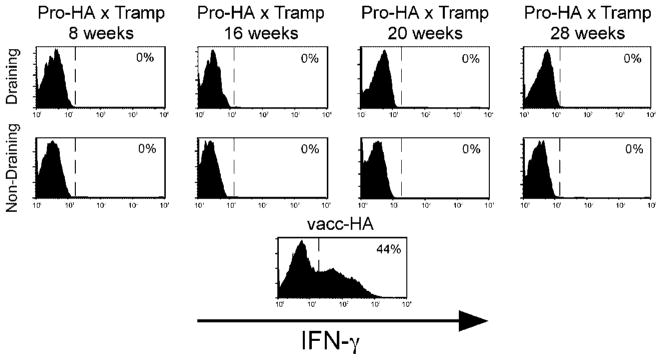
Since we had not previously performed clonotypic CD4 cell adoptive transfers using F1 FVB × B10.D2 donors and recipients, in addition to the Pro-HA × TRAMP double transgenic recipients, control recipients were included to verify that the basic functional properties of the clonotypic CD4 cells were not altered on the F1 background. Similar to the clonotypic CD4 cell responses previously observed on the pure B10.D2 background [6], robust CFSE-dilution was observed in F1 NT recipients that had been infected with a recombinant vaccinia virus that expresses HA (vacc-HA), and proliferation did not occur in either non-infected NT recipients or single transgenic Pro-HA recipients (Fig. 3A). Also similar to the pure B10.D2 background (refer to Fig. 2), clonotypic CD4 cell proliferation was observed in the prostate-draining LN of Pro-HA × TRAMP double transgenic recipients from 8 to 28 weeks of age (Fig. 3). The pattern of CFSE-dilution, however, was slightly different in the F1 recipients, which displayed an accumulation of clonotypic cells that had undergone multiple rounds of division. Additionally, CFSE-diluted clonotypic CD4 cells were often observed in prostate-non-draining LN, perhaps resulting from dissemination of HA antigen to other anatomical locations via metastases (which occur at higher frequency in F1 mice [19]), or alternatively, the more robust proliferation induced in the prostate-draining LN might have been associated with the acquisition of migratory capacity. Either way, the more extensive pattern of proliferation associated with the more aggressive pattern of tumorigenesis in the F1 mice suggested the possibility that the functional outcome of this response might be more immunogenic in nature than the non-immunogenic response we previously characterized in the pure B10.D2 background in which the clonotypic CD4 cells did not develop effector function [6]. This was not the case, however, as clonotypic CD4 cells that had proliferated in response to prostatic antigen in F1 Pro-HA × TRAMP mice were not able to express the effector cytokine IFN-γ in response to in vitro re-stimulation with HA peptide-pulsed APCs, in contrast to vacc-HA-primed clonotypic CD4 cells which had developed the capacity to express this effector cytokine (Fig. 4). Taken together, these results suggest that while a more aggressive pattern of tumorigenesis appears to result in greater release of prostatic antigen for cognate T cell recognition, these T cells still fail to develop effector function.
Fig. 3.
Time course of T cell prostatic antigen recognition in F1 FVB × B10.D2 Pro-HA × TRAMP mice. Representative CFSE-dilution histograms (A) and scatter plot showing the percentage of clonotypic CD4 cells with diluted CFSE for individual mice (B) are presented as in Figure 2.
Fig. 4.
IFN-γ expression potential of CFSE-diluted clonotypic CD4 cells recovered from F1 FVB × B10.D2 Pro-HA × TRAMP mice. Clonotypic CD4 cells recovered from F1 Pro-HA × TRAMP mice described in Figure 3 were restimulated in vitro with HA peptide-pulsed APCs prior to fixation, permeabilization, and intracellular staining for IFN-γ. Representative histogram plots (from two to four mice per group) of IFN-γ expression gated on CFSE-diluted clonotypic CD4 cells are shown with a dashed line indicating positive IFN-γ expression. Restimulated clonotypic CD4 cells recovered from the spleen of a vacc-HA-infected F1 mouse is shown as a positive control for IFN-γ expression.
Dendritic Cells, But Not CD4+CD25+ T Regulatory Cells, Play a Critical Role in Programming CD4 Cell Responses to Prostatic Antigen During Tumorigenesis
Having established that prostatic antigen is presented to cognate T cells in the draining LN at relatively early stages of prostate tumorigenesis, we wished to understand the pathways by which this antigen presentation and cognate T cell response was mediated. It has previously been shown that T cell tolerance induction to parenchymal self-antigens can be mediated through an indirect or cross-presentation pathway in which parenchymal antigen is acquired by bone marrow-derived antigen presenting cells (APCs) and subsequently presented to cognate T cells in the draining lymphoid organs [10,20]. Although all three major subsets of bone marrow-derived APCs (i.e., B cells, macrophages, and DC) are capable of cross-presenting antigen under certain conditions, previous studies have suggested that during steady state conditions, DC are the most likely to present parenchymal self-antigen to induce T cell tolerance. Thus, naïve CD8 cells are able to recognize their cognate parenchymally derived class I-restricted self-epitope in transgenic mice where DC are the only APC population genetically capable of presenting it [21]. Furthermore, when APCs are fractionated from LN draining a particular self-antigen, DC are the only subset capable of stimulating cognate T cell lines or hybridomas in vitro [22,23]. It had not been formally demonstrated, however, that DC play an essential role in facilitating T cell recognition of a self/tumor-antigen in vivo. To assess the relevance of DC in presenting prostatic antigen in our system, we generated bone marrow chimeras in which lethally irradiated single transgenic Pro-HA and double transgenic Pro-HA × TRAMP mice were reconstituted with bone marrow from either NT (control) or CD11c-DTR donors in which the DT receptor (DTR) is expressed under the control of the DC-specific CD11c promoter [8]. Following reconstitution, DC can be efficiently depleted in these chimeras via treatment with DT every 3rd day [13].
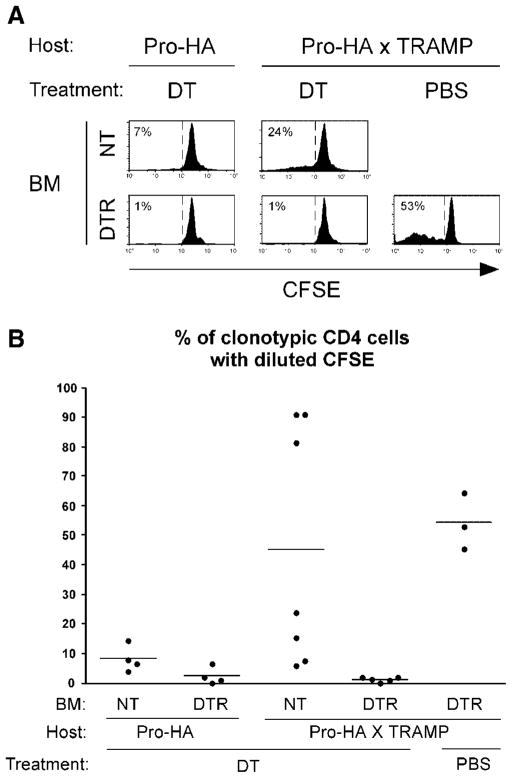
When CFSE-labeled naïve clonotypic CD4 cells were adoptively transferred into control DT-treated Pro-HA single transgenic chimeras that had been reconstituted with NT bone marrow (i.e., DC should not be depleted), a low level of CFSE-dilution was observed following recovery from the prostate-draining LN (Fig. 5), consistent with our previous results using non-chimeric mice [6]. This weak proliferative response was reduced to near background in DT-treated Pro-HA single transgenic chimeras that had been reconstituted with DTR bone marrow, indicating that DC are responsible for presenting low levels of prostatic antigen under steady state conditions. Clonotypic CD4 cell proliferation was more robust in DT-treated NT → Pro-HA × TRAMP double transgenic chimeras compared to NT → Pro-HA single transgenic counterparts, consistent with our previous observation that prostate tumorigenesis increases prostatic antigen presentation [6]. Clonotypic CD4 cell proliferation in DTR → Pro-HA × TRAMP chimeras was reduced to background following treatment with DT, but not PBS (Fig. 5), indicating that DC are responsible for presenting prostatic antigen during prostate tumorigenesis.
Fig. 5.
DC are essential for presenting prostatic antigen during tumorigenesis. Pro-HA single transgenic and Pro-HA × TRAMP double transgenic mice on the B10.D2 background were lethally irradiated and reconstituted with bone marrow from either control NT or CD11c-DTR (DTR) donors. At least 8 week slater, chimeras were treated with DT(4ηg/gbody weight)or PBS on Days −4, −1, and +2, and received adoptive transfers of naœve CFSE-labeled clonotypic CD4 cells on Day 0 which were subsequently recovered for analysis 5 days post-transfer from the prostate-draining LN. Chimeric recipients were between 3 and 4 months of age at the time of adoptive transfer. Representative CFSE-dilution histograms (A) and scatter plot showing the percentage of clonotypic CD4 cells with diluted CFSE for individual mice(B)are presented as in previous figures.
Although bone marrow-derived APCs presenting self-antigens under steady state conditions clearly play a critical role in facilitating T cell tolerance, CD4+CD25+ Tregs have also been shown to play an important role in maintaining peripheral T cell tolerance in numerous models [24,25]. The precise mechanisms by which CD4+CD25+ Tregs suppress autoreactive T cell responses in vivo have not been well defined, but given in vitro studies suggesting that CD4+CD25+ Tregs are most active when DC remain in non-activated or steady state [26,27], we hypothesized that Tregs might work in concert with steady state DC to program non-immunogenic prostate-specific T cell responses during tumorigenesis. To test this possibility, Treg function was neutralized in Pro-HA × TRAMP mice using the established protocol of treatment with the anti-CD25 mAb PC61 [14–17], which has previously been shown to augment anti-tumor T cell responses in various models [14,15,28,29].
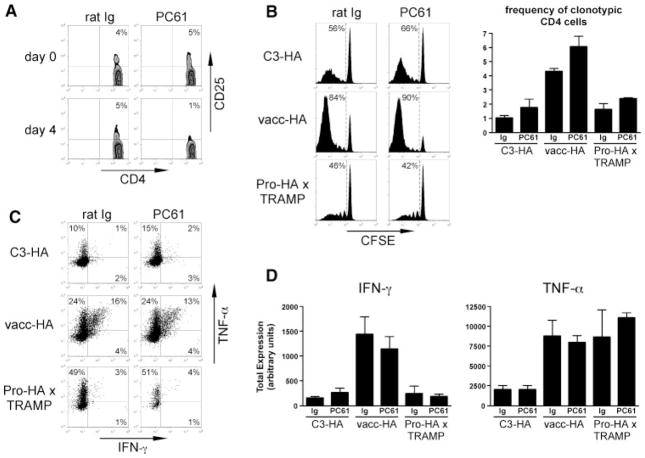
In addition to neutralizing Treg function in Pro-HA × TRAMP clonotypic CD4 cell adoptive transfer recipients, we included an immunogenic vacc-HA-infected NT control recipient group in which naïve clonotypic CD4 cells normally differentiate into Th1 effectors capable of producing IFN-γ and TNF-α. A non-immunogenic control group was also included in which naïve clonotypic CD4 cells transferred into C3-HA transgenic recipients (which express parenchymal self-HA in multiple tissues) normally undergo an initial proliferative response followed by the development of a tolerant/hyporesponsive phenotype [11,30,31]. Recipients were treated with either PC61 (anti-CD25) mAb or rat Ig (control Ab) 4 days prior to adoptive transfer, and the clonotypic CD4 cells were recovered from the prostate-draining LN 5 days post-transfer for analysis. PC61-treatment reduced the frequency of CD4+CD25+ cells from 5% of the total CD4+ T cell population to background as measured on the day of adoptive transfer (Fig. 6A). Neutralizing levels of the PC61 mAb did not appear to be present at this time point (i.e., the time of clonotypic CD4 cell adoptive transfer), however, given that the clonotypic CD4 cell response was not diminished in PC61-treated vacc-HA recipients compared to rat Ig controls. Thus, clonotypic CD4 cells transferred into vacc-HA recipients transiently express high levels of CD25 [11], which should render them susceptible to neutralization if the PC61 mAb remained at neutralizing levels. To the contrary, although PC61-treatment did not affect the ability of the vacc-HA-primed clonotypic CD4 cells to express IFN-γ or TNF-α (Fig. 6C,D), proliferation and accumulation were slightly enhanced (Fig. 6B). PC61-treatment also elicited slightly enhanced clonotypic CD4 cell expansion in the Pro-HA × TRAMP and C3-HA recipients (Fig. 6B), and minimally augmented IFN-γ (but not TNF-α) expression in C3-HA recipients. Clonotypic CD4 cells did not appear to undergo as profoundly a tolerogenic response in Pro-HA × TRAMP recipients as compared to C3-HA recipients, in as far as only IFN-γ (but not TNF-α) expression was impaired in the former, likely owing to lower expression levels of the tolerizing HA antigen [6,10]. Nevertheless, PC61-treatment did not augment cytokine expression potential in Pro-HA × TRAMP recipients. Taken together, these results suggest that during prostate tumorigenesis, steady state DC, but not CD4+CD25+ Tregs, play a critical role in programming non-immunogenic prostate-specific CD4 cell responses.
Fig. 6.
CD4+CD25+ T regulatory cells do not appreciably influence the naœve CD4 cell response to prostatic antigen during prostate tumorigenesis. C3-HA, vacc-HA-infected NT (vacc-HA), and Pro-HA × TRAMP recipients on the B10.D2 background were treated with either anti-CD25 (PC61) or control (rat Ig) antibody 4 days prior to receiving CFSE-labeled naœve clonotypic CD4 cells, which were subsequently recovered for analysis 5 days later from the prostate-draining LN. A: Representative FACS plots showing the frequency of CD4+CD25+cells within the total peripheral blood CD4+ population both before (Day 0) and 4 days following treatment with either PC61 or rat Ig.CD25 staining was performed using the 7D4 mAb, which does not cross-react with PC61. B: Representative CFSE-dilution histograms and graph showing frequencies of clonotypic CD4 cells. C: Representative FACS plots of IFN-γ versus TNF-α expression following in vitro restimulation with HA peptide-pulsed APCs, fixation/permeabilization, and intracellular staining, gated on CFSE-diluted clonotypic CD4 cells. D: Quantitative analysis of IFN-γ and TNF-α expression. Total cytokine expression is expressed in arbitrary units (calculated as the product of the % of cytokine positive clonotypic CD4 cells multiplied by the mean fluorescence intensity of cytokine staining per positively expressing cell) as previously described [11]. All quantitative data is expressed as the mean ± SEM, and n = 3 for each group.
DISCUSSION
A major impediment toward the development of effective anti-tumor vaccines is that tumor-reactive T cells are often rendered tolerant/non-functional prior to vaccination, particularly in the case of T cells that recognize tumor differentiation antigens that are expressed on both tumors as well as the normal tissues from which the tumors derived because the tolerance induction machinery will have had access to these antigens prior to the initiation of tumorigenesis [32,33]. A potential exception to this scenario is prostate cancer, since in the absence of tumorigenesis, T cells specific for prostate epithelial antigens remain functional, presumably because these antigens are secreted into the ejaculate rather than the draining lymphatics where they might be acquired by tolerance-inducing bone marrow-derived APCs [6]. That the development of advanced prostate cancer is associated with the presentation of prostate epithelial antigen in the draining LN and the systemic tolerization of cognate T cells [6], raised the possibility that there might be a window during the initial stages of tumorigenesis when prostatic antigen is not released into the draining lymphatics, either because the prostatic architecture has not been sufficiently altered to cause significant redirection of antigen drainage, or because the level of antigen expression has not substantially increased due to the limited growth of the primary tumor. If true, this would have suggested that T cell tolerance might not significantly impede the efficacy of prostate cancer vaccines administered during early disease stages. Unfortunately, this does not appear to be the case, as our current data indicate that prostatic antigen is presented in the draining LN at relatively early stages of disease progression when primary tumors are still small and well differentiated.
The question of whether tumors prime or tolerize tumor-reactive T cells has been somewhat confusing in as far as tolerance occurs in some models [6,34–36] and priming in others [37–39]. A model that has been postulated to explain these divergent results is that tumors vary in their ability to prime or tolerize cognate T cells depending upon whether they do or do not generate inflammation, respectively, with inflammation perhaps resulting from a more aggressive pattern of tumor growth, invasion of surrounding tissues and metastases [40]. We had previously demonstrated that prostate tumorigenesis can promote T cell tolerance [6], but to assess whether non-immunogenicity is a general property of prostate tumors, in the current study we also examined prostate epithelial-specific T cell response in mice that develop a more aggressive pattern of tumorigenesis. Thus, in contrast to our standard B10.D2 genetic background, Pro-HA × TRAMP mice on a F1 FVB × B10.D2 background develop larger primary tumors with more severe pathological scores, and develop metastases at higher frequency ([19] and data not shown). Despite this more aggressive pattern of tumorigenesis that could have potentially elicited a greater level of inflammation, and thus more immunogenic T cell responses, naïve prostate-specific CD4 cells still failed to develop effector function. Although these T cells did proliferate more robustly and extensively, this may have simply resulted from a greater level of antigen (due to increased tumor size), as we have previously observed that increased self-antigen expression levels result in increased proliferation of cognate CD4 cells without altering the tolerogenic outcome of their response [30,41]. Taken together, these results suggest that non-immunogenicity/tolerogenicity might be a general property of prostate tumors.
In probing the mechanisms by which prostatic antigen is presented to induce non-immunogenic T cell responses during tumorigenesis, we first assessed the role of steady state DC using a previously developed bone marrow chimera strategy in which DC expressing the CD11c-DTR transgene can be efficiently and selectively depleted following treatment with DT [13]. Steady state DC have been implicated in playing an essential role in programming T cell tolerance to parenchymal self-antigens (in contrast to activated DC that prime effector/memory T cell responses), in part because targeting exogenous antigens to DC in the absence or presence of inflammation induces either T cell tolerance or immunity, respectively [42,43]. Additionally, T cell recognition of a parenchymal self-antigen occurs in mice where DC are the only APC population genetically capable of cross-presenting that antigen [21], and ex vivo T cell stimulation assays suggest that DC are the predominant APC population that present parenchymal self-antigens [22,23]. Our current result that DC depletion completely abrogates prostatic antigen presentation during tumorigenesis, however, is the first in vivo demonstration that DC are essential for cross-presenting a self/tumor antigen. Given previous in vitro studies suggesting the possibility that CD4+CD25+ Tregs might work in concert with steady state DC [26,27], it was somewhat surprising that Treg neutralization did not alter the functional response of naïve prostate-specific CD4 cells during tumorigenesis. This result does not, however, rule out the possibility that Tregs influence prostate-specific immunity. For instance, tumor-infiltrating Tregs could potentially dampen the activity of prostate-specific effector T cells in the tumor microenvironment, as has been demonstrated or suggested in other tumor systems [44–46]. Nevertheless, our results do suggest that during tumorigenesis, steady state DC in the draining LN are sufficient to both cross-present prostatic antigen as well as program non-immunogenic cognate naïve T cell responses.
CONCLUSIONS
Our current results indicate that beginning at early stages of prostate tumorigenesis, prostatic antigen is presented in the draining LN by steady state DC to program non-immunogenic/tolerogenic naïve T cell responses irregardless of the level of aggressiveness by which tumorigenesis occurs. Thus, non-immunogenicity/tolerogenicity might be a general property of prostate tumors, and T cell tolerance towards prostatic/prostate tumor antigens might negatively impact the efficacy of prostate tumor vaccines even if they are administered during early stages of disease progression.
Acknowledgments
National Institutes of Health; Grant numbers: AI057441, CA109339.
We thank Dr. Barbara Foster for advice on TRAMP pathological analysis, as well as Dr. David Zammit and Dr. Leo Lefrancois for the CD11c-DTR mice. This work was supported by National Institutes of Health grants AI057441 and CA109339 to A.J.A.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18(2):201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Adler AJ. Mechanisms of T cell tolerance and suppression in cancer mediated by tumor-associated antigens and hormones. Curr Cancer Drug Targets. 2007;7(1):3–14. doi: 10.2174/156800907780006931. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- 6.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180(1):25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen presenting cells. J Exp Med. 1998;187(10):1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168(11):5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 12.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-alpha and IFN-gamma expression potentials. Cell Immunol. 2003;224(2):114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 15.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T Cells Regulate Virus-specific Primary and Memory CD8+ T Cell Responses. J Exp Med. 2003;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21(5):733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55(3):219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 19.Gingrich J, Barrios R, Foster B, Greenberg N. Pathologic progression of autochonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;6:1–6. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 20.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Ex Med. 1997;186(2):239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166(3):1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 22.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196(8):1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196(8):1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193(11):F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 26.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 27.Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173(12):7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 29.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61(24):8643–8646. [PubMed] [Google Scholar]
- 30.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 31.Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: Implications for adoptive immunotherapy. J Immunol. 2004;172(9):5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160(2):643–651. [PubMed] [Google Scholar]
- 33.Hu J, Kindsvogel W, Busby S, Bailey MC, Shi Y, Greenberg PD. An evaluation of the potential to use tumor-associated antigens as targets for antitumor T cell therapy using transgenic mice expressing a retroviral tumor antigen in normal lymphoid tissues. J Exp Med. 1993;177:1681–1690. doi: 10.1084/jem.177.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11(4):483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 35.Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: Deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur J Immunol. 1996;26(11):2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 36.Stavely-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13(2):265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 38.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411(6841):1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 39.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via cross-presentation by bone marrow-derived stromal cells. Immunity. 2002;17(6):737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 41.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169:3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157(4):1406–1414. [PubMed] [Google Scholar]
- 44.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 45.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201(5):779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]