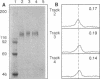
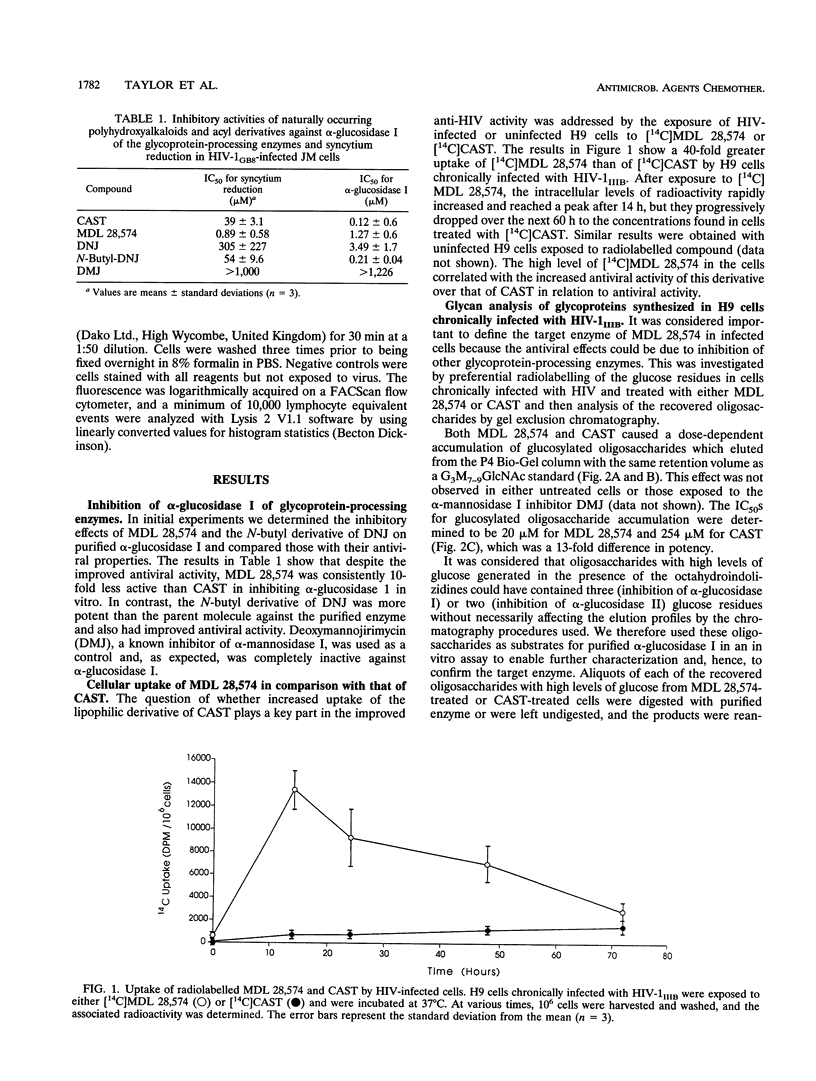
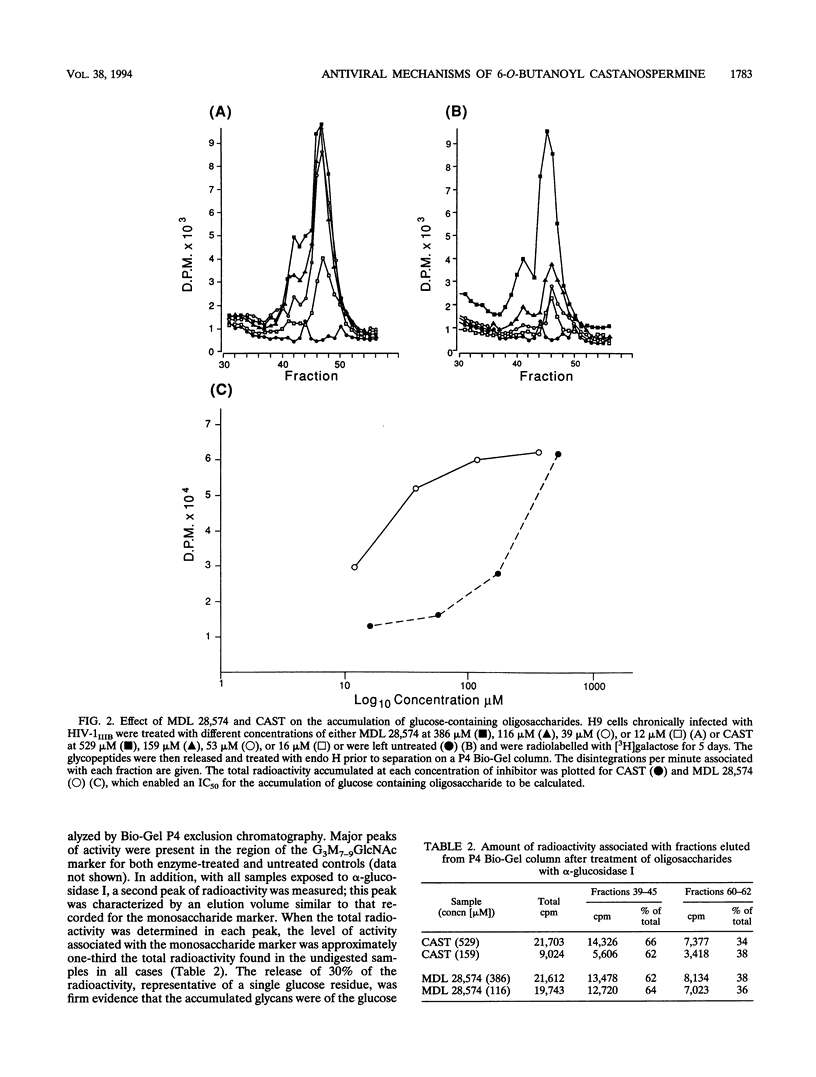
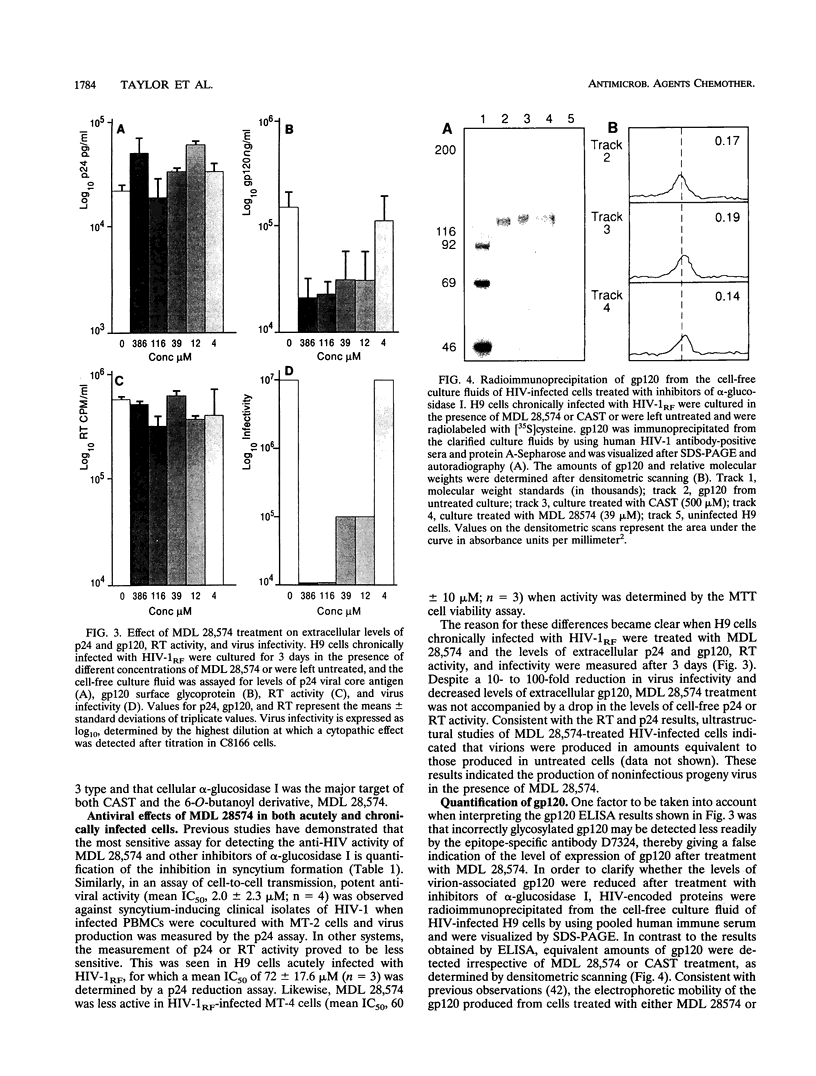
Abstract
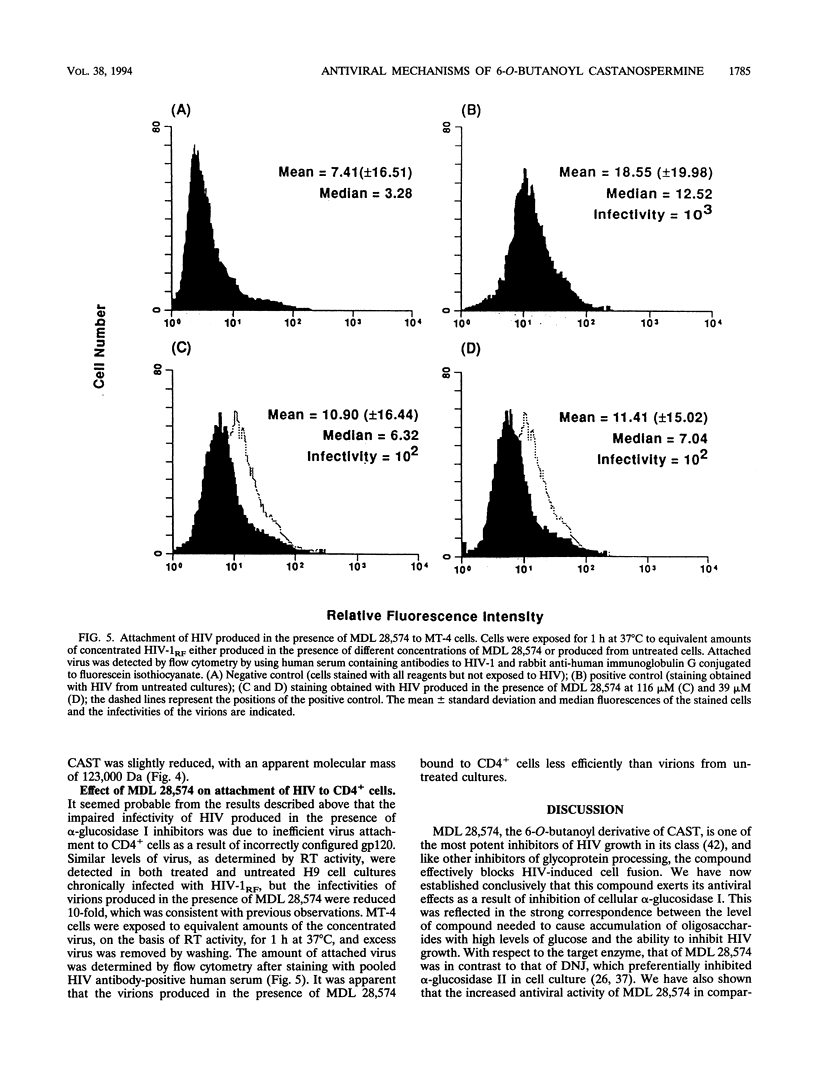
The 6-O-butanoyl derivative of castanospermine (MDL 28,574) was previously shown to be approximately 30-fold more potent than the naturally occurring molecule at inhibiting the replication of human immunodeficiency virus (HIV) (D. L. Taylor, P. S. Sunkara, P. S. Liu, M. S. Kang, T. L. Bowlin, and A. S. Tyms, AIDS 5:693-698, 1991). We now report that consistent with its improved anti-HIV activity, MDL 28,574 is more effective (50% inhibitory concentration [IC50], 20 microM) than the parent molecule (IC50, 254 microM) at causing the accumulation of glucosylated oligosaccharides in HIV-infected cells by inhibition of glycoprotein processing. These were predominantly of the glucose 3 type, as determined by P4 Bio-Gel analysis after digestion with purified alpha-glucosidase I, indicating that, intracellularly, this enzyme is the major target for inhibition. MDL 28,574, however, was less active (IC50, 1.27 microM) than castanospermine (IC50, 0.12 microM) against the mutual target enzyme, cellular alpha-glucosidase I, in a cell-free assay system. The increased effects of MDL 28,574 against alpha-glucosidase I in cell culture were attributed to the improved cellular uptake of the more lipophilic derivative. Inhibition of this enzyme activity in HIV-infected H9 cells impaired viral glycoprotein processing and resulted in the expression of abnormally configured gp120. This did not affect virus production, but the virions had decreased infectivity which was partially related to a reduced ability to bind to CD4+ T cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dedera D., Vander Heyden N., Ratner L. Attenuation of HIV-1 infectivity by an inhibitor of oligosaccharide processing. AIDS Res Hum Retroviruses. 1990 Jun;6(6):785–794. doi: 10.1089/aid.1990.6.785. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Doms R. W., Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990 Jan;87(2):648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Clerget-Raslain B., Gluckman J. C., Guétard D., Montagnier L., Bahraoui E. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J Exp Med. 1989 Mar 1;169(3):807–822. doi: 10.1084/jem.169.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C. Effect of a glucosidase inhibitor on the bioactivity and immunoreactivity of human immunodeficiency virus type 1 envelope glycoprotein. J Gen Virol. 1991 Aug;72(Pt 8):1919–1926. doi: 10.1099/0022-1317-72-8-1919. [DOI] [PubMed] [Google Scholar]
- Fleet G. W., Karpas A., Dwek R. A., Fellows L. E., Tyms A. S., Petursson S., Namgoong S. K., Ramsden N. G., Smith P. W., Son J. C. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988 Sep 12;237(1-2):128–132. doi: 10.1016/0014-5793(88)80185-6. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Hallenberger S., Bosch V., Angliker H., Shaw E., Klenk H. D., Garten W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature. 1992 Nov 26;360(6402):358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- Hettkamp H., Legler G., Bause E. Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides. Eur J Biochem. 1984 Jul 2;142(1):85–90. doi: 10.1111/j.1432-1033.1984.tb08253.x. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Jacob G. S. Anti-HIV drug mechanism. Nature. 1991 Jul 18;352(6332):198–198. doi: 10.1038/352198b0. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Zwolshen J. H., Harry B. S., Sunkara P. S. A simple and rapid microplate assay for glycoprotein-processing glycosidases. Anal Biochem. 1989 Aug 15;181(1):109–112. doi: 10.1016/0003-2697(89)90401-6. [DOI] [PubMed] [Google Scholar]
- Karpas A., Fleet G. W., Dwek R. A., Petursson S., Namgoong S. K., Ramsden N. G., Jacob G. S., Rademacher T. W. Aminosugar derivatives as potential anti-human immunodeficiency virus agents. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9229–9233. doi: 10.1073/pnas.85.23.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Jun 25;265(18):10373–10382. [PubMed] [Google Scholar]
- Li Y., Luo L., Rasool N., Kang C. Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993 Jan;67(1):584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- McDowell W., Schwarz R. T. Dissecting glycoprotein biosynthesis by the use of specific inhibitors. Biochimie. 1988 Nov;70(11):1535–1549. doi: 10.1016/0300-9084(88)90290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Spellman M. W., Larkin M., Solomon J., Basa L. J., Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J. 1988 Sep 1;254(2):599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Pal R., Kalyanaraman V. S., Hoke G. M., Sarngadharan M. G. Processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1 in the presence of trimming glucosidase inhibitor deoxynojirimycin. Intervirology. 1989;30(1):27–35. doi: 10.1159/000150073. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Peterson A., Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988 Jul 1;54(1):65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A., Bona C., Zaghouani H., Gorny M. K., Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., vander Heyden N., Dedera D. Inhibition of HIV and SIV infectivity by blockade of alpha-glucosidase activity. Virology. 1991 Mar;181(1):180–192. doi: 10.1016/0042-6822(91)90483-r. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Saunier B., Herscovics A. Comparison between 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin as inhibitors of oligosaccharide processing in intestinal epithelial cells. Biochem J. 1985 Mar 15;226(3):733–740. doi: 10.1042/bj2260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailubhai K., Pratta M. A., Vijay I. K. Purification and characterization of glucosidase I involved in N-linked glycoprotein processing in bovine mammary gland. Biochem J. 1987 Nov 1;247(3):555–562. doi: 10.1042/bj2470555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. S., Engleman E. G. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J Biol Chem. 1990 Feb 15;265(5):2640–2649. [PubMed] [Google Scholar]
- Sunkara P. S., Taylor D. L., Kang M. S., Bowlin T. L., Liu P. S., Tyms A. S., Sjoerdsma A. Anti-HIV activity of castanospermine analogues. Lancet. 1989 May 27;1(8648):1206–1206. doi: 10.1016/s0140-6736(89)92787-6. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Sunkara P. S., Liu P. S., Kang M. S., Bowlin T. L., Tyms A. S. 6-0-butanoylcastanospermine (MDL 28,574) inhibits glycoprotein processing and the growth of HIVs. AIDS. 1991 Jun;5(6):693–698. doi: 10.1097/00002030-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]