Summary
New technologies have emerged that enable the tracking of molecular motors and their cargos with very high resolution both in vitro and in live cells. Classic in vitro motility assays are being supplemented with assays of increasing complexity that more closely model the cellular environment. In cells, the introduction of probes such as quantum dots allows the high resolution tracking of both motors and vesicular cargos. The “bottom up” enhancement of in vitro assays and the “top down” analysis of motility inside cells are likely to converge over the next few years. Together, these studies are providing new insights into the coordination of motors during intracellular transport.
Introduction
Molecular motors drive a myriad of essential processes in the cell, including targeted delivery of vesicular cargos, localization of organelles and mRNAs, and chromosome and spindle dynamics during mitosis. The three classically described linear molecular motors, myosin, kinesin and dynein, carry their cargos along actin filaments (AFs) and microtubules (MTs) in these transport roles, in addition to their well-characterized functions in muscle contraction and flagellar beating. New single molecule fluorescence microscopy techniques and infrared optical traps have led to remarkable progress in understanding the transduction of metabolic energy into mechanical force and motion, using in vitro systems combining purified motors with their tracks on glass microscope slides (Box 1). Of course, transport in the cell occurs in an environment that is considerably more complex than is reflected in these simplified assays of motor function. Intersecting and bundled networks of cytoskeletal filaments, obstacles such as actin-binding or microtubule-associated proteins bound along motor tracks, and the coordination of multiple motor types bound to the same cargos are all likely to affect motility within the cell (Fig. 1). The gap between simplified in vitro assays of motor activity and the biology of intracellular transport in vivo is being bridged from both sides. Here we consider “bottom up” experimental approaches using purified components in motility assays modified to reflect elements of complexity present within cells, as well as “top down” studies of motors tagged with fluorescent reporters such as quantum dots (QDs) and introduced into cells. The results of these experimental studies on the coordination and collective properties of molecular motors can be compared to theoretical predictions, reviewed in this volume by Guérin et al. [1].
Box 1. Modeling Intracellular Motility With In vitro Assays.
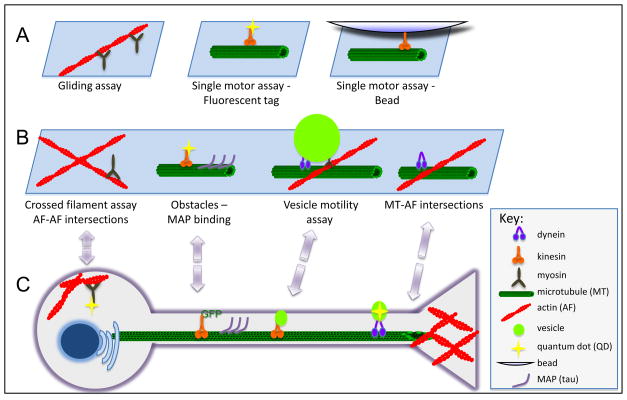
A.Gliding assays for all three types of molecular motors (myosins, kinesins, and dynein) involve attaching the motor to a glass microscope slide and monitoring the translocation of either actin filaments or microtubules across the surface upon addition of ATP (Box Figure 1). Sliding velocity, ATP dependence and some indications of population dynamics can be obtained. Single motor assays involve an inverse configuration: the actin filament or microtubule is attached to the glass surface and the movement of the motor is monitored, either directly via a fused fluorescent tag such as GFP, organic fluorophore or a quantum dot (QD). This approach yields nanometer resolution, allowing the measurement of step size and angular changes during translocation. In single motor assays with beads, motors are attached to small polymer spheres that are easily observed by differential interference constrast or phase microscopy. This configuration can be used in an optical trap to measure step size, processivity and stall force. Additionally, multiple motors of the same or different types can be bound to the same bead to study collective motor activity. B. Closer approximations of the cellular environment can be developed in crossed filament assays (Box Figure 1). In these assays, the translocation and/or switching of motors can be monitored through actin-actin (AF-AF), microtubule-microtubule (MT-MT), and microtubule-actin filament (MT-AF) intersections. Other obstacles to motility can be bound to cytoskeletal filaments, such as the microtubule-associated protein (MAP), tau. While bead assays provide information on motor dynamics, monitoring vesicle motility in vitro provides insight into the coordinate regulation of motors bound to their natural cargos. C. Finally, direct measurements of motility in a cell, such as the neuron shown here, can be made by expressing GFP-labeled motors, or by introducing motors or probes into the cell through pinocytosis or endocytosis.
Figure 1. Multiple motor proteins, including dynein, kinesin, and myosin-V, drive vesicle motility along the complex cellular cytoskeleton.
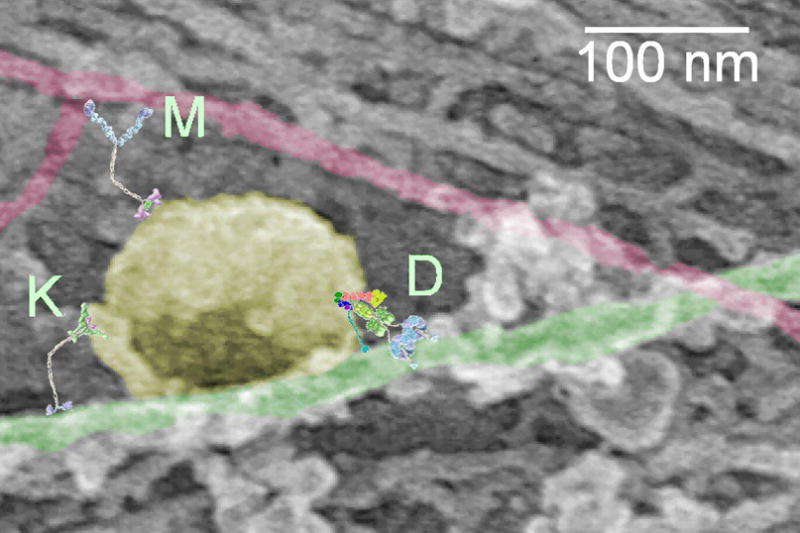
In this electron micrograph showing a platinum replica of an unroofed rat embryonic fibroblast, a vesicle pseudo-colored in yellow is bound along a microtubule (green), in the vicinity of an actin filament highlighted in red. Schematic structures of the motors dynein (D), kinesin (K), and myosin-V (M) are drawn approximately to scale. The authors gratefully acknowledge Tatyana Svitkina of the University of Pennsylvania for providing the electron micrograph.
Processivity and Gating
The three major motor families, kinesins, dyneins, and myosins, show considerable variation in structure, speed, and number of steps taken per diffusional encounter with their cytoskeletal track (mechanical processivity). The paradigm is a highly processive, two-headed motor, such as kinesin-1, which drives cargos toward the plus end of microtubules. Cytoplasmic dynein is also a highly processive two-headed motor, but moves toward MT minus ends. Similarly, myosin-V and myosin VI move in opposite directions along actin filaments. Kinesin and dynein produce repeated steps along microtubules at the 8 nm periodicity of the tubulin dimers, although dynein can exhibit larger steps as well as reversals [2,3]. Myosin V takes 36 nm steps toward the barbed end of actin, corresponding approximately to the half-pitch of actin’s double helix; whereas myosin VI exhibits pointed end directed steps of 20 – 40 nm [4]. Each of these examples has been shown to move with a “hand-over-hand” gait [5–8] in which both heads can bind simultaneously to their track. Their stride involves detachment and forward motion of the trailing head to become the leader (Fig. 2A→ B ).
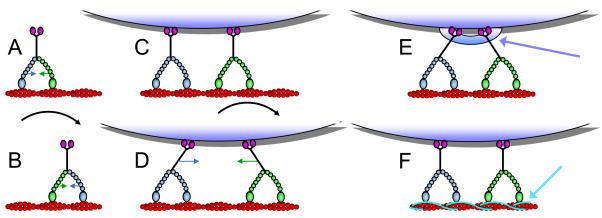
Figure 2. Mechanisms for interaction between the motor components.
A, a double-headed motor, such as myosin V is bound to its track, actin (red), by both heads. The trailing head (blue) detaches and steps forward (A → B) to become the leading head. Due to stretch of the molecule required to reach the next binding site, the leading head is pulled backward (blue arrow in B) and the trailing head is pulled forward (green arrow in B). C and D, two myosin V molecules bound to the same cargo particle and to the same actin filament. When one of the motors (green) steps forward (C → D), the cargo moves half as far as the motor steps. Attachment and motor compliances strain the stepping motor backwards and the non-stepping motor (blue) forward (arrows in D). E, an extra regulatory component (blue arrow) may control the activity of the motors. F, a filament binding protein, such as tropomyosin (light blue arrow) drawn here or MAPs, might regulate motor-track interactions and cooperative behavior of nearby motors.
High processivity implies a mechanism to prevent both heads from dissociating from the track at the same time. For kinesin and myosin V, thermal fluctuations stretch the two heads apart to reach the next filament binding site at the completion of the step, leading to an intramolecular force that pulls the leading head backward and the trailing head forward (colored arrows in Fig. 2A and B). The intramolecular forces on the two heads are the most likely signals that cause the trailing head to detach while the leading head remains bound [9,10]. Steps in the biochemical or mechanical pathways are said to be gated by internal strain in the molecule. Besides the intramolecular force, physical interactions between the two heads (for instance between the two ring-shaped heads of dynein if they are adjacent on the microtubule [11]) or structural changes in the filament could also mediate head-head communication.
How Do Multiple Motors Interact on a Cargo?
The same concepts potentially influence motions of a vesicle or filament transported by several motors mechanically coupled to each other through the cargo. The restraining force that stops motility, the stall force, is expected to increase with the number of motors, although not necessarily in proportion [12,13]. The distance a cargo is transported before it dissociates from its track is markedly lengthened by increased numbers of motors [14,15]. At typical transport velocities of artificial cargos in vitro and probably also in cells, the viscous drag of the environment is relatively small and the transport speed is determined mainly by the motor dynamics.
Coupling between several identical motors
By imaging GFP-labeled kinesins in a gliding filament assay at low surface density, and by observing swiveling of the MT when only one kinesin was bound, Leduc et al. [16] could identify intervals corresponding to one, exactly two, or higher numbers of motors. MTs bound to single kinesins exhibited 8.1 nm steps in this assay, in agreement with earlier studies of kinesin motility [17]. MTs driven by two kinesins, however, took twice as many steps per second, averaging 4.2 nm displacements per step, leading to the same velocity. The implication is that intermolecular forces transmitted through the microtubule did not greatly modulate the stepping dynamics, but reduced the step size for the ensemble as expected from the cargo-mediated strain in Fig. 1C→ D . Double-headed kinesin molecules assembled into pairs on an artificial scaffold did not move faster than single ones, also indicating low kinetic interaction through the cargo [18]. On the other hand, several single-headed kinesin molecules assembled onto a scaffold enhanced motility speed, indicating cooperation among single heads via the mechanical coupling [19].
Interaction between fast and slow motors
In many types of intracellular transport, motors with differing dynamic properties cooperate to move the same cargo. When several motors with different speeds are present, the aggregate velocity is expected to reach an intermediate value determined by a balance of forces between their individual force-velocity curves. Examples are the faster and slower isoforms of myosin II that are intermixed in individual muscle fibers [20], and the heterotrimeric kinesin-II and homodimeric OSM-3 (both members of the kinesin-2 family) that carry intraflagellar transport particles (IFTs) toward the tips of cilia [21]. In gliding filament assays using pairwise mixtures of myosin II isoforms differing over a 50-fold range of maximal velocities [22], or kinesin-related motors, kinesin II and OSM-3, differing 3-fold in velocity [23], intermediate velocities were observed. In both of these cases, a half-and-half mixture of the two isoforms translocated the filaments at speeds less than half-way between the individual velocities. This dominance of the slower isoform can be explained by changes in the detachment rate from the actin or MT at the end of the working stroke, so that a relatively small number of the slower motor produces a strong component of drag.
When the number of motors of each type is small enough that stochastic probabilities allow periods of complete detachment, the net velocity can be envisioned as an “alternating action” mechanism of one motor type and then the other. Or, the motors might be nearly continuously engaged and interact via forces transmitted between them through the cargo. In this situation, when one molecule steps forward on its track, the other bound motors not stepping at that moment are pulled forward by the cargo (Fig. 2C→ D ). Their elasticity resists this motion thereby producing a differential, oppositely directed tensile component between the stepping and non-stepping molecules (a “mechanical competition” model, Fig. 1D). The transition rates governing attachment and detachment may depend on force applied to the motor, similar to the gating described above for the individual heads [24]. For the two IFT kinesins, either mechanical competition in which the slower kinesin-II generated a drag force or else alternating periods of fast and slow velocity could explain the in vitro motility results. But evidence from in vivo studies of IFT transport with knockout constructs suggests that the two motors exert forces on each other in a mechanical competition [23].
In contrast, with mixtures of kinesin-1 and a mutant purposefully slowed 15-fold [25], the transport velocity was dominated by the faster wild-type motor. The active motor is able to dissociate the weaker mutant, possibly due to the known asymmetry of kinesin-MT dissociation rates under forward (plus-end directed) and backward (minus-end directed) forces [26]. Similarly, when a weak binding myosin, such as unphosphorylated smooth muscle myosin, was mixed with an active myosin isoform, the more strongly bound motor could dissociate the weaker one to maintain a high velocity ([22] and other papers cited therein).
Besides interactions mediated via forces through their load, motors associated with a cargo could potentially interact directly or share structural or modulatory components (Fig. 1E). Features of the cytoskeletal tracks could also affect the operation of nearby motors. For instance, muscle myosin shows cooperative attachment caused by the regulatory component, tropomyosin, that winds along the actin helix (Fig. 1E) [27]. The various mechanisms for interactions between multiple motors through shared cargos and substrates are presumably applicable to the regulation of directionality, velocity and processivity of transport in cells.
Obstacles and Crossovers
To further study motor interactions, in vitro assays have been developed with added complexity, such as filament intersections or filament-binding proteins. For example, kinesin-1 motors were imaged by fluorescence as single molecules or bound in ensembles to polymer beads as they encountered MT-MT intersections. If the approach was along the overpass microtubule, motility was observed to continue through the microtubule intersection, without a switch or a dissociation [28], consistent with their highly directed motility parallel to the protofilament axis [29,30]. However, to a bead arriving at an intersection from an underpass, an overlying MT is an obstacle. It only takes one or a few kinesins at the front of the bead to switch the cargo onto the crossing microtubule, presumably due to kinesin’s high processivity. The methodical nature of kinesin motility helps to explain its interactions with the neuronal microtubule binding protein, tau. Encounters with patches of tau on microtubules caused individual kinesins to dissociate as if they were pried off of the MT, unable to walk around [13,31].
Dynein, consistent with its much more varied stepping dynamics [2,32], exhibits a less uniform response than kinesin at MT-MT intersections: passing, pausing, switching, dissociating and even reversing its direction [28]. The stepping and directional variability of dynein on individual bare MTs and at obstacles presumably also explains how dynein navigates around or through patches of tau better than kinesin [31]. However, lack of cooperation of the dynein motors becomes more apparent at higher motor densities, as the dynein-bound beads become tethered at MT-MT intersections under these conditions [28].
Myosin V passes and switches at branches and intersections of actin filaments [33]. During motility, a myosin V head stepping from the trailing to the leading position averages 72 nm, twice the distance of the molecule’s center (Fig. 1A → B, [5,33]). But this distance varies over a broad range, 50 – 95 nm, for individual steps, indicating flexibility, and the molecule sometimes tilts sideways around the filament and continues at a new azimuth [34]. The high likelihood for switching at AF-AF intersections [35], suggests a random search for the target monomer unbiased by stiffness at the head-tail junction.
A novel finding made by Ali et al. [35] was that at a MT overpass, myosin V often switched tracks onto the microtubule and exhibited passive one-dimensional diffusive sliding along the MT. Myosin V is known to interact with microtubules in various ways [36], but the diffusive motion was unexpected. Myosin V and kinesin increase each other’s processive run lengths, a kind of intermolecular cooperation that may enhance transport of cargos containing both motors [37]. Although these non-cognate filament interactions in vitro are greatly diminished at physiological salt concentrations, they may nevertheless be relevant in cells because several motors on a cargo could compensate for the weak individual affinity. These findings raise the interesting possibility that myosin searches along MTs for cargos, preparing to switch them to the actin filament network.
Mixtures of motor classes
Several different types of motors can be added to a quantum dot or polymer cargo enabling direct testing of the relative efficacy of the motors under competing loads or to facilitate switching onto another filament type. With mixtures of dynein/dynactin and myosin V on polymer beads in vitro, an apparent tug of war takes place at AF-MT intersections. The likelihood of switching onto an actin filament or MT is closely related to the maximum force and the density of each type of motor on the bead, the outcome being determined most noticeably by the relative force between the two motor types [38]. Surprisingly, whether the starting filament is the MT or actin and whether it’s an underpass or overpass hardly affects the outcome, suggesting that the motors commonly interact with both filaments at the intersection. The tug of war model for interactions between motors at a filament intersection, or between two oppositely directed motors on a single filament, is similar to the mechanical competition model discussed above for two motor types with the same directionality that share a cargo. In these mechanisms, both types of motor act simultaneously, rather than alternating, one engaged, then the other.
These in vitro assays show that motors and cargos interact in a number of ways. Their mechanics, force, directionality and velocity, are coupled to each other via elastic connections with the cargo, leading to cooperative modulation of their stepping dynamics and directionality. Measurements of corresponding parameters in the cellular environment may indicate if the biophysical properties of the motors are similar and whether the same phenomena take place in the cell.
Motors Moving in Cells
The question of how in vitro measures of motor function compare to motility observed in the complex cellular environment may be addressed in two ways. First, the behavior of exogenous, labeled motors introduced into cells can be studied. In this experimental approach, motors move along the complex cellular cytoskeleton, operating independently of any association with intracellular cargos. Alternatively, the movement of native organelles and vesicles can be analyzed, usually by the introduction of a fluorescent probe such as a quantum dot taken up by endocytosis. In this experimental design, motors work collectively as they associate with endogenous cargo. As multiple motors of the same type or different types may be associated with the same cellular cargo, motility may be more complex. Motility of endogenous cargos is also more likely to be affected by the regulatory environment of the cell. Both approaches have been employed, and the results from these studies have many striking similarities to data obtained from in vitro studies.
Microtubule-based motility
The introduction of fluorescently labeled motors into cells has allowed the high resolution tracking of motors moving along the endogenous cytoskeleton under physiologically relevant conditions of ionic strength and ATP concentration. The velocities observed in these assays are remarkably similar to those observed in vitro. In a direct comparison, a truncated kinesin-1 construct labeled by the tandem fusion of three monomeric citrine tags was expressed in transfected COS cells. The cells were then either lysed to generate cytosolic extracts for in vitro experiments or imaged using total internal reflection fluorescence (TIRF) microscopy in live cell experiments [39]. Kinesin-1 moved processively in both assays, with similar velocities (0.8 μm/sec) and run lengths (0.8 to 1.2 μm/run). Kinesin motors bound to QDs and internalized into HeLa cells by osmotic lysis of pinocytic vesicles also exhibited velocities and run lengths similar to those observed for the same kinesin construct in vitro [40]. In contrast to these observations, in a study of kinesin-bound QDs internalized via lipid transfection [41], directed motility in the cell was frequently interrupted by pausing. This “stop-and-go” behavior differed significantly from observations with the same kinesin construct in vitro. Maximum velocities also differed, with cellular velocities up to twice that observed in vitro [41]. The differences observed among these studies merit further analysis, but may be due either to distinct properties of the specific kinesin constructs tested and their relative susceptibility to regulatory control within the cell, or to the various cell types used in these assays, which may contain cytoskeletal tracks with differing properties.
The overall effect of the complex organization of the cellular cytoskeleton on motility in vivo is still not clear. Motors may select particular tracks in the cell; for example kinesin-1 but not kinesin-2 or kinesin-3 preferentially moves along a subset of microtubules marked by post-translational acetylation [42,43]. Track-associated proteins such as microtubule-bound MAPs did not appear to affect kinesin in some studies [39] but may have affected kinesin in other work [41]. While these observations are in apparent contrast to the strong inhibition of kinesin by MAPs seen in vitro [13,31], there are a few caveats. First, following preferential tracks in the cell may allow motors to avoid filaments with associated roadblocks, such as microtubules heavily decorated with MAPs. Second, variability in the cellular complements of MAPs may explain some of the observed differences in motility; both the level of expression and the types of MAPs expressed vary from one cell type to another. For example, in neurons, increasing levels of tau expression led to a significant inhibition of kinesin-mediated organelle transport [44], in contrast to the apparent lack of inhibition of kinesin by MAPs seen in COS and HeLa cells, which do not normally express tau.
Actin-based motility
Myosin-V motors have also been introduced into cells and monitored with single molecule resolution. Both Nelson et al. [45] and Pierobon et al. [46] introduced single myosin-V motors into cultured cells (COS-7 and HeLa, respectively). Both groups found that single myosin molecules moved with velocities and step sizes similar to those measured in vitro. At short time scales (several steps) the introduced myosin-V motors moved processively. However, movement over longer time scales or distances was more random than directed. Monte Carlo simulations suggest that the apparent random walk is due to complexity of the actin network rather than reflecting a lack of processivity of the motor [45]. The random walk pattern of myosin-V-bound QDs moving near the cortex is best understood as a processive motor moving along a dense, intersecting, and randomly oriented cortical actin network.
Other studies have similarly suggested that myosin motor activity in the cell is influenced by the geometry of cytoskeletal tracks [47,48]. This has been shown most dramatically for myosins in a study by Brawley and Rock [49] in which fluorescently labeled myosins V, VI, and X were introduced into extracted COS, S2 and U2OS cells, which retained the overall organization of the actin cytoskeleton seen in intact cells. The introduced myosins moved preferentially along some actin structures but not others. For example, myosin VI but not myosins V and X moved along stress fiber bundles, while myosin X was recruited to filopodia. Track switching was seen at actin filament intersections in regions with high filament density, similar to the results of Nelson et al. [45] in intact cells, as well as to the in vitro filament switching assays described above.
Motors on Cargos
Perhaps the major difference between purified motors studied in vitro and organelle movement within the cell is the association of motors with native cargos, which has the potential to affect motor function in several ways. For some motors, binding to cargo may drive dimerization, changing a relatively nonprocessive motor to a processive form that can drive transport effectively over longer distances. Cargo-induced dimerization was initially proposed for the kinesin motor KIF1A/Unc104 [50,51], although this model is not supported by more recent work [52]. Cargo-induced dimerization of myosin-VI was similarly controversial, but recent developments now favor this model [53]. Even for motors known to form stable dimers, cargo binding is likely to increase motor density, so that motors are functioning in arrays rather than individually. In vitro studies have shown that coupling multiple motors to a single artificial bead cargo can significantly increase stall force, directionality, and/or processivity [12,13,54].
Further, intracellular cargos may recruit multiple types of motors, such as the oppositely oriented motors kinesin and dynein, or motors that move along distinct cytoskeletal filaments such as myosin and dynein. For at least some cargos, multiple types of motors remain stably bound to vesicles undergoing bidirectional motility [55–57]. Cargo binding may also affect motor coordination and/or regulation, for example through the activities of cargo-bound scaffolding proteins (reviewed in [58]).
Recent work suggests that motors of opposite polarity bound to the same cargo are required to activate bidirectional cargo transport. Ally et al. [59] found that the mislocalization of peroxisomes that occurs in S2 cells upon depletion of either kinesin-1 or cytoplasmic dynein was rescued by the expression of a peroxisome-targeted construct encoding an unrelated motor with the same polarity (Unc104 or Ncd, respectively). While the exogenous constructs restored organelle distribution and some features of bidirectional movement, it is unclear if a full restoration of wild type motility was achieved. However, this work does suggest a novel concept – that there is a mechanical coupling of oppositely-oriented microtubule-based motors, and that this coupling is required for activation of motility in either direction along the microtubule. This observation differs significantly from results of in vitro studies on purified motors bound to artificial bead cargos, so it is clear that more work is required to explore the mechanisms that lead to mechanical, biochemical, or regulatory coupling of motors in the cell.
How Many Motors?
Since in vitro studies have demonstrated that motility characteristics are strongly dependent on the number of motors simultaneously bound to a cargo, it is important to know how many motors are associated with endogenous vesicles and organelles moving in cells. A range of experimental approaches suggests that very few motors are actively engaged during transport along the microtubule. Both classic EM studies (reviewed in [14]) and force measurements on lipid droplets either in Drosophila embryos [60] or mammalian cells [61] have led to estimates that range from 1 to 7 actively engaged motors. Studies on purified axonal transport vesicles indicate that the total number of tightly associated motors is also low: ~1 kinesin-1 and 6 dyneins per vesicle [62]. Interestingly, if we consider the stall force measurements of ~6 pN for kinesin-1 [63] and ~1 pN for dynein [2], then the number of motors stably associated with motile cargos approaches a balance of forces, as recently shown for endosomal motility in cell extracts from Dictyostelium [64]. This situation can be modeled as a tug-of-war [24]. If these motors are simultaneously engaged, then unproductive back-and-forth motility might result, such as that seen in many cellular experiments. However, either a temporary dominance of one motor, due to force-dependent dissociation [24], or a regulatory switch that preferentially activates or inhibits one motor population would result in directed movement. Identification of a small complement of microtubule motors tightly bound to a vesicle suggests an efficient regulatory scheme where small changes in the number of engaged motors can manifest in large changes in the directed motility of the cargo along the cellular cytoskeleton [62].
The number of myosin motors associated with intracellular cargo, or engaged with the actin filament track during active transport, is still unclear. For melanosomes, which move along both the microtubule and actin cytoskeletons in a highly regulated process [65], the number of cargo-associated myosin-V motors ranges from ~65 during aggregation to ~88 motors during dispersal, when myosin-V activity is key [66]. Estimates of myosin-V motors associated with axonal transport vesicles from immuno-EM studies range from 6 to 120, dependent on vesicle size, with many fewer kinesin motors detected on the same vesicles [67].
Tracking Vesicular Cargos in Cells
To examine the activities of motors moving endogenous biological cargos, high-resolution imaging has been performed using a range of reporters, including GFP-fusion proteins targeted to specific organelles [68], or internalized markers such as gold nanoparticles [69] or QDs, that allow tracking of vesicle motility with high spatial precision. QDs, either derivatized by attachment of specific ligands, such as nerve growth factor (NGF) [70–72] or nonspecific peptides (polyarginine [73]), or QDs that have been aggregated by crosslinking to an antibody [74], are taken up by endocytosis.
Tracking of QDs internalized into endosomes shows multiple forms of motility. Movement of QD-labeled endosomes near the membrane is consistent with myosin-driven motility along the actin cytoskeleton [74]. The observed centripetal velocities (0.3 – 0.8 μm/sec) and step sizes (15 and 29 nm) suggest this motility at the cell periphery is driven by myosin VI. Away from the cortex, endosomes often exhibit saltatory movements suggestive of a tug-of-war between oppositely oriented microtubule motors [62]. Directed runs of several microns are also observed, consistent with unidirectional movement of endosomes along the microtubule cytoskeleton [70,73].
For microtubule-based vesicular motility in the cell, a broad range of velocities is observed, centered at ~1 μm/s. Step size analysis shows minimum steps either toward or away from the cell center of 8 nm, the repeat length for motor binding sites along the microtubule [68,73,74]. Plus end-directed steps are almost exclusively limited to 8 nm [68,69,74]. Analysis of minus end-directed motility reveals occasional larger steps of 16 and 24 nm that may occur consecutively during a run [69,73]. These cellular observations are consistent with the variable stepping of purified mammalian dynein observed in vitro [2].
Analysis of NGF-coupled QDs taken up by neurons through receptor-mediated endocytosis reveals long-range unidirectional motility directed toward the cell center [70]. While pausing was frequent, plus end-directed runs were rare. Imaging of NGF-QD motility in a cellular model of neurodegeneration indicates more frequent pausing, shorter runs, slower velocities, and generally less directed motility than seen in control neurons [72]. These observations suggest that transport properties are carefully regulated within the cell, and that transport may become mis-regulated during cellular stress responses.
Conclusions and Perspectives
The studies described here are closing the gulf between the “bottom up” approaches using in vitro assays with increasing complexity, and “top down” approaches that study motility in live cells with increasing temporal and spatial resolution. Intermediate methods have also been described, such as the isolation of cellular cargos with bound motors for in vitro studies of motility, and the “unroofing” of cells to make the cytoskeleton accessible to exogenously added motors. New geometries and technologies that reveal biophysical and cellular mechanisms are leading to rapid progress in understanding how motors operate in cells. Heisenberg said in a different context, “Science describes nature as exposed to our method of questioning.” Expanding our ways of viewing biological transport will reduce the uncertainties about its mechanisms down to their natural limits.
Box Figure 1.
Acknowledgments
The authors gratefully acknowledge funding from P01 GM087253, Dr. Tatyana Svitkina of the University of Pennsylvania for the micrograph shown in Fig. 1, and John F. Beausang, Adam G. Hendricks and Harry (Trey) W. Schroeder III for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated Bibliography
- 1.Guerin T, Prost J, Martin P, Joanny J-F. Coordination and collective properties of molecular motors: theory. Current Opinion in Cell Biology. 2010 doi: 10.1016/j.ceb.2009.12.012. this volume. [DOI] [PubMed] [Google Scholar]
- 2.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 3.Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur EL. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- 4.Rock RS, Rice SE, Wells AL, Purcell TJ, Spudich JA, Sweeney HL. Myosin VI is a processive motor with a large step size. Proc Natl Acad Sci U S A. 2001;98:13655–13659. doi: 10.1073/pnas.191512398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science. 2003;300:2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz A, Park H, Safer D, Yang Z, Chen LQ, Selvin PR, Sweeney HL. Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J Biol Chem. 2004;279:37223–37226. doi: 10.1074/jbc.C400252200. [DOI] [PubMed] [Google Scholar]
- 8.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc Natl Acad Sci U S A. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellers JR, Veigel C. Walking with myosin V. Curr Opin Cell Biol. 2006;18:68–73. doi: 10.1016/j.ceb.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Gennerich A, Vale RD. Walking the walk: how kinesin and dynein coordinate their steps. Curr Opin Cell Biol. 2009;21:59–67. doi: 10.1016/j.ceb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno H, Yasunaga T, Shingyoji C, Hirose K. Dynein pulls microtubules without rotating its stalk. Proc Natl Acad Sci U S A. 2008;105:19702–19707. doi: 10.1073/pnas.0808194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Gross SP, Vershinin M, Shubeita GT. Cargo transport: two motors are sometimes better than one. Curr Biol. 2007;17:R478–486. doi: 10.1016/j.cub.2007.04.025. A thoughtful discussion of the interaction of multiple motors on cargo in intracellular transport. [DOI] [PubMed] [Google Scholar]
- 15.Beeg J, Klumpp S, Dimova R, Gracia RS, Unger E, Lipowsky R. Transport of beads by several kinesin motors. Biophys J. 2008;94:532–541. doi: 10.1529/biophysj.106.097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Leduc C, Ruhnow F, Howard J, Diez S. Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors. Proc Natl Acad Sci U S A. 2007;104:10847–10852. doi: 10.1073/pnas.0701864104. In gliding assays at low densities of kinesin, microtubules were translocated by individual motors or pairs. Step size with two motors is half that with one motor demonstrating mechanical coupling of the motors through the microtubule and the substrate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svoboda K, Schmidt CF, Schnapp BJ, Block SM. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993;365:721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- 18.Rogers AR, Driver JW, Constantinou PE, Kenneth Jamison D, Diehl MR. Negative interference dominates collective transport of kinesin motors in the absence of load. Phys Chem Chem Phys. 2009;11:4882–4889. doi: 10.1039/b900964g. [DOI] [PubMed] [Google Scholar]
- 19.Diehl MR, Zhang K, Lee HJ, Tirrell DA. Engineering cooperativity in biomotor-protein assemblies. Science. 2006;311:1468–1471. doi: 10.1126/science.1122125. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier GF, Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979;81:10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 22.Cuda G, Pate E, Cooke R, Sellers JR. In vitro actin filament sliding velocities produced by mixtures of different types of myosin. Biophys J. 1997;72:1767–1779. doi: 10.1016/S0006-3495(97)78823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. Interactions between the two motors were tested by in vitro giding assays with mixed proportions of the two kinesins and by live cell imaging with knockouts of IFT components. The combined results suggest a mechanical competition model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Muller MJ, Klumpp S, Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci U S A. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. The authors develop a mathematical model to explain bidirectional cargo transport based on the load-dependent kinetic properties of kinesin and dynein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson AG, Landahl EC, Rice SE. Mechanism of cooperative behaviour in systems of slow and fast molecular motors. Phys Chem Chem Phys. 2009;11:4890–4898. doi: 10.1039/b900968j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uemura S, Kawaguchi K, Yajima J, Edamatsu M, Toyoshima YY, Ishiwata S. Kinesin-microtubule binding depends on both nucleotide state and loading direction. Proc Natl Acad Sci U S A. 2002;99:5977–5981. doi: 10.1073/pnas.092546199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Ross JL, Shuman H, Holzbaur EL, Goldman YE. Kinesin and dynein-dynactin at intersecting microtubules: motor density affects dynein function. Biophys J. 2008;94:3115–3125. doi: 10.1529/biophysj.107.120014. When tested at intersecting microtubules, kinesin and dynein showed distinctly different responses. Kinesin-bound beads moved smoothly through intersections at all concentrations tested, while the response of dynein-bound beads varied with motor density, indicating a switch in behavior from motor to tether. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray S, Meyhofer E, Milligan RA, Howard J. Kinesin follows the microtubule’s protofilament axis. J Cell Biol. 1993;121:1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitzsche B, Ruhnow F, Diez S. Quantum-dot-assisted characterization of microtubule rotations during cargo transport. Nat Nanotechnol. 2008;3:552–556. doi: 10.1038/nnano.2008.216. [DOI] [PubMed] [Google Scholar]
- 31**.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. Direct visualization of encounters of kinesin and dynein motors with microtubule-bound tau at single molecule resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Khan S, Sheetz MP. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys J. 1995;69:2011–2023. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warshaw DM, Kennedy GG, Work SS, Krementsova EB, Beck S, Trybus KM. Differential labeling of myosin V heads with quantum dots allows direct visualization of hand-over-hand processivity. Biophys J. 2005;88:L30–32. doi: 10.1529/biophysj.105.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syed S, Snyder GE, Franzini-Armstrong C, Selvin PR, Goldman YE. Adaptability of myosin V studied by simultaneous detection of position and orientation. EMBO J. 2006;25:1795–1803. doi: 10.1038/sj.emboj.7601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Ali MY, Krementsova EB, Kennedy GG, Mahaffy R, Pollard TD, Trybus KM, Warshaw DM. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc Natl Acad Sci U S A. 2007;104:4332–4336. doi: 10.1073/pnas.0611471104. Myosin V was attached to quantum dots and motility was studied on actin branches and AF-MT intersections. Switching tracks was highly likely and myosin V even traveled along microtubules by one-dimensional diffusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammer JA, 3rd, Wu X. Slip sliding away with myosin V. Proc Natl Acad Sci U S A. 2007;104:5255–5256. doi: 10.1073/pnas.0701071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali MY, Lu H, Bookwalter CS, Warshaw DM, Trybus KM. Myosin V and Kinesin act as tethers to enhance each others’ processivity. Proc Natl Acad Sci U S A. 2008;105:4691–4696. doi: 10.1073/pnas.0711531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder HW, Shuman H, Holzbaur ELF, Goldman YE. Cargo switching at actin-microtubule intersections: a case for strength in numbers. ASCB Abstracts. 2008;48:42. [Google Scholar]
- 39**.Cai D, Verhey KJ, Meyhofer E. Tracking single Kinesin molecules in the cytoplasm of mammalian cells. Biophys J. 2007;92:4137–4144. doi: 10.1529/biophysj.106.100206. This study directly compared the motility of fluorescently labeled kinesin-1 motors both in vitro and in cells, monitored by TIRF microscopy. Speeds and run lengths were remarkably similar in both assays. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Lett. 2006;6:1491–1495. doi: 10.1021/nl060921t. [DOI] [PubMed] [Google Scholar]
- 41.Yoo J, Kambara T, Gonda K, Higuchi H. Intracellular imaging of targeted proteins labeled with quantum dots. Exp Cell Res. 2008;314:3563–3569. doi: 10.1016/j.yexcr.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 42*.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. This study demonstrated that acetylation of the microtubule track can lead to preferential association of kinesin-1, thus supporting a model for filament-based regulation of transport in the cell. [DOI] [PubMed] [Google Scholar]
- 43.Cai D, McEwen DP, Martens JR, Meyhofer E, Verhey KJ. Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 2009;7:e1000216. doi: 10.1371/journal.pbio.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Nelson SR, Ali MY, Trybus KM, Warshaw DM. Random walk of processive, quantum dot-labeled myosin Va molecules within the actin cortex of COS-7 cells. Biophys J. 2009;97:509–518. doi: 10.1016/j.bpj.2009.04.052. QD-labeled myosin-V was introduced into COS-7 cells by pinocytosis and tracked with TIRF microscopy. The unexpected observation of an apparent random walk for a motor known to move processively along actin filaments in vitro was interpreted as a consequence of a dense cortical actin network; this hypothesis was supported by Monte Carlo simulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierobon P, Achouri S, Courty S, Dunn AR, Spudich JA, Dahan M, Cappello G. Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys J. 2009;96:4268–4275. doi: 10.1016/j.bpj.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kural C, Serpinskaya AS, Chou YH, Goldman RD, Gelfand VI, Selvin PR. Tracking melanosomes inside a cell to study molecular motors and their interaction. Proc Natl Acad Sci U S A. 2007;104:5378–5382. doi: 10.1073/pnas.0700145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruno L, Echarte MM, Levi V. Exchange of microtubule molecular motors during melanosome transport in Xenopus laevis melanophores is triggered by collisions with intracellular obstacles. Cell Biochem Biophys. 2008;52:191–201. doi: 10.1007/s12013-008-9034-3. [DOI] [PubMed] [Google Scholar]
- 49*.Brawley CM, Rock RS. Unconventional myosin traffic in cells reveals a selective actin cytoskeleton. Proc Natl Acad Sci U S A. 2009;106:9685–9690. doi: 10.1073/pnas.0810451106. Labeled myosins V, VI, and X were introduced into triton-extracted cells. Preferential binding and transport were observed along some actin tracks, consistent with a filament-based model for the regulation of myosin activity in the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomishige M, Klopfenstein DR, Vale RD. Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science. 2002;297:2263–2267. doi: 10.1126/science.1073386. [DOI] [PubMed] [Google Scholar]
- 51.Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammond JW, Cai D, Blasius TL, Li Z, Jiang Y, Jih GT, Meyhofer E, Verhey KJ. Mammalian Kinesin-3 motors are dimeric in vivo and move by processive motility upon release of autoinhibition. PLoS Biol. 2009;7:e72. doi: 10.1371/journal.pbio.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. doi: 10.1016/j.cell.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 54.Sivaramakrishnan S, Spudich JA. Coupled myosin VI motors facilitate unidirectional movement on an F-actin network. J Cell Biol. 2009;187:53–60. doi: 10.1083/jcb.200906133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma S, Chisholm RL. Cytoplasmic dynein-associated structures move bidirectionally in vivo. J Cell Sci. 2002;115:1453–1460. doi: 10.1242/jcs.115.7.1453. [DOI] [PubMed] [Google Scholar]
- 56.Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc Natl Acad Sci U S A. 1997;94:3720–3725. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers SL, Gelfand VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 58.Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. Journal of Cell Biology. 2009;187 doi: 10.1083/jcb.200908075. Mislocalization of peroxisomes upon depletion of either kinesin-1 or dynein in S2 cells can be rescued by expression of an unrelated motor with the same directionality, suggesting that oppositely-oriented motors bound to the same cargo are required to activate motility in the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. Lipid droplets in Drosophila embryos are found to be transported toward the plus ends of microtubules by kinesin-1. Varying the number of motors on each droplet increased stall force, but had surprisingly little influence on velocity or processivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sims PA, Xie XS. Probing dynein and kinesin stepping with mechanical manipulation in a living cell. Chemphyschem. 2009;10:1511–1516. doi: 10.1002/cphc.200900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross JL, Hendricks AG, Perlson E, Schroeder HW, Holzbaur EL. Motor coordination via tug-of-war mechanism drives bidirectional vesicle transport. ASCB Abstracts. 2009 doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svoboda K, Block SM. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 64.Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci U S A. 2009;106:19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kashina A, Rodionov V. Intracellular organelle transport: few motors, many signals. Trends Cell Biol. 2005;15:396–398. doi: 10.1016/j.tcb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111 (Pt 21):3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- 68.Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 69**.Nan X, Sims PA, Xie XS. Organelle tracking in a living cell with microsecond time resolution and nanometer spatial precision. Chemphyschem. 2008;9:707–712. doi: 10.1002/cphc.200700839. Two dimensional tracking of endocytosed gold nanoparticles with high temporal and spatial resolution reveals individual steps of cargo moving toward either the plus or minus end of the microtubule. [DOI] [PubMed] [Google Scholar]
- 70*.Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci U S A. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. Endocytosed QDs were tracked in neurons grown in compartmentalized microfluidic chambers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Echarte MM, Bruno L, Arndt-Jovin DJ, Jovin TM, Pietrasanta LI. Quantitative single particle tracking of NGF-receptor complexes: transport is bidirectional but biased by longer retrograde run lengths. FEBS Lett. 2007;581:2905–2913. doi: 10.1016/j.febslet.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 72.Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, Holzbaur EL. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nan X, Sims PA, Chen P, Xie XS. Observation of individual microtubule motor steps in living cells with endocytosed quantum dots. J Phys Chem B. 2005;109:24220–24224. doi: 10.1021/jp056360w. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe TM, Higuchi H. Stepwise movements in vesicle transport of HER2 by motor proteins in living cells. Biophys J. 2007;92:4109–4120. doi: 10.1529/biophysj.106.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]