Abstract
When Th1 effector CD4 cells encounter tolerizing Ag in vivo, their capacity to express the effector cytokines IFN-γ and TNF-α is lost more rapidly than noneffector functions such as IL-2 production and proliferation. To localize the relevant intracellular signaling defects, cytokine expression was compared following restimulation with Ag vs agents that bypass TCR-proximal signaling. IFN-γ and TNF-α expression were both partially rescued when TCR-proximal signaling was bypassed, indicating that both TCR-proximal and -distal signaling defects impair the expression of these two effector cytokines. In contrast, bypassing TCR-proximal signaling fully rescued IL-2 expression. T-bet, a transcription and chromatin remodeling factor that is required to direct the differentiation of naive CD4 cells into IFN-γ -expressing Th1 effectors, was partially down-modulated in tolerized Th1 effectors. Enforcing T-bet expression during tolerization selectively rescued the ability to express IFN-γ, but not TNF-α. Conversely, expression of a dominant-negative T-bet in Th1 effectors selectively impaired the ability to express IFN-γ, but not TNF-α. Analysis of histone acetylation at the IFN-γ promoter further suggested that down-modulation of T-bet expression during Th1 effector CD4 cell tolerization does not impair IFN-γ expression potential through alterations in chromatin structure.
Self-reactive T cells have generally been thought to undergo tolerization at either of two separate stages in their development. The majority of self-reactive T cells undergo negative selection during development in the thymus, where immature T cells expressing TCRs that recognize MHC molecules presenting self-epitopes at high affinity/avidity undergo deletion (1–4). Subsequently, mature T cells that recognize parenchymal self-Ags that are not presented in the thymus can be subject to a variety of peripheral tolerization mechanisms such as deletion (5–7), functional inactivation (also referred to as adaptive tolerance or anergy; Ref. 8) or suppression (9, 10). Although it had been suggested that only naive self-reactive T cells were susceptible to peripheral tolerization, it has recently been demonstrated that virally primed self-reactive effector (11) and memory (12) T cells are equally susceptible. Tolerization of self-reactive effector and memory T cells that are initially primed by pathogens expressing cross-reactive Ags (i.e., molecular mimicry; Ref. 13) could potentially limit their potential to inflict autoimmune damage, while tolerization of tumor-reactive effector and memory T cells might negatively impact tumor immunity (14, 15).
Given the relevance of effector and memory T cell tolerization to both autoimmunity and tumor immunity, dissection of the underlying mechanisms will be important for designing approaches to manipulate these pathways for therapeutic benefit. To study Th1 effector CD4 cell peripheral tolerization induced by cognate self-Ag, we previously developed an adoptive retransfer system in which naive clonotypic TCR transgenic CD4 cells specific for the model Ag influenza hemagglutinin (HA)3 are initially transferred into recipients that have been infected with a recombinant HA-expressing vaccinia virus (which induces Th1 differentiation), and subsequently retransferred into secondary recipients that express HA as a parenchymal self-Ag. Our initial findings indicated that self-Ag-induced Th1 effector CD4 cell tolerization is a complex process in which different functions are lost with different kinetics. Thus, the ability to express the effector cytokines IFN-γ and TNF-α are lost more rapidly than noneffector functions such as IL-2 production and proliferation (11, 16).
Because IFN-γ and TNF-α can both play critical roles in various models of autoimmunity (17–20) and tumor immunity (21–25), in the current study, we sought to uncover the basis by which their expression potentials are impaired during Th1 effector CD4 cell tolerization. To begin to localize the intrinsic intracellular signaling defects, cytokine expression in tolerized effectors was compared following restimulation with Ag vs agents that bypass the TCR-proximal signaling machinery. IFN-γ and TNF-α expression in tolerized effectors were both partially rescued when TCR-proximal signaling was bypassed, indicating the existence of both TCR-proximal and -distal signaling defects that impair the expression potentials of these two effector cytokines. In contrast, IL-2 expression was fully rescued when TCR-proximal signaling was bypassed. Expression of T-bet, a transcription and chromatin remodeling factor that is required to direct the differentiation of naive CD4 cells into effectors that express the hallmark Th1 cytokine IFN-γ (26–29), was partially down-modulated in tolerized effectors, as was the IL-12R β-chain (which is regulated by T-bet; Refs. 27 and 30). Enforcing T-bet expression during Th1 effector CD4 cell tolerization rescued the ability to express IFN-γ, but not TNF-α. Conversely, expression of a dominant-negative T-bet in Th1 effectors impaired the ability to express IFN-γ, but not TNF-α. Taken together, these data indicate that down-modulation of T-bet expression in tolerized Th1 effector CD4 cells selectively impairs IFN-γ expression potential, thus extending the range of the known functions of T-bet in CD4 cells to include the regulation of peripheral tolerance. Analysis of histone acetylation at the IFN-γ promoter in tolerized effectors further suggested that down-modulated T-bet expression does not impair IFN-γ expression potential through alterations in chromatin structure.
Materials and Methods
Adoptive transfer and flow cytometry
Adoptive transfers of CFSE-labeled 6.5 TCR transgenic naive and Th1 effector Thy1.1+ clonotypic CD4 cells (31) into Thy1.2+ recipients, vaccinia inoculations, and subsequent functional analyses were performed as previously described (16, 32), with the following modifications. Intracellular cytokine staining of nonretrovirally transduced clonotypic CD4 cells (identified as Thy1.1+ and CFSE-divided) was performed following in vitro stimulation for 3 h with 5 μg/ml brefeldin A (Sigma-Aldrich) and the cognate I-Ed-restricted HA peptide (100 μg/ml) or PMA (125 ng/ml; Sigma-Aldrich) plus ionomycin (2.5 μg/ml; Sigma-Aldrich), or for 11 h with IL-12 (10 U/ml; a gift from Genetic Institute, Cambridge, MA) plus IL-18 (50 ng/ml; Cell Sciences) with brefeldin A added for the final 2 h. For anti-CD3 stimulations, enriched clonotypic CD4 cells were incubated with brefeldin A for 3 h in flat-bottom 96-well plates that had been pre-coated with anti-CD3ε (eBioscience) in PBS at 25 μg/ml. Cells were stained with anti-Thy1.1 PerCP (eBioscience), followed by fixation, permeabilization and staining with anti-TNF-α allophycocyanin and anti-IFN-γ PE or anti-IL-2 PE (eBioscience) as indicated, and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Total intracellular cytokine expression (expressed in arbitrary units) was calculated as the product of the percentage of cytokine-expressing clonotypic CD4 cells and the mean fluorescence intensity (MFI) of cytokine expression in these positively staining cells, as previously described (32). Adoptive transfers and intracellular cytokine staining of retrovirally transduced clonotypic Th1 effector CD4 cells were performed similarly, except that the transferred cells were not labeled with CFSE, and following in vitro restimulation, were stained with anti-Thy1.1 PE (eBioscience), anti-CD4 PerCP-Cy5 (BD Biosciences), anti-IFN-γ allophycocyanin (eBioscience), anti-TNF-α PE-Cy7 (BD Biosciences) and analyzed on a LSR II flow cytometer (BD Biosciences). The Animal Care Committee of the University of Connecticut Health Center approved all mouse protocols used in this study.
Immunomagnetic enrichment of Thy1.1+ clonotypic CD4 cells
Adoptively transferred Thy1.1+ clonotypic CD4 cells were enriched from recipient spleens using MACS technology as per the manufacturer’s instructions (Miltenyi Biotec) with minor modifications. Briefly, total spleen was labeled with PE-conjugated anti-Thy1.1 (eBioscience) in sterile PBS with 2% BSA and 2.5 mM EDTA for 10 min at 4°C, washed, and then labeled with anti-PE microbeads for an additional 15 min. The microbead-labeled Thy1.1+ cells were subsequently enriched by serial passage over a MS MACS column, a Lympholyte-M gradient (Cedarlane Laboratories), and finally a second MS MACS column. With an initial clonotypic CD4 cell frequency of 1.5% or greater, this enrichment protocol generally yielded purities of 85–90%. Chromatin immunoprecipitation (ChIP) and RT-PCR analyses were only performed on samples that were enriched to at least 85%.
Reverse transcription
Approximately 1–2 × 105 enriched clonotypic CD4 cells were homogenized and expunged of genomic DNA using a QIAshredder kit, total RNA was isolated using a RNeasy mini isolation kit, and reverse transcription was performed using an Omniscript Reverse Transcription kit with oligo(dT)16 as primer (Qiagen), all according to the manufacturer’s instructions.
Real-time PCR
Real-time quantitative PCR was performed on cDNA (RT-PCR) and precipitated genomic DNA (ChIP) using an iCycler iQ system and iQ SYBR Green Supermix (Bio-Rad). Primer sequences were as follows: IFN-γ promoter: forward (F): 5′-TCAGCTGATCCTTTG GACCC, reverse (R): 5′-CTCAGAGCTAGGCCGCAGG; CD3ε enhancer: F: 5′-TTCCAAGTGACGTGGAGCAG, R: 5′-AGGTGTCTGAACCCCACACAG; TNF-α promoter: F: 5′-TTTTCCGAGGGTTGAATGAGA, R: 5′-AGACGGCCGCCTTTATAGC; hypoxanthine phosphoribosyltransferase cDNA: F: 5′CTCCTCAGACCGCTTTTTGC, R: 5′-TAACCTGGTTCATCATCGCTAATC; IL-12Rβ cDNA: F: 5′-ACAACCTGAGCTCTGCGAAATT, R: 5′-TGTAGGCTGCTTATTGGATGTGAG; IL-18Rα cDNA: F:5′-ACACCTTGGAATTCTGGCCA, R: 5′-TGCGACGATCATTTCCGAC; T-bet cDNA: F:5′-CGGGAGAACTTTGAGTCCATGT, R: 5′-GCTGGCCTGGAAGGTCG, and IFN-γ cDNA: F: 5′-CATTGAAAGCCTAGAAAGTCTGAATAAC, R: 5′-TGGCTCTGCAGGATTTTCATG-3′. All samples were run in duplicate using cycling conditions of 95°C for 5 min, then 45 cycles of 95°C for 20 s and 60°C for 60 s. SYBR Green fluorescence was measured during the 60°C annealing step. Melting curve analysis and agarose gel electrophoresis were performed to verify that the amplified products constituted discrete molecular species (data not shown). Quantitation of RT-PCR and ChIP data were performed by first normalizing for input template amounts using hypoxanthine phosphoribosyltransferase- and CD3ε-specific primers, respectively, and subsequently calculating the fold-difference between experimental and a representative naive CD4 cell sample using the Δ-Δ cycle threshold method (described in User Bulletin No. 2; Applied Biosystems).
Retroviral transductions
The MigR1-based retroviral vectors that express wild-type (wt) T-bet and dominant-negative (DN) T-bet bicistronically with GFP have been described (27, 33). The retroviral packaging cell line Phoenix-Eco was originally developed by Dr. G. Nolan (Stanford University, Stanford, CA), and obtained from the American Type Culture Collection. Phoenix-Eco cells were transfected with retroviral vectors using TransIT-293 reagent (Mirus Bio) according to the manufacturer’s instructions, and 48 h later, retrovirus was harvested from culture supernatants. Spleen cells from 6.5 TCR transgenic mice containing naive clonotypic CD4 cells were cultured (1 × 107/ml) in 24-well plates with 100 μg/ml synthetic I-Ed-restricted HA peptide plus IL-2 (50 U/ml; National Cancer Institute Biological Research Branch, Frederick, MD) and IL-12 (2.5 ng/ml; Genetic Institute) in IMDM medium containing 10% FBS. Twenty-four hours later, the activated clonotypic CD4 cells were mixed with freshly prepared retrovirus plus 8 μg/ml polybrene (Sigma-Aldrich) and 50 U/ml IL-2 and centrifuged at 2500 rpm for 90 min at 30°C. Subsequently, 70% of the supernatant was removed, and fresh medium containing 50 U/ml IL-2 and 2.5 ng/ml IL-12 were added, and the cells incubated at 37°C. Following an additional 6 days of culture, the transduced cells were washed twice in Hank’s and adoptively transferred into recipients at 2.5 × 106 clonotypic CD4 cells per mouse.
Chromatin immunoprecipitation
ChIP analysis was performed using the Acetyl-Histone H3 Immunoprecipitation (ChIP) Assay kit (Upstate Biotechnology) according to the manufacturer’s instructions. Briefly, enriched clonotypic CD4 cells were fixed with 0.5% formaldehyde for 10 min at 37°C, washed twice with ice-cold PBS, and resuspended in ChIP lysis buffer. The lysates were subsequently sonicated to shear the genomic DNA into 200–1000 bp fragments using a Misonix S3000 sonicator: each sample was sonicated on ice 20 times for 10 s at an output level of 1.5 with a 20 s rest between each pulse. Sonicated lysates were microcentrifuged at 14,000 rpm for 10 min, and supernatants were added to ChIP dilution buffer (1/10) and precleared with protein A-agarose beads before overnight incubation at 4°C with antiacetylated histone H3 (4 μl per immunoprecipitation). Protein A-agarose beads were subsequently added and the incubation was extended for an additional 1 h, followed by sequential washing one time each with low-salt buffer, high-salt buffer, and LiCl buffer, and finally two washes with TE buffer. Precipitated chromatin complexes were eluted off of the washed beads with 0.1 M NaHCO3 and 1% SDS, cross-links were reversed at 65°C for 4 h following addition of NaCl to a final concentration of 200 mM, and then 20 μg of proteinase K along with EDTA (final concentration 10 mM) and Tris (pH 6.5 at final concentration of 40 mM) were added for a final 1 h incubation at 45°C. DNA was extracted with phenol and chloroform, ethanol-precipitated along with 20 μg of glycogen as a carrier, and subsequently analyzed by real-time PCR. The minimum number of clonotypic CD4 cells required to detect a reproducible difference in histone H3 acetylation in the IFN-γ promoter region comparing naive vs Th1 effector clonotypic CD4 cells is 1 × 105 (data not shown), although experimental samples contained 3–5 × 105 clonotypic cells.
Results
Multiple signaling defects impair IFN-γ and TNF-α expression in tolerized Th1 effector CD4 cells
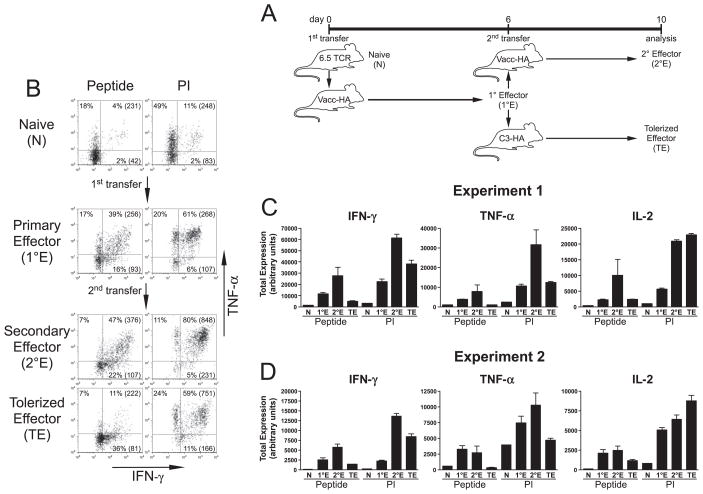
We have previously demonstrated that when naive clonotypic HA-specific TCR transgenic CD4 cells are adoptively transferred into nontransgenic (NT) recipients that have been infected with a recombinant vaccinia virus that expresses HA (vacc-HA) they differentiate into Th1 effectors (32), but that these effectors can subsequently be induced to undergo peripheral tolerization following retransfer into transgenic secondary recipients that express HA as a parenchymal self-Ag (11). Interestingly, self-HA induces a rapid loss in the ability of these Th1 effectors to express IFN-γ and TNF-α, while their ability to express IL-2 and proliferate is lost more slowly (11, 16). To begin to localize the signaling defect(s) associated with the inability to express these cytokines during effector tolerization, we assessed whether cytokine expression could be rescued by stimulation with PMA plus ionomycin (PI) (Fig. 1). Because PMA and ionomycin directly activate protein kinase C and release intracellular Ca2+, respectively, thus bypassing TCR-proximal signaling events, comparing cytokine expression in tolerized effectors restimulated with HA peptide-pulsed APCs (peptide) vs PI should indicate whether the underlying signaling defect(s) is proximal or distal to the TCR.
FIGURE 1.
Th1 effector CD4 cells tolerized following retransfer into cognate self-Ag-expressing recipients exhibit multiple signaling defects that impair IFN-γ and TNF-α expression. A, Diagram of experimental design. Naive 6.5 TCR transgenic HA-specific clonotypic CD4 cells expressing the Thy1.1+ congenic marker (N) were labeled with CFSE and adoptively transferred into vacc-HA-infected NT Thy1.2+ recipients, and 6 days later when they had differentiated into resting Th1 effectors (primary effectors or 1°E, identified as Thy1.1+ and CFSE-diluted), they were harvested from spleens, relabeled with CFSE, and retransferred into either a second set of vacc-HA-infected NT recipients (secondary effectors or 2°E) or transgenic self-HA-expressing secondary recipients (tolerized effectors or TE) and subsequently harvested from spleens 4 days later for analysis. B, Representative intracellular staining plots of IFN-γ vs TNF-α expression in clonotypic CD4 cells following in vitro restimulation with either HA peptide-pulsed APCs (Peptide) or PI. Shown are the percentages of cytokine-expressing cells, and level of IFN-γ expression (MFI) in parentheses. Note that the percentage of cytokine-expressing naive clonotypic CD4 cells stimulated with peptide is underestimated by ~3-fold because CFSE dilution could not distinguish clonotypic from nonclonotypic cells during the brief in vitro stimulation that was insufficient in duration to induce division of the clonotypic cells. C, Quantitative analysis corresponding to A. Total intracellular cytokine expression (expressed in arbitrary units, mean ± SEM) was calculated as the product of the percentage of cytokine-expressing clonotypic CD4 cells and the MFI of cytokine expression as previously described (32); n = 4, 2, and 3 for 1°E, 2°E, and TE, respectively, and the naive group was a single pooled sample generated from three separate mice. D, Quantitative analysis of a second independent experiment performed similarly to that described in A and B; n = 4 for each group, except for naive which was a single pooled sample generated from four separate mice.
To establish baseline cytokine expression potentials before tolerization, the ability of naive and day 6 primary Th1 effector clonotypic CD4 cells to express intracellular IFN-γ, TNF-α, and IL-2 was compared following in vitro stimulation with HA peptide vs PI (Fig. 1). Although naive CD4 cells expressed negligible levels of IFN-γ in response to both peptide and PI stimulation, TNF-α and IL-2 expression potentials were more apparent. Day 6 virally primed Th1 effectors (recovered from spleens of primary adoptive transfer recipients) expressed markedly enhanced levels of all three cytokines in response to peptide stimulation, and PI stimulation could, in some cases, further enhance these levels by as much as an additional 2-fold.
To assess cytokine expression potentials in self-Ag-tolerized Th1 effector CD4 cells, day 6 primary effectors were retransferred into C3-HA transgenic (line 142; Ref. 34) recipients that express HA as a parenchymal self-Ag, and recovered from spleens 4 days later for analysis. As a control, primary effectors were also retransferred into a second set of vacc-HA-infected NT recipients (i.e., 2° effectors). This second round of viral-HA priming generally further enhanced cytokine expression potentials. Consistent with our previous observations (11, 16), 1° effectors exposed to self-HA underwent numerous rounds of division as indicated by CFSE dilution (data not shown), and expressed substantially reduced levels of IFN-γ, TNF-α, and IL-2 following in vitro peptide stimulation, compared with counterparts re-exposed to viral HA. Interestingly, PI restimulation rescued IL-2 expression in tolerized effectors to a level that was comparable to control 2° effectors, however, IFN-γ and TNF-α expression were only partially rescued by PI. Thus, TNF-α expression in tolerized effectors remained ~2-fold lower than in control 2° effectors, and although IFN-γ expression in tolerized effectors was only ~30 – 40% reduced, compared with control 2° effectors, this difference was observed in two independent experiments (compare Fig. 1C to 1D). To further assess the reproducibility of this result, effector CD4 cell tolerization was induced via bolus soluble HA peptide injections (11). Similar to the results with self-HA (Fig. 1), in either primary (Fig. 2) or secondary (Fig. 3) recipients, PI stimulation partially rescued IFN-γ and TNF-α expression in clonotypic effector CD4 cells tolerized by four consecutive daily peptide injections, compared with nontolerized controls. Taken together, these results suggested that while tolerized effectors have a TCR-proximal signaling defect(s) that impairs the expression potentials of IFN-γ, TNF-α, and IL-2, there is also a TCR-distal defect(s) that selectively impairs IFN-γ and TNF-α expression potentials.
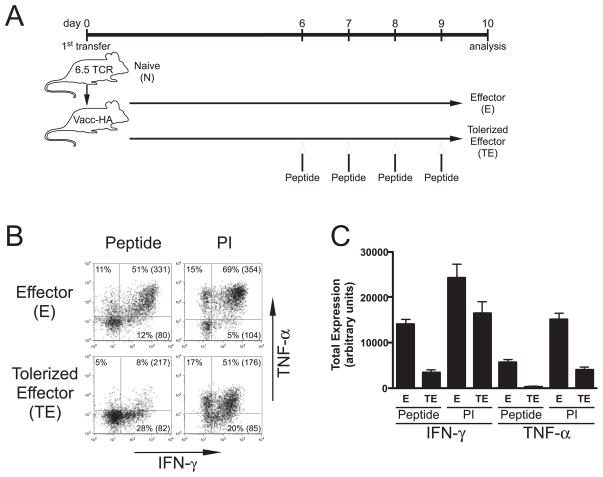
FIGURE 2.
Th1 effector CD4 cells tolerized in primary recipients following exposure to bolus injections of soluble HA peptide exhibit similar impairments in IFN-γ and TNF-α expression potentials as those induced by self-HA. A, Diagram of experimental design. vacc-HA-infected NT 1° recipients were treated with or without soluble HA peptide boluses on days 6, 7, 8, and 9, and tolerized clonotypic effectors (TE) and nontolerized effectors (E), respectively, were harvested from spleens on day 10 for analysis. Representative IFN-γ vs TNF-α intracellular staining plots (B) and quantitative analysis (C) are presented as in Fig. 1; n = 4 for both groups.
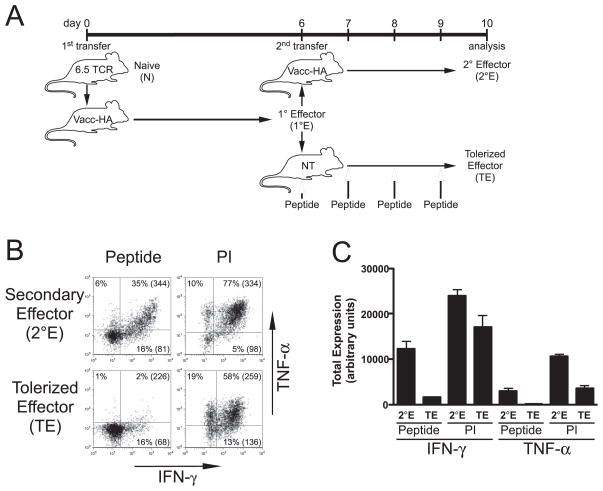
FIGURE 3.
Th1 effector CD4 cells tolerized following retransfer and exposure to bolus injections of soluble HA peptide exhibit similar impairments in IFN-γ and TNF-α expression potentials as those induced by self-HA. A, Diagram of experimental design. Secondary effectors (2°E) and tolerized effectors (TE) were generated as in Fig. 1, except that TE were harvested from NT 2° recipients that had been treated on days 6, 7, 8, and 9 with soluble HA peptide boluses. Representative IFN-γ vs TNF-α intracellular staining plots (B) and quantitative analysis (C) are presented as in Fig. 1; n = 4 for both groups
T-bet is down-modulated in tolerized Th1 effector CD4 cells
To begin characterizing the molecular basis for impaired cytokine expression in tolerized Th1 effector CD4 cells, tolerized and non-tolerized clonotypic effector CD4 cells were enriched from spleens of adoptive transfer recipients by MACS column chromatography. Because the frequency of effectors tolerized by retransfer into self-HA-expressing 2° recipients is generally lower than effectors tolerized by bolus soluble HA peptide in 1° recipients (data not shown), the latter were used in the following experiments to maximize the yield and purity of enriched clonotypic CD4 cells (purities ranged between 85 and 90%, data not shown). Additionally, because the frequency of 1° effectors drops from ~2% of splenocytes at day 6 to ~1% at day 10, and clonotypic frequencies of <1.5% generally result in substantially reduced purities following MACS enrichment (data not shown), day 6 primary effectors were used as controls for tolerized effectors that were exposed to bolus soluble HA peptide on days 6–9 and harvested on day 10 (the frequency being ~2%). To validate this approach, we directly compared the potential of day 6 vs day 10 nonpeptide-treated 1° effectors to express IFN-γ and TNF-α following restimulation with either peptide or PI, and found that day 10 effectors expressed comparable if not slightly greater levels of both cytokines in response to either stimulant (data not shown), indicating that impaired cytokine expression in day 10 peptide-treated effectors relative to day 6 nontreated counterparts is the consequence of Ag-induced tolerance rather than an inherent time-dependent diminution in effector function.
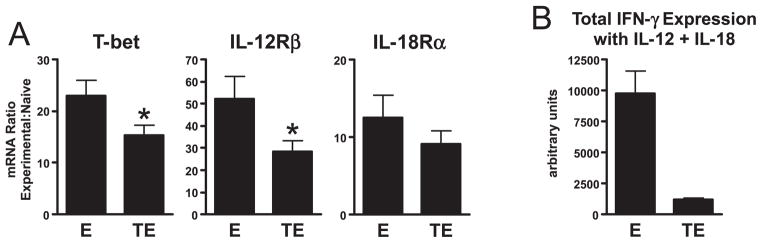
Using quantitative real-time RT-PCR, expression of the mRNA encoding the Th1 differentiation factor T-bet (26) was found to be ~30% lower in tolerized effector compared with control nontolerized clonotypic Th1 effector CD4 cells (Fig. 4A). Although this difference was relatively modest, it was statistically significant, and intriguingly was comparable in magnitude to the difference in the ability of tolerized and nontolerized clonotypic Th1 effector CD4 cells to express IFN-γ following PI stimulation (Figs. 1–3). T-bet protein levels in these cells were not directly measured (in part due to the relatively small number of clonotypic cells that could be recovered from adoptive transfer recipients), however, it did appear that tolerized effectors possessed a reduced level of T-bet activity. Thus, expression of the IL-12 receptor β subunit mRNA (whose expression is T-bet dependent; Refs. 27, 30, and 33) but not the IL-18R α subunit mRNA (whose expression is T-bet independent; Ref. 30) was significantly reduced in tolerized, compared with control nontolerized clonotypic Th1 effector CD4 cells (Fig. 4A). Furthermore, this reduction in IL-12R β subunit mRNA appeared to be associated with a reduction in the level of the functional IL-12R because stimulation with IL-12 plus IL-18 (which can induce Ag-independent IFN-γ expression in Th1 effectors; Ref. 35) elicited substantially reduced IFN-γ expression in tolerized, compared with control nontolerized clonotypic effectors (Fig. 4B). Taken together, these data are consistent with tolerized Th1 effector CD4 cells expressing reduced levels of T-bet functional activity.
FIGURE 4.
T-bet is down-modulated in tolerized Th1 effector CD4 cells. A, 1° effectors (E) and HA peptide bolus-tolerized clonotypic effectors (TE) generated as in Fig. 2 were enriched from recipient spleens, and T-bet, IL-12Rβ, and IL-18Rα mRNA levels were compared by real-time RT-PCR. Data is presented as the ratio of mRNA expression between either E or TE and a representative naive clonotypic CD4 cell sample; n = 15 for both groups; *, p < 0.05 using an unpaired two-tailed t test. B, Quantitative analysis of intracellular IFN-γ expression (presented as in Fig. 1C) in E and TE following in vitro restimulation with IL-12 + IL-18; n = 7 and 8 for E and ET, respectively.
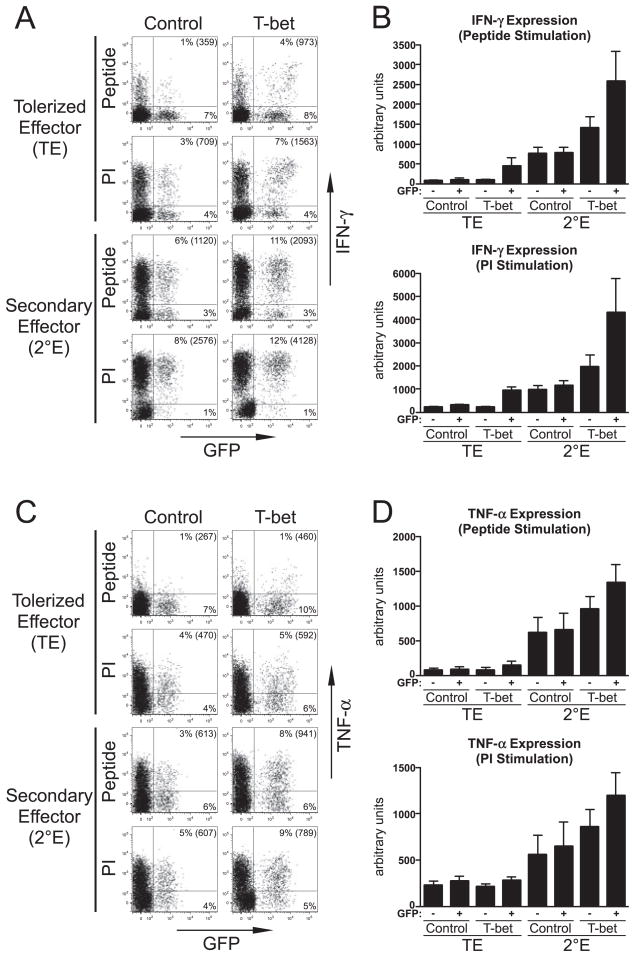
Enforcing T-bet expression during Th1 effector CD4 cell tolerization selectively rescues IFN-γ expression
Given the reduction in T-bet expression and functional activity in tolerized Th1 effector CD4 cells, and that T-bet regulates IFN-γ expression at the chromatin/transcriptional level (26–28, 36–39), this reduced T-bet expression associated with tolerization might have been playing a role in mediating the TCR-distal signaling defect that impairs IFN-γ expression. To test this possibility, we asked whether enforcing T-bet expression during tolerization could rescue IFN-γ expression potential. Thus, naive clonotypic CD4 cells were stimulated in vitro under Th1-differentiating conditions, transduced 24 h later with either a recombinant retrovirus expressing T-bet and GFP bicistronically or a control retrovirus expressing only GFP (27). Six days later (when the clonotypic CD4 cells had differentiated into Th1 effectors, data not shown) they were transferred into self-HA or viral-HA recipients, and recovered from spleens 4 days later for analysis (Fig. 5). Overall, the pattern of tolerization for in vitro-differentiated Th1 effectors encountering self-HA was similar to that observed for in vivo-differentiated effectors (compare Fig. 5 to Figs. 1–3). In particular, clonotypic effectors that had been transduced with the control retrovirus and recovered from self-HA recipients exhibited substantially reduced IFN-γ and TNF-α expression following restimulation with HA peptide-pulsed APCs, compared with viral-HA recipients, and both IFN-γ and TNF-α expression were partially rescued by PI stimulation.
FIGURE 5.
Enforcing T-bet expression in Th1 effector CD4 cells undergoing tolerization selectively rescues IFN-γ expression. Naive clonotypic CD4 cells were stimulated in vitro under Th1-differentiating conditions, transduced with either a T-bet-expressing or control retrovirus, at day 6 posttransduction transferred into either self-HA (TE) or viral-HA (2°E) recipients, and 4 days later recovered from spleens for analysis; n = 8, 7, 9, and 9 for TE control, TE T-bet, 2°E control, and 2°E T-bet, respectively. A, Representative IFN-γ vs GFP expression plots for recovered clonotypic CD4 cells (Thy1.1+) restimulated with either HA peptide or PI, with the percentage and MFI for IFN-γ expression in GFP+ clonotypic cells shown. B, Quantitative analysis of data corresponding to A is presented as in Fig. 1B. C, Representative TNF-α vs GFP expression plots for the cells shown in A. D, Quantitative analysis of data corresponding to C.
Clonotypic Th1 effector CD4 cells recovered from self-HA-expressing recipients that had been transduced (i.e., GFP+) with the T-bet retrovirus expressed 3- to 4-fold greater levels of IFN-γ following restimulation with either peptide or PI, compared with clonotypic effectors that had either been transduced with the control retrovirus or nontransduced (i.e., GFP−). Furthermore, IFN-γ expression in PI-stimulated T-bet-transduced clonotypic effectors recovered from self-HA recipients was rescued to a level that was equivalent to clonotypic cells transduced with the control retrovirus that were recovered from viral-HA recipients, and only partial rescue was observed when peptide was used as a stimulant (Fig. 5, A and B). Thus, in effector CD4 cells exposed to tolerizing Ag, enforced T-bet expression rescued IFN-γ expression potential more effectively when the TCR-proximal defect(s) was bypassed, indicating that down-modulated T-bet expression plays a role in mediating the TCR-distal defect that impairs IFN-γ expression. Interestingly, retrovirally mediated T-bet expression in clonotypic effectors recovered from viral-HA recipients enhanced IFN-γ expression potential, compared with counterparts transduced with the control retrovirus (Fig. 5, A and B), apparently indicating that endogenous T-bet expression is not saturating, and that retroviral transduction might result in T-bet levels that exceed normal. In viral-HA recipients there was also a more modest increase in IFN-γ expression potential in the GFP− fraction of T-bet-transduced clonotypic cells (Fig. 5B), which might have been the result of a CD4 cell-extrinsic mechanism (see below).
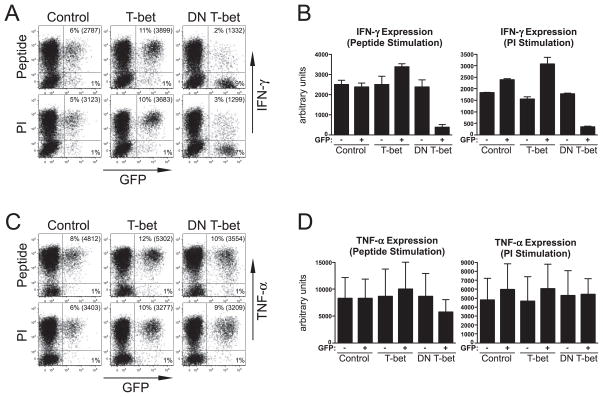
Although enforced T-bet expression effectively rescued IFN-γ expression potential in self-HA recipients (Fig. 5, A and B), it only had a marginal effect in rescuing TNF-α expression potential (Fig. 5, C and D), suggesting that down-modulated T-bet expression in tolerized Th1 effector CD4 cells selectively impairs IFN-γ expression potential. Nevertheless, retroviral T-bet expression did elicit a modest increase in TNF-α expression potential in clonotypic effector CD4 cells recovered from viral-HA recipients (Fig. 5, C and D). Given the lack of evidence that T-bet directly regulates TNF-α this effect might have been indirect (e.g., perhaps resulting from expression (in particular, naive CD4 cells do not express T-bet the increased IFN-γ expression from the CD4 cells that was (Fig. 4A) but are able to express TNF-α (Fig. 1)), we suspected that activating other components of the immune system that, in turn, stimulate the CD4 cells to produce greater TNF-α levels). To more directly assess the CD4 cell-intrinsic ability of T-bet to directly regulate IFN-γ and TNF-α expression in differentiated Th1 effectors, day 6 in vitro-differentiated Th1 clonotypic effector CD4 cells were restimulated and transduced with either the wt T-bet or a DN T-bet retroviral vector (33), and IFN-γ and TNF-α expression potentials were analyzed following an additional 4 days of in vitro culture (Fig. 6). Similar to the result observed in the preceding in vivo transfer experiments (Fig. 5, A and B), Th1 effectors transduced with the wt T-bet retrovirus expressed higher levels of IFN-γ following restimulation with either peptide or PI, compared with control retrovirus-transduced cells. The magnitude of this increase, however, was substantially less in the in vitro system, perhaps because the effect of augmented T-bet expression in enhancing IFN-γ expression in nontolerized effectors can be amplified in vivo through a CD4 cell-extrinsic (i.e., indirect) pathway analogous to that proposed above to explain enhanced TNF-α expression. In contrast to wt T-bet, effectors transduced with the DN T-bet retrovirus exhibited a 7- or 5-fold reduction in IFN-γ expression, compared with controls, following restimulation with peptide or PI, respectively (Fig. 6, A and B). TNF-α expression potential was not substantially altered when T-bet activity was either increased using wt T-bet, or decreased using DN T-bet (Fig. 6, C and D). Taken together, these results indicate that alterations in T-bet activity in differentiated Th1 effector CD4 cells directly regulate IFN-γ, but not TNF-α, expression potential, and that down-modulated T-bet expression in tolerized effector CD4 cells selectively impairs IFN-γ expression potential.
FIGURE 6.
Expression of a DN T-bet in Th1 effectors selectively impairs IFN-γ expression potential. Day 6 in vitro-differentiated Th1 clonotypic effector CD4 cells were restimulated and transduced with either a control, wt T-bet, or a DN T-bet retroviral vector. IFN-γ and TNF-α expression in response to restimulation with either HA peptide or PI was analyzed following an additional 4 days of culture; n = 4 for each group. A, Representative IFN-γ vs GFP expression plots are presented as in Fig. 5A. B, Quantitative analysis of data corresponding to A. C, Representative TNF-α vs GFP expression plots. D, Quantitative analysis of data corresponding to C.
Down-modulated T-bet expression appears to impair IFN-γ mRNA expression potential without altering the chromatin structure of the IFN-γ promoter
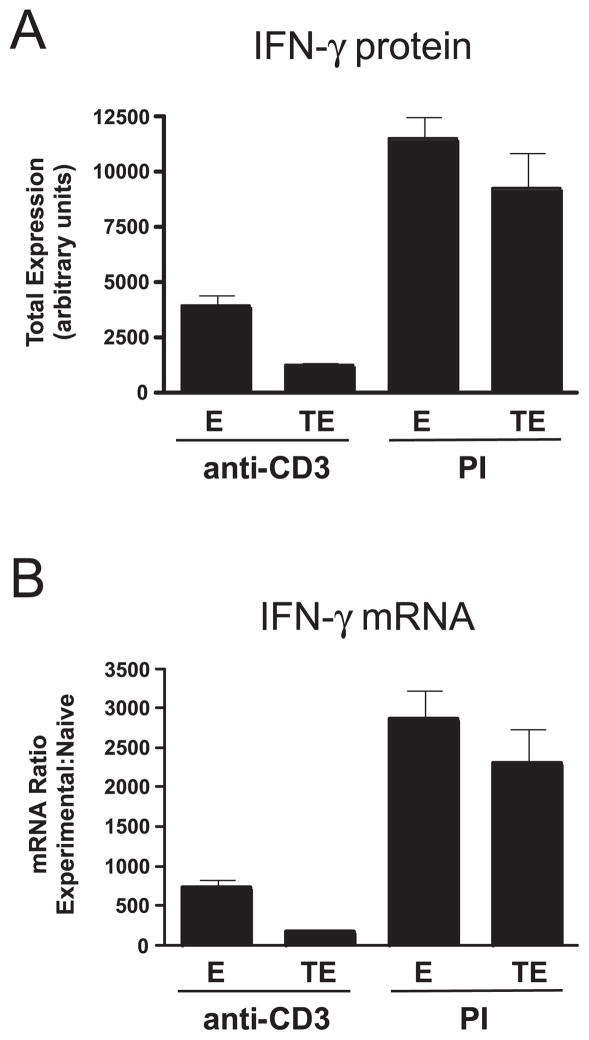
Based on the ability of T-bet to regulate IFN-γ expression at the transcriptional level, we predicted that the reduced ability of tolerized Th1 effector CD4 cells to express IFN-γ resulting from down-modulated T-bet expression would correlate with reduced levels of IFN-γ mRNA. To test this possibility, tolerized Th1 effector clonotypic CD4 cells and nontolerized controls were enriched from adoptive transfer recipients and stimulated with either anti-CD3 or PI, and subsequently, each sample was divided and subjected to intracellular staining to measure IFN-γ protein (Fig. 7A) as well as real-time RT-PCR to measure IFN-γ mRNA levels (Fig. 7B). The differences in IFN-γ protein expression between enriched effector and tolerized effector clonotypic CD4 cells following stimulation with anti-CD3 or PI were similar to that earlier observed in non-enriched cells stimulated with HA peptide-pulsed APCs or PI, respectively (compare with Fig. 2). Importantly, the relative differences in IFN-γ mRNA levels between samples (Fig. 7B) was very similar to the differences in IFN-γ protein levels (Fig. 7A), confirming that in tolerized effector CD4 cells, reduced IFN-γ protein expression is associated with a proportional decrease in IFN-γ mRNA levels.
FIGURE 7.
Decreased IFN-γ protein expression in tolerized Th1 effector CD4 cells is associated with a proportional decrease in IFN-γ mRNA levels. Primary effectors (E) and HA peptide bolustolerized clonotypic effectors (TE) were enriched from recipient spleens and stimulated with either anti-CD3 or PI. Subsequently, a portion of each sample was subjected to intracellular staining to measure IFN-γ protein (A, presented as total IFN-γ expression as in previous figures) and the remaining cells analyzed by real-time RT-PCR to measure IFN-γ mRNA levels (B, presented as in Fig. 4A); n = 8 for E plus anti-CD3, 5 for E plus PI, 10 for TE plus anti-CD3 and 7 for TE plus PI.
T-bet regulates expression of the IFN-γ gene in CD4 cells by multiple mechanisms. During Th1 differentiation, T-bet facilitates remodeling of the IFN-γ promoter region from a condensed/closed to a decondensed/open configuration that is competent for transcription (27, 28, 36), and in primary Th1 effectors, continued T-bet expression appears to be required to maintain this open chromatin configuration (33). Additionally, once the IFN-γ promoter has been remodeled, T-bet can bind to specific sequence elements to enhance transcription (26, 37–40). Acetylation of histones bound to the IFN-γ promoter is an early T-bet-dependent chromatin remodeling event that correlates with transcriptional competence (28, 36). Thus, to assess whether decreased T-bet expression in tolerized Th1 effector CD4 cells might be limiting IFN-γ expression potential by reverse-modeling the chromatin structure of the IFN-γ promoter region from a decondensed back to a condensed configuration, we analyzed the extent of histone H3 acetylation in the proximal promoter region using a ChIP assay (28, 36). Because it was only possible to enrich a relatively small number of clonotypic CD4 cells from adoptive transfer recipients (typically 3–5 × 105, data not shown), quantitative real-time PCR was used to amplify a DNA fragment corresponding to the IFN-γ proximal promoter that was precipitated with an anti-acetylated histone H3 Ab, and normalized for the amount of input DNA using primers specific for the CD3ε enhancer (which should be associated with acetylated histones in all samples given that FACS analysis indicated that surface CD3ε protein expression is comparable between clonotypic naive, effector, and tolerized effector CD4 cells, data not shown).
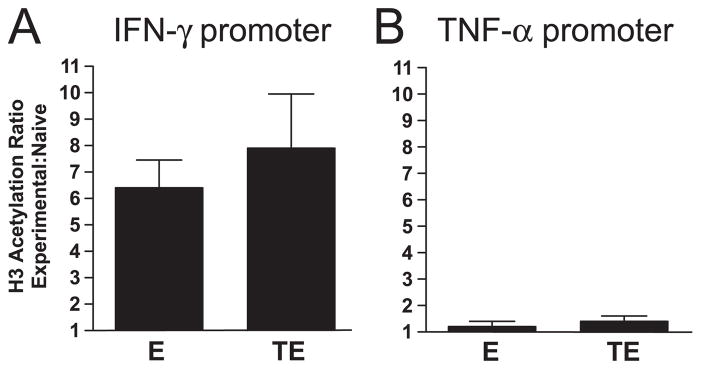
Th1 clonotypic effectors exhibited a 6.5-fold increase in histone acetylation at the IFN-γ promoter relative to naive clonotypic CD4 cells (Fig. 8A). As an additional validation of this assay, no difference in the extent of histone acetylation was observed at the TNF-α promoter between clonotypic naive and Th1 effector CD4 cells (Fig. 7B), consistent with the potential of naive CD4 cells to express TNF-α (Fig. 1). Interestingly, histone acetylation at the IFN-γ promoter in tolerized effectors was comparable, if not slightly greater, than nontolerized counterparts (Fig. 8A), and similarly, histone acetylation at the TNF-α promoter was not altered during effector tolerization (Fig. 8B). These data suggest that down-modulated T-bet expression in tolerized Th1 effector CD4 cells does not impair IFN-γ (or TNF-α) expression potential through alterations in chromatin structure.
FIGURE 8.
Down-modulation of T-bet expression in tolerized Th1 effector CD4 cells does not alter histone acetylation at the IFN-γ promoter. Primary effectors (E) and HA peptide bolus-tolerized clonotypic effectors (TE) were enriched from recipient spleens, and ChIP analysis performed using real-time PCR to assess acetylation of histone H3 at the IFN-γ (A) and TNF-α (B) promoters. Data are presented as the ratio of H3 acetylation between either E or TE and a representative naive clonotypic CD4 cell sample; n = 7 and 6 for E and TE, respectively.
Discussion
The mechanisms that impair the ability of CD4 cells to proliferate and express IL-2 during the development of nonresponsiveness/anergy have been the subject of considerable investigation (reviewed in Ref. 8), however, relatively little is known regarding the mechanisms that impair the expression of effector cytokines such as IFN-γ. This is largely because peripheral CD4 cell tolerance has been studied primarily using either in vitro anergy models in which proliferative and IL-2, but not IFN-γ, expression potentials are impaired (8), or in vivo adoptive transfer systems in which naive CD4 cells (whose IFN-γ gene locus has not yet undergone chromatin remodeling to become transcriptionally competent; Refs. 28, 36, 41, 42) are exposed to tolerizing forms of Ag (e.g., Refs. 43–45). Our Th1 effector CD4 cell tolerization system is somewhat unique in this regard given that the loss of IFN-γ, and also TNF-α, expression potential is lost more rapidly than IL-2 expression and proliferative potentials (11, 16).
To begin probing the intracellular signaling defects responsible for impaired cytokine expression potentials in tolerized Th1 effector CD4 cells, we assessed whether these defects could be overcome using agents that bypass TCR-proximal signaling. This strategy fully rescued IL-2 expression, consistent with previous in vitro (46–50) and in vivo (51) tolerance studies that have found TCR-proximal signaling defects that impair proliferative and IL-2 expression potentials. Interestingly, bypassing TCR-proximal signaling in our system only partially rescued IFN-γ and TNF-α expression potentials, indicating the existence of a TCR-distal signaling defect(s) that impairs effector cytokine expression potentials. In attempting to identify this putative TCR-distal signaling defect(s), we quantitatively analyzed expression levels of mRNA encoding factors that play important roles in Th1 differentiation and function. Expression of T-bet, which regulates IFN-γ expression in CD4 cells (26, 29) at the transcriptional and chromatin structural levels (26–28, 36 – 40), was down-modulated ~30%, compared with nontolerized counterparts. Although it appeared to be rather modest, this reduction in T-bet expression was similar in magnitude to the reduction in the ability of these cells to express IFN-γ in response to PI stimulation. Additionally, there was also a coordinate reduction in expression of the IL-12R β subunit mRNA (which is regulated by T-bet; Refs. 27, 30, 33) but not the IL-18R α subunit mRNA (which is not regulated by T-bet; Ref. 30), as well as a reduced ability to express IFN-γ in response to IL-12 + IL-18 stimulation. Interestingly, it has previously been shown that naive CD4 cells that have been induced to undergo peripheral tolerization while simultaneously receiving enforced OX40 co-stimulation are able to express robust levels of IFN-γ in response to subsequent stimulation with either IL-12 + IL-18 or PI (52), consistent with the possibility that enforced OX40 costimulation is able to induce and/or maintain T-bet expression under tolerizing conditions. Nevertheless, our current data indicate that T-bet can become down-modulated in Th1 effector CD4 cells that have encountered tolerizing Ag under steady-state conditions.
Enforcing T-bet expression during Th1 effector CD4 cell tolerization selectively rescues the ability to express IFN-γ, but not TNF-α, and conversely blocking T-bet activity in nontolerized Th1 effectors using a DN T-bet selectively impairs the ability to express IFN-γ, but not TNF-α. Because Th1 clones no longer require T-bet to express IFN-γ (33), our current observation that alterations in T-bet activity influence IFN-γ expression potential in day 6 primary Th1 effectors (Figs. 5 and 6) might reflect that the IFN-γ promoter is only partially demethylated at this time point (33), and that continued T-bet expression might be required to prevent binding of the methylated DNA-associated corepressor mSin3a to the IFN-γ promoter (39). Thus, while our current study indicates that down-modulated T-bet expression can contribute to a TCR-distal signaling defect that selectively impairs IFN-γ expression potential in primary Th1 effector CD4 cells exposed to tolerizing Ag, it might be possible that altered T-bet expression levels play a less important role in the tolerization of more terminally differentiated Th1 effector or memory CD4 cells in which the IFN-γ promoter is likely to be more fully demethylated (33).
It is not clear what causes T-bet to become down-modulated in tolerized Th1 effector CD4 cells, although given that T-bet is regulated by STAT1 (30), one possibility is that suboptimal exposure to IFN-γ during encounter with tolerizing Ag results in suboptimal STAT1 activation and hence down-modulated T-bet expression. Nevertheless, it initially appeared that the resulting decrease in IFN-γ expression potential could have been caused by at least two separate mechanisms. During naive → Th1 effector differentiation, T-bet induces chromatin remodeling of the IFN-γ promoter region from a condensed to a decondensed (i.e., transcriptionally competent) configuration (27, 28, 36). Although histone acetylation coinciding with transcriptional competence occurs before the day 6 postpriming time point (28, 36) when tolerization was induced in the current study, DNA demethylation which might stabilize this open chromatin configuration is only partial at this time point, and inhibition of T-bet activity via DN T-bet results in reverse-modeling of the IFN-γ gene as indicated by the loss of a T-bet-induced hypersensitive site in the first intron (33). This prompted us to ask whether the partial down-modulation of T-bet expression during Th1 effector tolerization might promote reverse-modeling of the IFN-γ promoter region back to a condensed configuration, thus limiting IFN-γ expression potential. Analysis of histone acetylation at the IFN-γ promoter in tolerized and nontolerized Th1 effector CD4 cells suggests, however, that reverse-modeling does not occur in this system. It thus appears that maximal T-bet expression is not required to maintain the IFN-γ gene in an open chromatin configuration, but is nevertheless required for maximal IFN-γ expression. Given that down-modulated T-bet expression in tolerized Th1 effectors does not appear to limit IFN-γ expression through alterations in the chromatin structure of the IFN-γ gene, this effect could be mediated via reduced transcriptional initiation at a decondensed promoter (26, 37–40).
Our current data are consistent with the possibility that down-modulated T-bet expression in tolerized Th1 effector CD4 cells plays a pivotal role in mediating the TCR-distal defect that selectively impairs IFN-γ expression potential. First, the magnitude of diminished IFN-γ expression following stimulation with PI (i.e., when the TCR-proximal signaling defect is bypassed) is comparable to the decrease in T-bet mRNA, and a direct correlation between the levels of T-bet and IFN-γ expression has previously been observed in Th1 effectors generated from T-bet haploinsufficient mice (29). Additionally, enforcing T-bet expression during tolerization via retroviral transduction prevents the TCR-distal defect from developing. It might be possible, however, that down-modulated expression of other critical transcription factors also contributes to impaired IFN-γ expression given the possibility that retroviral transduction might result in a supraphysiological level of T-bet expression which could potentially compensate for additional deficiencies. The transcription factors AP-1 and NFAT are unlikely to be involved in such a scenario, given that they both play a role in regulating IL-2 expression (8), and PI stimulation (which activates the respective protein kinase C and calcium pathways that activate these factors) elicits comparable IL-2 expression in both tolerized and nontolerized Th1 effectors.
In summary, this study extends the range of the known functions of T-bet in CD4 cells. Thus, in addition to its well-established role as an essential factor that is required to direct the differentiation of naive CD4 cells into Th1 effectors, it can also play a role in the peripheral tolerization of Th1 effectors.
Acknowledgments
We thank L. Lefrancois, Z. Li, W. Nelson, and Q. Yang for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grants AI057441 and CA109339 (to A.J.A.).
Abbreviations used in this paper: HA, hemagglutinin; MFI, mean fluorescence intensity; ChIP, chromatin immunoprecipitation; wt, wild type; DN, dominant negative; NT, nontransgenic; vacc-HA, recombinant vaccinia expressing HA; PI, PMA plus ionomycin.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kappler J, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 3.Sebzda E, V, Wallace A, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 5.Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science. 1990;250:1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- 6.Carlow DA, Teh SJ, van Oers NS, Miller RG, Teh HS. Peripheral tolerance through clonal deletion of mature CD4+CD8+ T cells. Int Immunol. 1992;4:599–610. doi: 10.1093/intimm/4.5.599. [DOI] [PubMed] [Google Scholar]
- 7.Zhang LI, Martin DR, Fung-Leung WP, Teh HS, Miller RG. Peripheral deletion of mature CD8+ antigen-specific T cells after in vivo exposure to male antigen. J Immunol. 1992;148:3740–3745. [PubMed] [Google Scholar]
- 8.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 10.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169:3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreuwel HT, Aung S, Silao C, Sherman LA. Memory CD8+ T cells undergo peripheral tolerance. Immunity. 2002;17:73–81. doi: 10.1016/s1074-7613(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 13.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler AJ. Peripheral tolerization of effector and memory T cells: implications for autoimmunity and tumor-immunity. Curr Immunol Rev. 2005;1:21–28. doi: 10.2174/1573395052952879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-α and IFN-γ expression potentials. Cell Immunol. 2003;224:114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor α on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF α in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 19.von Herrath MG, Oldstone MB. Interferon-γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seewaldt S, Thomas HE, Ejrnaes M, Christen U, Wolfe T, Rodrigo E, Coon B, Michelsen B, Kay TW, von Herrath MG. Virus-induced autoimmune diabetes: most β-cells die through inflammatory cytokines and not perforin from autoreactive (antiviral) cytotoxic T-lymphocytes. Diabetes. 2000;49:1801–1809. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 21.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Z, Blankenstein T. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN γ receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda H, Old LJ, Schreiber RD. The roles of IFN γ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 24.Poehlein CH, Hu HM, Yamada J, Assmann I, Alvord WG, Urba WJ, Fox BA. TNF plays an essential role in tumor regression after adoptive transfer of perforin/IFN-γ double knockout effector T cells. J Immunol. 2003;170:2004–2013. doi: 10.4049/jimmunol.170.4.2004. [DOI] [PubMed] [Google Scholar]
- 25.Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Immunotherapy of melanoma: a dichotomy in the requirement for IFN-γ in vaccine-induced antitumor immunity versus adoptive immunotherapy. J Immunol. 2001;166:7370–7380. doi: 10.4049/jimmunol.166.12.7370. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 27.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 28.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 29.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 30.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 31.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 33.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable TH1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 34.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Murphy TL, Ouyang W, Murphy KM. Induction of interferon-γ production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999;29:548–555. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 37.Cho JY, Grigura V, Murphy TL, Murphy K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-γ promoter. Int Immunol. 2003;15:1149–1160. doi: 10.1093/intimm/dxg113. [DOI] [PubMed] [Google Scholar]
- 38.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Tong Y, Aune T, Boothby M. T-bet antagonizes mSin3a recruitment and transactivates a fully methylated IFN-γ promoter via a conserved T-box half-site. Proc Natl Acad Sci USA. 2005;102:2034–2039. doi: 10.1073/pnas.0409510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki Y, Ihara K, Matsuura N, Kohno H, Nagafuchi S, Kuromaru R, Kusuhara K, Takeya R, Hoey T, Sumimoto H, Hara T. Identification of a novel type 1 diabetes susceptibility gene, T-bet. Hum Genet. 2004;115:177–184. doi: 10.1007/s00439-004-1146-2. [DOI] [PubMed] [Google Scholar]
- 41.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 43.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 44.Lanoue A, Bona C, von Boehmer H, Sarukhan A. Conditions that induce tolerance in mature CD4+ T cells. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaSalle JM, Tolentino PJ, Freeman GJ, Nadler LM, Hafler DA. Early signaling defects in human T cells anergized by T cell presentation of autoantigen. J Exp Med. 1992;176:177–186. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quill H, Riley MP, Cho EA, Casnellie JE, Reed JC, Torigoe T. Anergic Th1 cells express altered levels of the protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:2887–2893. [PubMed] [Google Scholar]
- 48.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 49.Fields PE, Gajewski TF, Fitch FW. Blocked ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 50.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 51.McKay DB, Irie HY, Hollander G, Ferrara JL, Strom TB, Li Y, Burakoff SJ. Antigen-induced unresponsiveness results in altered T cell signaling. J Immunol. 1999;163:6455–6461. [PubMed] [Google Scholar]
- 52.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]