Abstract
The ability of mature T lymphocytes to develop effector capacity after encounter with cognate Ag is generally dependent upon inflammatory signals associated with infection that induce dendritic cell activation/maturation. These inflammatory signals can derive directly from pathogens or can be expressed by host cells in response to infection. Heat shock proteins (HSPs) are a class of host-derived inflammatory mediators that perform the duel function of both chaperoning MHC class I-restricted epitopes into the cross-presentation pathway of DCs and inducing the activation/maturation of these DCs to allow priming of cognate CD8+ T cell effector responses. Although the ability of HSPs to elicit effector CD8 cell responses has been well established, their potential to prime CD4 cell effector responses has been relatively unexplored. In the current study we compared the ability of the endoplasmic reticulum-resident HSP gp96 to prime CD4 vs CD8 cells using TCR transgenic adoptive transfer systems and soluble gp96-peptide complexes. As expected, gp96 facilitated the cross-presentation of a class I-restricted peptide and priming of effector function in cognate CD8 cells. Interestingly, gp96 also facilitated the in vivo presentation of a class II-restricted peptide; however, the resulting CD4 cell response did not involve the development of effector function. Taken together, these data suggest that gp96 is an inflammatory mediator that selectively primes CD8 cell effector function.
Mature T lymphocytes can be divided into two major subsets based on their expression of either CD4 or CD8 co-receptors. Both subsets remain in a functionally quiescent state before contact with their cognate Ags, but can be primed to differentiate into effector T cells after exposure to these Ags either in the context of infection or when admixed with adjuvant. In contrast, antigenic exposure in the absence of inflammation generally results in nonproductive or tolerogenic T cell responses.
Inflammatory mediators that promote effector T cell responses appear to function by inducing the activation/maturation of dendritic cells (DCs)3 that present cognate Ags (1–4) and can derive directly from pathogens (e.g., LPS) (5–7) or can be host factors that are induced by infection (e.g., type I IFNs) (8). A third class of inflammatory signals includes intracellular host factors that are present constitutively, but only made accessible to DCs after necrosis (e.g., genomic DNA (9) and uric acid (10)). A particularly interesting example falling into this category is the heat shock proteins (HSPs). HSPs are abundant, extremely well conserved in evolution, function primarily as chaperones during protein synthesis and folding, and protect cells from mechanical and thermal stresses. HSPs can also induce Ag-specific effector CD8 cell responses that can protect hosts from both infectious and malignant challenges (reviewed in Ref. 11). The immunogenic properties of HSPs appear to stem from at least two separate activities. Similarly to other inflammatory mediators, HSPs can induce dendritic cell maturation/activation (12–15) and migration into draining lymphoid organs (16), but also possess the unique ability to chaperone antigenic peptides into the cross-presentation pathway of dendritic cells via specific surface receptors (14, 17–19). Given that HSPs are released from necrotic cells (13, 20) along with their abilities to facilitate Ag presentation and to activate APCs, it has been postulated that they might play a role in priming effector T cell responses to lytic intracellular pathogens (11).
The ability of HSPs to facilitate the cross-presentation of MHC class I-restricted epitopes and to prime CD8 cell effector responses has been well established (11); however, the potential for HSPs to facilitate the presentation of MHC class II-restricted epitopes and to prime CD4 cells has been relatively unexplored. In the current study we compared the ability of the endoplasmic reticulum-resident HSP gp96 to prime CD4 vs CD8 cells using TCR-transgenic adoptive transfer systems and soluble gp96-peptide complexes. As expected, gp96 facilitated the cross-presentation of a class I-restricted peptide and priming of effector function in cognate CD8 cells. Interestingly, gp96 also facilitated the in vivo presentation of a class II-restricted peptide; however, the resulting CD4 cell response did not involve the development of effector function. Taken together, these data suggest that gp96 is an inflammatory mediator that selectively primes CD8 cell effector function.
Materials and Methods
Mice and adoptive transfers
6.5 TCR transgenic mice (clonotypic CD4 cell adoptive transfer donors) express a clonotypic TCR that recognizes an I-Ed-restricted HA epitope (110SFERFEIFPKE120) (21) and were backcrossed to a B10.D2 (H-2d), Thy1.1+ congenic background. Clone 4 TCR transgenic mice (clonotypic CD8 cell adoptive transfer donors) express a clonotypic TCR that recognizes a Kd-restricted HA epitope (542IYSTVASSL550) (22) and were also on the Thy1.1+, B10.D2 congenic background. Nontransgenic (NT) adoptive transfer recipients were on the B10.D2, Thy1.2+ background.
Adoptive transfers of 2.5 × 106 naive Thy1.1+ CFSE-labeled 6.5 clonotypic CD4 cells into Thy1.2+ nontransgenic (NT) recipients were performed as previously described (23–25). In short, naive clonotypic CD4 cells were prepared from lymph nodes (LN) of 6.5 donors, depleted of CD8 cells using CD8-conjugated magnetic beads (Dynal Biotech, Lake Success, NY), labeled with the fluorescent dye CFSE and injected into the retro-orbital sinus of recipient mice. Adoptive transfers of 1 × 106 naive Thy1.1+ CFSE-labeled clone 4 clonotypic CD8 cells were performed similarly, except that donor cell preparations were depleted of CD4 cells using magnetic beads.
Generation of soluble gp96-peptide complexes, immunizations, and FACS analysis
gp96-peptide complexes were generated following the previously described protocol (26). In short, soluble synthetic peptides were mixed with soluble gp96 purified from mouse liver and kidney (27) at a molar ratio of 50:1 in PBS, heated to 50°C for 10 min, cooled at room tenperatute for 30 min, and subsequently washed several times using Amicon Ultra filters with a 30 kDa cut-off (Millipore, Bedford, MA) to remove noncomplexed peptides. The same preparation of gp96 was used throughout this study. Mouse serum albumin (MSA; Sigma-Aldrich, St. Louis, MO)-peptide complexes were generated using a similar protocol, except that Amicon filters with a 10-kDa cut-off were used.
Immunizations with gp96 and MSA peptide complexes, soluble peptide, and 105 PFU of recombinant vaccinia virus expressing HA (vacc-HA) were performed intradermally at the midline of the abdomen, and the clonotypic T cells were recovered 4 days later from the draining inguinal, brachial, and axillary LN for analysis.
Intracellular cytokine staining of clonotypic T cells after 5-h restimulation with the appropriate class I- or class II-restricted HA peptides (100 μg/ml) was performed as previously described (23–25) with the following modifications. Clonotypic CD4 cells (gated as Thy1.1+ and CFSEdim) were stained with PE-conjugated anti-IFN-γ and allophycocyanin-conjugated anti-IL-4 or PE-conjugated anti-IL-2 and allophycocyanin-conjugated anti-TNF-α, and clonotypic CD8 cells (also gated as Thy1.1+ and CFSEdim) were stained with PE-conjugated anti-IFN-γ and allophycocyanin-conjugated TNF-α (BD PharMingen, San Diego, CA).
Results
gp96 can facilitate the in vivo presentation of MHC class II-restricted epitopes
It has been well established that several HSPs can chaperone MHC class I-restricted epitopes into in vivo cross-presentation pathways and elicit CD8 cell effector responses (11). These HSPs can derive from either the cytoplasm (e.g., hsp70) or the endoplasmic reticulum (e.g., gp96), and chaperone the endogenous peptides that they naturally complex with. Comparable CD8 cell effector responses can also be elicited by HSPs that are complexed to synthetic peptides in vitro (26). To examine the potential of HSP-peptide complexes to induce CD4 cell responses, we used our previously established model in which CFSE-labeled naive Thy1.1+ clonotypic 6.5 TCR transgenic CD4 cells specific for influenza hemagglutinin (HA) are adoptively transferred into Thy1.2-expressing NT recipient mice, and their proliferative and functional responses are tracked after exposure to various forms of HA (23).
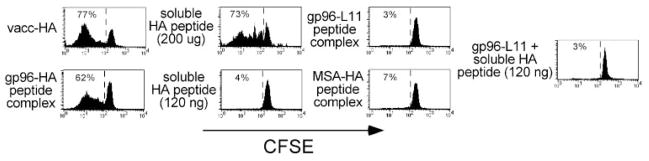
When 70 μg of gp96 complexed to the cognate I-Ed-restricted HA peptide was injected intradermally into adoptive transfer recipients, and the response of the clonotypic CD4 cells was assessed 4 days later after recovery from the draining LN, these clonotypic CD4 cells were found to have undergone a significant proliferative response, as evidenced by their diluted levels of the fluorescent dye CFSE (Fig. 1). Roughly similar proliferative responses were elicited by vacc-HA as well as a large bolus of soluble HA peptide (200 μg). Several controls indicated that clonotypic CD4 cell proliferation elicited by gp96-HA peptide complexes was mediated through the ability of gp96 to act as a chaperone in delivering the class II-restricted HA peptide to be presented by APCs. First, clonotypic CD4 cells exposed to 120 ng of soluble HA peptide (the amount that would be complexed to 70 μg of gp96 if the complexing efficiency were 10%, which is the upper limit of complexing efficiencies (26)) underwent negligible proliferation. Additionally, 70 μg of gp96 complexed to the unrelated I-Ed-restricted peptide L11 (28), as well as HA peptide complexed to 70 μg of MSA (a non-HSP control) both elicited negligible proliferation. Finally, admixture of noncomplexed soluble HA peptide (120 ng) plus 70 μg of gp96-L11 complexes resulted in negligible proliferation, indicating that gp96 and HA peptide must be physically associated to induce clonotypic CD4 cell proliferation.
FIGURE 1.
gp96 can facilitate in vivo presentation of MHC class II-restricted epitopes. Naive Thy1.1+ CFSE-labeled clonotypic CD4 cells were adoptively transferred into Thy1.2+ NT recipients that were simultaneously immunized intradermally with vacc-HA, 200 μg or 120 ng of soluble HA peptide, 70 μg of gp96-HA peptide complexes, 70 μg of gp96-L11 peptide complexes, 70 μg of MSA-HA peptide complexes, or 70 μg of gp96-L11 complexes and 120 ng of soluble HA peptide. Four days later, clonotypic CD4 cells (Thy1.1+) were recovered from the draining LNs and analyzed for their proliferative response (i.e., CFSE dilution). Each histogram is representative of three separate replicates.
gp96 cannot elicit CD4 cell effector function
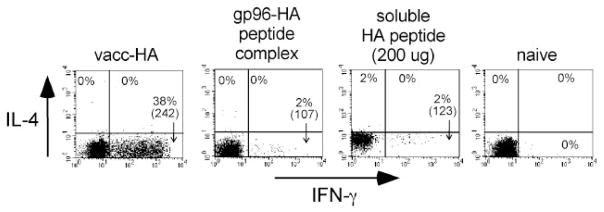
To characterize the functional capacity of the clonotypic CD4 cells that had been induced to undergo proliferation by gp96-HA peptide complexes, we assessed their ability to express effector cytokines by intracellular staining after in vitro restimulation with HA peptide-pulsed APCs (Fig. 2). Consistent with our previous results (23–25), naive clonotypic CD4 cells could not express either the Th1 effector cytokine IFN-γ or the Th2 effector cytokine IL-4 (presumably because the promoter regions of the these effector cytokine genes exist in a condensed or inaccessible configuration (reviewed in Ref. 29), whereas clonotypic CD4 cells primed with vacc-HA developed the capacity to express high levels of IFN-γ, but not IL-4. Also as predicted, clonotypic CD4 cells exposed to a large bolus of soluble HA peptide (200 μg) that is known to induce tolerogenic differentiation (24, 30) could not express significant levels of either effector cytokine. Importantly, although gp96-HA peptide complexes elicited clonotypic CD4 cell proliferation (Fig. 1), these clonotypic CD4 cells did not develop the capacity to express either IFN-γ or IL-4 (Fig. 2). Titration experiments indicated that decreasing the dose of immunizing gp96-HA peptide complex from 70 to 7 μg and 1 μg resulted in a progressive decline in the percentage of clonotypic CD4 cells that underwent division (with 1 μg eliciting negligible division), whereas increasing the dose to 140 μg did not increase proliferation compared with 70 μg. Furthermore, at none of these immunizing doses did the divided clonotypic CD4 cells acquire the capacity to express effector cytokines (data not shown).
FIGURE 2.
gp96 cannot elicit CD4 cell effector function. Clonotypic CD4 cells recovered from recipients described in Fig. 1 that had undergone significant proliferation were restimulated in vitro with HA peptide-pulsed APCs and subsequently stained for intracellular IFN-γ and IL-4 expression. The naive group derived from the undivided cells that had been exposed to gp96-L11 peptide complexes. FACS plots show IFN-γ vs IL-4 expression, with the percentage of clonotypic CD4 cells expressing each cytokine (as well as the level of IFN-γ expression (mean fluorescence intensity) in parentheses) shown for each quadrant. Each plot is representative of three separate replicates. MiCK-2 cells (BD PharMingen) were used to verify that staining reactions contained a saturating concentration of anti-IL-4 mAb (data not shown).
gp96 elicits both CD8 cell proliferation as well as effector function
Given the ability of gp96-peptide complexes to elicit CD8 cell effector responses in normal mice (26), the preceding experiments using adoptively transferred TCR transgenic CD4 cells, which indicated that gp96 can chaperone MHC class II-restricted peptides to be presented in vivo, but that the ensuing CD4 cell response does not involve the development of effector function, suggested that there might be an inherent difference in the ability of gp96-peptide complexes to prime CD8 vs CD4 cell effector functions. Although the TCR transgenic CD4 cells developed effector function (i.e., IFN-γ expression potential) in response to vacc-HA, we wanted to confirm that the inability of gp96-peptide complexes to elicit CD4 cell effector function was not due to a limitation in the adoptive transfer technique. Thus, we performed analogous adoptive transfer experiments using TCR transgenic CD8 cells specific for a Kd-restricted HA epitope (i.e., clone 4 CD8 cells) (22) and cognate gp96-HA peptide complexes.
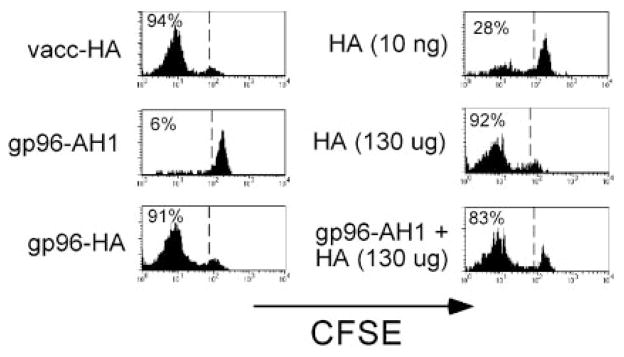
As predicted based on previous adoptive transfer studies using clonotypic clone 4 CD8 cells (31), naive clonotypic CD8 cells underwent robust CFSE dilution in response to both vacc-HA as well as a large bolus of soluble Kd-restricted HA peptide (130 μg; Fig. 3). Immunization with 7 μg of gp96 complexed to the Kd-restricted HA peptide also induced the clonotypic CD8 cells to under robust CFSE dilution. This proliferative response was mediated through the ability of gp96 to chaperone the class I-restricted HA peptide into the cross-presentation pathway, as confirmed by the following controls. gp96 (7 μg) complexed to the irrelevant H-2d class I-restricted peptide AH1 (32) did not induce clonotypic CD8 cell proliferation, and 10 ng of soluble HA peptide (the amount that would be complexed to 7 μg of gp96 if the complexing efficiency were 10%) elicited relatively weak proliferation.
FIGURE 3.
gp96 can facilitate in vivo presentation of MHC class I-restricted epitopes. Naive Thy1.1+ CFSE-labeled clonotypic CD8 cells were adoptively transferred into Thy1.2+ NT recipients that were simultaneously immunized intradermally with vacc-HA, 130 μg or 10 ng of soluble HA peptide, 7 μg of gp96-HA peptide complexes, 7 μg of gp96-AH1 peptide complexes, or 7 μg of gp96-AH1 complexes and 130 μg of soluble HA peptide. Four days later, clonotypic CD8 cells (Thy1.1+) were recovered from the draining LNs and analyzed for their proliferation response (i.e., CFSE dilution). Each histogram is representative of three separate replicates, except for the 7 μg of gp96-AH1 complexes and 130 μg of soluble HA peptide group, which is representative of two replicates.
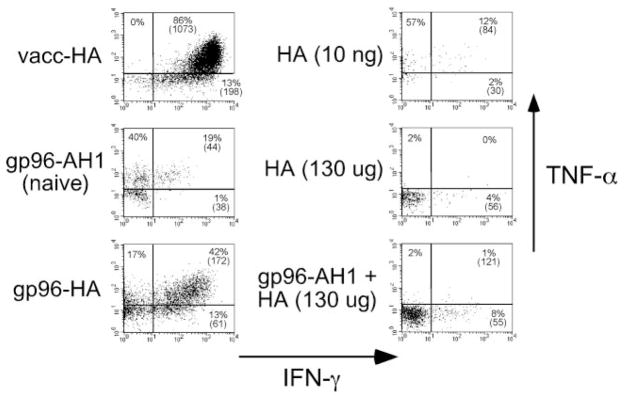
Clonotypic CD8 cells that remained in a naive state after exposure to 7 μg of gp96-AH1 peptide complexes, or that had undergone limited division after exposure to 10 ng of soluble peptide, expressed moderate levels of TNF-α and low levels of IFN-γ after in vitro restimulation with HA peptide-pulsed APCs (Fig. 4). Immunization with vacc-HA induced a dramatic increase in IFN-γ expression potential as well as a slight increase in TNF-α expression potential. In contrast, exposure to a large bolus of HA peptide (130 μg) resulted in a loss of TNF-α expression potential and no development of IFN-γ expression potential, consistent with the capacity of soluble peptide to induce tolerogenic clonotypic CD8 cell responses (31). Importantly, clonotypic CD8 cells immunization with 7 μg of gp96 complexed to HA peptide developed the capacity to express substantial levels of IFN-γ (albeit to a lesser extent than with vacc-HA) and also retained the capacity to express TNF-α. Immunization with 70 μg of gp96-HA peptide complex induced a similar response (data not shown). Clonotypic CD8 cells primed with 7 μg of gp96-AH1 peptide complexes and 130 μg of soluble HA peptide expressed minimal levels of both IFN-γ and TNF-α, indicating that gp96 and HA peptide must be physically associated to elicit clonotypic CD8 cell effector function. Thus, gp96 peptide complexes can elicit the development of effector function in cognate, adoptively transferred, clonotypic CD8 cells.
FIGURE 4.
gp96 can elicit CD8 cell effector function. Clonotypic CD8 cells recovered from the recipients described in Fig. 3 were restimulated in vitro with HA peptide-pulsed APCs and subsequently stained for intracellular IFN-γ and TNF-α expression. The naive group derived from the undivided cells that had been exposed to gp96-AH1 peptide complexes. Representative FACS plots of IFN-γ vs TNF-α expression, with the percentage of clonotypic CD8 cells expressing each cytokine (as well as the level of IFN-γ expression (mean fluorescence intensity) in parentheses) shown for each quadrant.
gp96 does not induce CD4 cell tolerance
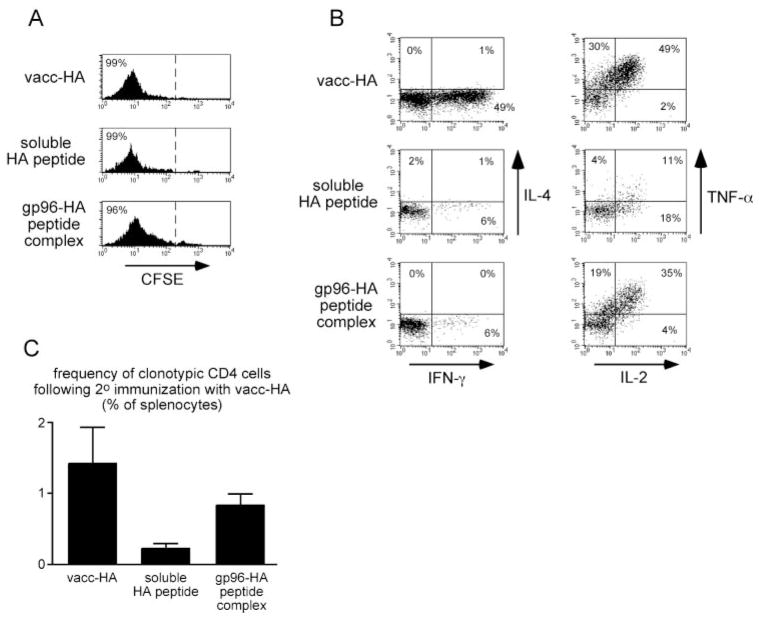
The data presented above indicate that gp96-HA peptide complexes can induce naive clonotypic CD4 cells to undergo proliferation without developing the capacity to express Th1 or Th2 effector cytokines. This response is consistent with the development of a tolerant phenotype (23), although it could have also been immunologically neutral (i.e., these CD4 cells would retain the capacity to respond to a stronger immunogenic signal). We therefore asked whether naive clonotypic CD4 cells that were initially primed with gp96-HA peptide complexes (or exposed to soluble peptide boluses as a tolerogenic control) would be able to respond to secondary immunization with vacc-HA. Pilot experiments indicated that naive clonotypic CD4 cells must be exposed to multiple soluble peptide boluses (at least three) before they lose the capacity to respond to subsequent immunization with vacc-HA (data not shown). Thus, to ascertain whether gp96 induces clonotypic CD4 cell tolerization, adoptive transfer recipients were immunized on 4 consecutive days with either gp96-HA peptide complexes or soluble HA peptide boluses (tolerogenic control) or once with vacc-HA (immunogenic control). After recovery from the draining LNs on the fifth day, virtually all the clonotypic CD4 cells were found to have undergone CFSE dilution in all three recipient groups (Fig. 5A). As expected, vacc-HA-primed clonotypic CD4 cells expressed high levels of IFN-γ, but no IL-4 after in vitro restimulation, such as where soluble HA peptide and gp96-HA peptide complex-primed counterparts could not express much of either effector cytokine. Interestingly, gp96-HA peptide complex-immunized clonotypic CD4 cells were able to express IL-2 and TNF-α (cytokines whose expression do not require Th1 or Th2 differentiation) at levels that were almost as high as in the vacc-HA controls. In contrast, IL-2 and TNF-α expression potentials were lower in the soluble peptide-tolerized controls (Fig. 5B). This result suggests that although gp96 cannot prime Th1 or Th2 CD4 cell effector function, it does not induce tolerance. To extend this analysis, 3.5 × 104 clonotypic CD4 cells from each of the primary adoptive transfer recipient groups were retransferred into secondary recipients that had been immunized with vacc-HA, and 6 days later spleens were analyzed to assess expansion of clonotypic CD4 cells (Fig. 5C). Clonotypic CD4 cells could not be detected in nonimmunized control recipients that received 3.5 × 104 naive clonotypic CD4 cells (data not shown). In response to secondary immunization with vacc-HA, clonotypic CD4 cells that had initially been primed with gp96-HA peptide complexes underwent expansion to ~0.8% of the splenocyte population, such as where soluble peptide-tolerized counterparts expanded 4-fold less. The ability of the peptide-tolerized clonotypic CD4 cells to undergo limited expansion might have been related to their ability to express lower levels of IL-2 (Fig. 5B). Clonotypic CD4 cells that were initially primed with vacc-HA underwent slightly (~70%) greater expansion then counterparts initially primed with gp96-HA peptide complexes, suggesting that vacc-HA-primed effectors are hyper-responsive to secondary immunization. Taken together, these data suggest that gp96-peptide complexes induce neither CD4 cell effector differentiation nor tolerization.
FIGURE 5.
gp96 does not induce CD4 cell tolerance. Naive clonotypic CD4 cell adoptive transfer recipients were immunized intradermally with 200 μg of soluble HA peptide or 52 μg of gp96-HA peptide complex on 4 consecutive days or once with vacc-HA. Clonotypic CD4 cells were recovered from the draining LNs on day 5 and analyzed for CFSE dilution (gating on Thy1.1+ and 6.5 (anti-clonotypic TCR) + cells); A) and intracellular cytokine expression after in vitro restimulation with HA peptide-pulsed APCs (gating on Thy1.1+ and CFSEdim cells; B). Each histogram is representative of three separate replicates. C, Clonotypic CD4 cells (3.5 × 104) recovered from each of the primary adoptive transfer recipients described in A and B were retransferred into vacc-HA-immunized secondary recipients, and their frequencies were measured 6 days later after recovery from spleens. Data are expressed as the mean ± SEM (n = 3 for each group).
Discussion
CD8 cells have previously been shown to have a greater proliferative capacity than CD4 cells (33, 34) as well as more stable memory after viral infection (34, 35). Additionally, in vitro studies have suggested that whereas CD8 cells only require a short period of antigenic stimulation to become programmed to differentiate into effectors (36, 37), CD4 cell effector differentiation generally requires longer periods of antigenic stimulation and/or extrinsic cytokines (38– 40). Our current study appears to be consistent with these previous observations by suggesting that CD4 and CD8 cells might differ in their immunization requirements for developing the capacity to express the effector cytokine IFN-γ. Thus, an immunogen (i.e., gp96-peptide complex) that induces cognate naive CD8 cells to both proliferate and develop the capacity to express IFN-γ can induce cognate naive CD4 cells to proliferate, but not to differentiate into effectors that have the capacity to express IFN-γ. These CD4 cells are not tolerant, however, as they can express high levels of IL-2 and TNF-α and can also undergo expansion in response to secondary immunization with virus. It is interesting to note that a previous study using a tumor model found that immunization with gp96 at doses approximating those used in our study (50 μg) elicited a suppressive CD4 cell activity that inhibited tumor immunity (41). Although our current observation that relatively high dose gp96 immunization does not lead to Ag-specific CD4 cell tolerance might appear surprising given this previous study, it should be pointed out that whereas high dose gp96 immunization suppresses tumor immunity, it does not suppress the development of CTL (P. Srivastava, unpublished observations). Thus, the ability of high dose gp96 immunization to suppress tumor immunity might not necessarily be related to the response of naive CD4 cells specific for peptides chaperoned by gp96. The ability of gp96 to selectively induce IFN-γ expression potential in our system appears to be the consequence of an inherent difference in the immunization requirements for CD4 and CD8 cells to develop effector capacity, rather than a lower avidity of the 6.5 compared with the clone 4 TCR for its respective MHC-peptide ligand or a lower complexing efficiency for the class II-restricted peptide for gp96 compared with the class I-restricted peptide for the following reasons. First, the 6.5 TCR has a high avidity for its cognate I-Ed-peptide ligand (42), and 6.5 clonotypic CD4 cells readily develop IFN-γ expression potential in response to vacc-HA. Additionally, if either of the above-mentioned alternate explanations were correct, immunizing with higher doses of complex or, conversely, adoptively transferring fewer clonotypic CD4 cells (to increase the ratio of immunizing Ag to CD4 cells) should elicit effector function. To the contrary, when 1 × 105 naive clonotypic CD4 cells were adoptively transferred rather than the usual 2.5 × 106, 70 μg of gp96-HA peptide complex was still unable to induce IFN-γ expression potential (data not shown). Along similar lines, immunizing with lower doses of vacc-HA results in a smaller percentage clonotypic CD4 cells undergoing division, but those divided cells do acquire the potential to express IFN-γ (data not shown), suggesting that the absolute level of Ag presentation per se does not determine whether clonotypic CD4 cells develop effector function in this system. Finally, although clonotypic CD4 cells primed with gp96-peptide complexes undergo fewer rounds of division (2–5) compared with vacc-HA (6 or greater), this weaker proliferative response is unlikely to fully account for the lack of effector function, as clonotypic CD4 cells primed with vacc-HA develop the ability to express very high levels of IFN-γ by their second or third division (23). Taken together, these data support the idea that gp96 is an inflammatory mediator that selectively primes CD8 cell effector function.
It is not clear what mechanisms allow gp96 to selectively elicit IFN-γ expression potential in CD8 cells, although they might be related to differences in both the costimulatory signals that are required to elicit CD4 vs CD8 cell effector responses (43– 46) as well as the transcriptional pathways that regulate IFN-γ expression in the two cell types (47, 48). With regard to costimulatory signals, it is worth noting that gp96 can induce B7.2, but not CD40, expression on immature DCs (13), and CD40 has been shown to be important for CD4 cell, but not CD8 cell, responses to lymphocytic choriomeningitis virus (45).
The immunological properties of HSPs such as gp96 were first characterized by the ability of purified preparations to induce CTL and anti-tumor immunity (11). Interestingly, tumor immunity elicited by gp96-based vaccines is less dependent on CD4 than on CD8 cells (49, 50), consistent with our current observation that gp96 can prime CD8, but not CD4, cell effector capacity. As CD4 cell help is not required for primary CD8 cell effector responses, but is required for CD8 memory (51, 52), our data suggest that CD8 cell memory might not be efficiently generated by HSP-peptide complexes. Consistent with this possibility, hsp70-peptide complexes elicit strong primary CD8 cell effector responses, but relatively weak memory (53).
Given that HSPs are released from necrotic cells (13, 20) and can promote DC activation/maturation (12–16), HSPs might play a role in priming effector T cell responses against intracellular lytic pathogens. Our current data suggest that whereas the HSP gp96 can facilitate in vivo presentation of both MHC class I- and class II-restricted peptides as well as prime CD8 cell effector function, overall it is less immunogenic than the lytic virus vaccinia. Thus, gp96 possesses some capacity to prime effector T cell responses, but it (and possibly other HSPs) might need to act in concert with pathogen-derived or pathogen-induced inflammatory mediators to elicit maximal effector responses. Thus, release of HSPs after necrosis induced by noninfectious mechanisms (e.g., physical trauma) would be less likely to elicit effector T cell responses to self-Ags.
Acknowledgments
We are grateful to Zihai Li, Toyoshi Matsutake, and Pramod Srivastava for helpful discussions and reagents, as well as to Linda Sherman for providing the clone 4 mice.
Footnotes
This work was supported by National Institutes of Health Grant AI49813 and Research Scholar Grant RSG-02-235-01-LIB from the American Cancer Society (to A.J.A.).
Abbreviations used in this paper: DC, dendritic cell; HA, hemagglutinin; HSP, heat shock protein; LN, lymph node; MSA, mouse serum albumin; NT, nontransgenic; vacc-HA, recombinant vaccinia expressing HA.
References
- 1.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 2.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 6.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med. 1998;187:763. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 9.Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 12.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12:1539. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 14.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, et al. The endoplasmic reticulum-resident heat shock protein gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 15.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 16.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 17.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 20.Berwin B, Reed RC, Nicchitta CV. Virally induced lytic cell death elicits the release of immunogenic GRP94/gp96. J Biol Chem. 2001;276:21083. doi: 10.1074/jbc.M101836200. [DOI] [PubMed] [Google Scholar]
- 21.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan DJ, Liblau R, Scott S, Fleck HO, McDevitt N, Sarvetnick D, Lo D, Sherman LA. CD8+ cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978. [PubMed] [Google Scholar]
- 23.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 24.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169:3622. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-α and IFN-γ expression potentials. Cell Immunol. 2003;224:114. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- 28.Matsutake T, Srivastava PK. The immunoprotective MHC II epitope of a chemically induced tumor harbors a unique mutation in a ribosomal protein. Proc Natl Acad Sci USA. 2001;98:3992. doi: 10.1073/pnas.071523398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KM, Reiner SL. Decision making in the immune system: The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 30.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719. [PubMed] [Google Scholar]
- 31.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8+ T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 34.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 35.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 36.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 38.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 39.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. Two distinct stages in the transition from naive CD4 T cells to effecters, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunol. 2000;165:5017. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 40.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 43.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 44.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859. [PubMed] [Google Scholar]
- 45.Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R, Slifka MK. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194. [PubMed] [Google Scholar]
- 46.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 47.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295:338. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 48.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 49.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163:5178. [PubMed] [Google Scholar]
- 51.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 52.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumaraguru U, Gierynska M, Norman S, Bruce BD, Rouse BT. Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J Virol. 2002;76:136. doi: 10.1128/JVI.76.1.136-141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]