Abstract
We compared how CD4 vs CD8 cells attain the capacity to express the effector cytokine IFN-γ under both immunogenic and tolerogenic conditions. Although the Ifng gene locus was epigenetically repressed in naive Ag-inexperienced CD4 cells, it had already undergone partial remodeling toward a transcriptionally competent configuration in naive CD8 cells. After TCR stimulation, CD8 cells fully remodeled the Ifng locus and gained the capacity to express high levels of IFN-γ more rapidly than CD4 cells. Enforced dual costimulation through OX40 and 4-1BB redirected CD8 cells encountering soluble exogenous peptide to expand and differentiate into IFN-γ and TNF-α double-producing effectors rather than becoming tolerant. Despite this and the stronger tendency of CD8 compared with CD4 cells to differentiate into IFN-γ-expressing effectors, when parenchymal self-Ag was the source of tolerizing Ag, enforced dual costimulation selectively boosted expansion but did not push effector differentiation in CD8 cells while both expansion and effector differentiation were dramatically boosted in CD4 cells. Notably, enforced dual costimulation was able to push effector differentiation in CD8 cells encountering cognate parenchymal self-Ag when CD4 cells were simultaneously engaged. Thus, the ability of enforced OX40 plus 4-1BB dual costimulation to redirect CD8 cells to undergo effector differentiation was unexpectedly influenced by the source of tolerizing Ag and help was selectively required to facilitate CD8 cell effector differentiation when the tolerizing Ag derived from self.
Following exposure to cognate pathogen-derived Ags, naive CD4 T cells differentiate into either Th1, Th2, Th17, in-ducible regulatory T cell, or Tr1 functional subsets that are marked by the ability to express IFN-γ, IL-4, IL-17, TGF-β, and IL-10, respectively (1, 2). In contrast, CD8 T cells typically develop both lytic capacity as well as the ability to express IFN-γ (1, 3). Although under certain circumstances CD8 cells develop the capacity to express IL-4 (4) and CD4 cells can develop lytic activity (5, 6), IFN-γ represents the most common effector molecule whose expression potential can develop in response to antigenic priming in both T cell populations.
The differentiation of naive CD4 cells into IFN-γ-expressing Th1 effectors is a complex process that is linked to cell cycle progression and epigenetic remodeling of the Ifng gene locus toward an open structure that allows accessibility to the transcriptional machinery (7–13). The T-box transcription factor T-bet drives this process (14) by facilitating chromatin remodeling of the Ifng locus (15), inducing expression of the IL-12 receptor (15, 16) and acting as a transcriptional activator at the Ifng promoter (14, 17). In CD8 cells, T-bet is not essential (18) due to the expression of Eomesodermin (Eomes) which can program IFN-γ expression either by itself or in cooperation with T-bet (19).
Costimulatory molecules belonging to the TNF/TNFR superfamily exert a powerful effect in programming effector T cell responses (20–22), and modulation of these pathways is a strategy being explored to treat cancer and autoimmunity (23–28). In particular, enforced stimulation of the CD134 (OX40) plus CD137 (4-1BB) costimulatory pathways potently redirects CD8 cells responding to exogenous soluble Ags to undergo both robust expansion and effector differentiation rather than tolerization and can also augment tumor immunity (29–31).
In the current study, we used a combination of in vitro- priming and in vivo TCR-transgenic adoptive transfer systems to compare several parameters of CD4 vs CD8 cell function that relate to effector differentiation and tolerization. In contrast to naive CD4 cells that are unable to express IFN-γ (7–12), in naive CD8 cells the Ifng locus was already partially remodeled and low-level IFN-γ expression was elicited by stimulants that bypass TCR signaling. Following antigenic priming, naive CD8 cells fully remodeled their Ifng loci and gained the capacity to express high levels of IFN-γ more rapidly than CD4 cells. Despite this stronger tendency of CD8 cells to undergo effector differentiation and the ability of enforced OX40 plus 4-1BB dual costimulation to redirect CD8 cells responding to soluble foreign Ag to undergo robust expansion and effector differentiation, in response to parenchymally derived self-Ag enforced OX40 plus 4-1BB dual costimulation augmented clonal expansion of both CD8 and CD4 cells, but only pushed effector differentiation in CD4 cells. Additionally, similar to the previous observation that CD4+ helper T cells can facilitate Ifng locus remodeling in CD8 cells primed in vitro or by viral infection in vivo (32, 33), we found that when CD4 and CD8 cells simultaneously encountered cognate self-Ag during enforced OX40 plus 4-1BB dual costimulation, the former facilitated effector differentiation in the latter. Thus, help was selectively required to facilitate enforced OX40 plus 4-1BB dual costimulation-mediated effector differentiation when CD8 cells encounter cognate self-Ag but not soluble peptide. Taken together, these surprising results indicate that the response of CD8 cells to enforced OX40 plus 4-1BB dual costimulation is strongly influenced by the source of tolerizing Ag. Since tumor Ags are in and of themselves a form of self-Ag and can induce T cell tolerance (34–37), these results raise important considerations in translating the use of costimulatory agonists to enhance tumor immunity.
Materials and Methods
TCR-transgenic T cell adoptive transfers and flow cytometry
All mice were on the B10.D2 genetic background. Adoptive transfer of the indicated number of naive hemagglutinin (HA)3-specific Thy1.1+ CFSE-labeled CD8-depleted 6.5 TCR-transgenic CD4 cells (38) and CD4-depleted clone 4 TCR-transgenic CD8 cells (39) into Thy1.2+ recipients that either express influenza HA as a generic parenchymal self-Ag (self-HA, transgenic founder line no.137 (40)), nontransgenic (NT) mice infected i.p. with 106 PFU of a recombinant vaccinia virus expressing HA (viral HA, given 1 day before adoptive transfer) or NT mice treated with i.p. boluses (100 μg) of soluble Kd-restricted HA peptide (given concurrent with adoptive transfer) and subsequent functional analyses were performed as previously described (41–43). In brief, TCR-transgenic CD4 or CD8 cells (identified as Thy1.1+) were recovered from spleens at the indicated times and analyzed for CFSE dilution or intracellular granzyme B expression directly ex vivo and intracellular cytokine expression following 5 h of in vitro restimulation with the respective cognate I-Ed- or Kd-restricted HA peptide or PMA plus ionomycin (PMA plus I) in the presence of brefeldin A. Total intracellular cytokine expression (expressed in arbitrary units) was calculated as the percentage of cytokine-expressing CD4 or CD8 cells multiplied by the mean fluorescence intensity (MFI) of cytokine staining on the cytokine+ cells as previously described (41). As indicated, Thy1.1+ double-transgenic mice containing both the clone 4 and the human Bcl-2 (44) transgenes were used as adoptive transfer donors. Also as indicated, agonistic anti-4-1BB (25 μg) and/or anti-OX40 (50 μg) mAbs were administered i.p. to adoptive transfer recipients 1 day following adoptive T cell transfer as previously described (29).
Intracellular IFN-γ staining of naive polyclonal single-positive thymocytes and peripheral T cells
NT lymph node (LN)-derived CD4 or CD8 cells and CD4+CD8− or CD8+CD4− single-positive thymocytes (double-positive and the reciprocal single-positive thymocytes were removed using the appropriate anti-CD4 or anti-CD8-conjugated magnetic beads; Dynal) were stimulated in vitro for 5 h with brefeldin A and either plate-bound anti-CD3 mAb or PMA plus I, and subsequently stained with anti-CD44 allophycocyanin and either anti-CD4 PerCP (BD Pharmingen) or anti-CD8 FITC (eBioscience), fixed, permeabilized, and then stained with anti-IFN-γ PE (eBioscience) as previously described (43).
In vitro priming of naive polyclonal T cells
NT LN cells were immunomagnetically depleted of CD44+ cells and cultured 2 days with soluble anti-CD3 mAb (5 μg/ml; eBioscience) plus IL-2 (50 U/ml; NCI Biological Research Branch), IL-12 (2.5 U/ml; Sigma-Aldrich), and IFN-γ (50 ng/ml; R&D Systems), with fresh medium and cytokines added daily. Cells harvested at the indicated times were either restimulated for 5 h with PMA plus I plus brefeldin A and subsequently stained for CD44, CD4, or CD8 and intracellular IFN-γ as described above, or RNA was directly extracted for real-time RT-PCR analysis.
Enrichment of T cells, chromatin immunoprecipitation (ChIP), and RT-PCR
NT naive CD8+CD4− and CD4+CD8− thymocytes and LN-derived naive CD4 and CD8 cells were enriched by first depleting CD44+ cells (to remove memory CD8 and CD4 cells as well as NKT cells) and then depleting either CD4+ or CD8+ cells using the appropriate magnetic beads (Dynal). At the indicated times before or after in vitro priming, CD8 and CD4 cells were positively enriched via MACS columns using anti-CD8 or CD4 PE, respectively, and anti-PE microbeads (Miltenyi Biotec), resulting in purities of at least 98% (data not shown). These purified naive or primed CD8 and CD4 cells were then either subjected directly to ChIP analysis or re-stimulated for 3 h with PMA plus I for RT-PCR analysis (3 × 105 cells/sample were used for each assay). Adoptively transferred clone 4 × Bcl-2 double-transgenic CD8 cells (Thy1.1+) were purified for ChIP using a FACSVantage SE cell sorter (BD Biosciences) (purities generally exceeded 99%). Adoptively transferred wild-type clone 4 TCR-transgenic CD8 cells subjected to enforced OX40 plus 4-1BB dual costimulation were purified for ChIP via depletion of B220+ and CD4+ cells using magnetic beads followed by positive enrichment of Thy1.1+ cells using MACS columns (purities were 98–99%).
Quantitative real-time PCR-based ChIP analysis to measure acetylated histone H3 (AcH3) within the Ifng proximal promoter was performed as previously described (43), except that primers specific for the GPDH promoter (forward, 5′-ACGGGGACCCAGGAGAACTG and reverse, 5′-TAGTTGCCGCTGCCAAACAC (45)) were used as the normalizing standard for ΔCT calculations. Additionally, the amount of AcH3 in experimental samples was calculated as the fold difference (ΔΔCT value) relative to either naive CD4 cells (for in vitro-priming experiments) or naive TCR-transgenic CD8 cells (for adoptive transfer experiments), which were both assigned an arbitrary value of 1.0.
Quantitative real-time PCR-based RT-PCR was performed as previously described (43), with the following modifications. T-bet and Eomes mRNA levels in in vitro-primed T cells were calculated by directly comparing CT values for each sample (containing 3 × 105 cell equivalents) with naive CD4 cells that were assigned an arbitrary value of 1.0. T-bet and Eomes mRNA levels in recovered adoptively transferred TCR-transgenic CD8 cells were determined by first calculating ΔCT values between T-bet or Eomes with HPRT and then calculating the fold difference (ΔΔCT value) between each experimental sample and naive TCR-transgenic CD8 cells which were assigned an arbitrary value of 1.0. The T-bet primers have been described elsewhere (43) and the Eomes primers were forward, 5′-TATAACGGTGAGAGAACCGT GCCA and reverse, 5′-ACCATATGGGAGCAAGGTACTGGA.
All ChIP and RT-PCR samples were run in duplicate using the cycling conditions of 95°C for 10 min, then 45 cycles of 95°C for 20 s and 59°C for 60 s. SYBR Green fluorescence was measured during the annealing/extension step.
Results
Naive CD8 cells have a stronger tendency to differentiate into IFN-γ-expressing effectors compared with naive CD4 cells
In response to both strong and weak immunogenic forms of HA Ag, adoptively transferred naive HA-specific clone 4 TCR-transgenic CD8 cells develop a more robust capacity to express IFN-γ compared with naive HA-specific 6.5 TCR-transgenic CD4 cells (42). Since these experiments used transgenic T cells expressing fixed TCRs, it could not be ruled out that the different capacities to express IFN-γ simply resulted from differences in either the affinities/avidities of the two transgenic TCRs for their cognate MHC-peptide complexes or processing/presentation of the cognate peptides. To examine the intrinsic abilities of naive CD4 vs CD8 cells to express IFN-γ, naive polyclonal T cells from NT mice were primed in vitro with Ag-nonspecific stimuli. To first establish the baseline of IFN-γ expression potential in mature naive T cells, single-positive thymocytes and LN cells were directly stimulated with either anti-CD3 mAb or PMA plus I (Fig. 1). Since memory T cells constitute a portion of both the normal peripheral T cell repertoire as well as the single-positive thymocyte population (which have reentered the thymus following priming in the periphery (46)), IFN-γ expression was analyzed in relation to the memory marker CD44. As expected, CD44high CD4 and CD8 memory T cells in both the thymus and LN expressed high levels of IFN-γ following stimulation with either anti-CD3 or PMA plus I (albeit PMA plus I-induced expression was higher). Also, memory CD8 cells expressed more IFN-γ than memory CD4 cells (Fig. 1A), mirroring the previous observation that adoptively transferred TCR-transgenic CD8 cells express more IFN-γ following immunization with a recombinant vaccinia virus compared with TCR-transgenic CD4 cells (42). A negligible proportion of CD44low naive CD4 cells from both the thymus and LN expressed IFN-γ in response to either anti-CD3 or PMA plus I (Fig. 1A), consistent with the well-established finding that naive CD4 cells cannot express IFN-γ because the Ifng gene locus exists in a closed/repressed epigenetic configuration before Th1 differentiation (7–12). In contrast, ∼10% of CD44low naive CD8+CD4− single-positive thymocytes and ∼20% of peripheral CD44low CD8 cells expressed moderate levels of IFN-γ in response to PMA plus I stimulation (Fig. 1A). A similar CD8 cell IFN-γ expression pattern was also observed when the memory marker CD11a (47) was used to distinguish naive from memory cells (data not shown). The ability of PMA plus I, but not anti-CD3, to elicit IFN-γ expression in naive CD8 cells is likely due to the less efficient TCR-proximal signaling apparatus in naive compared with memory T cells (48, 49) that can be bypassed by PMA plus I, but not anti-CD3. Nevertheless, the fact that PMA plus I did not elicit IFN-γ expression in naive CD4 cells indicates that naive CD8 cells selectively acquire a partial ability to express this effector cytokine.
FIGURE 1.
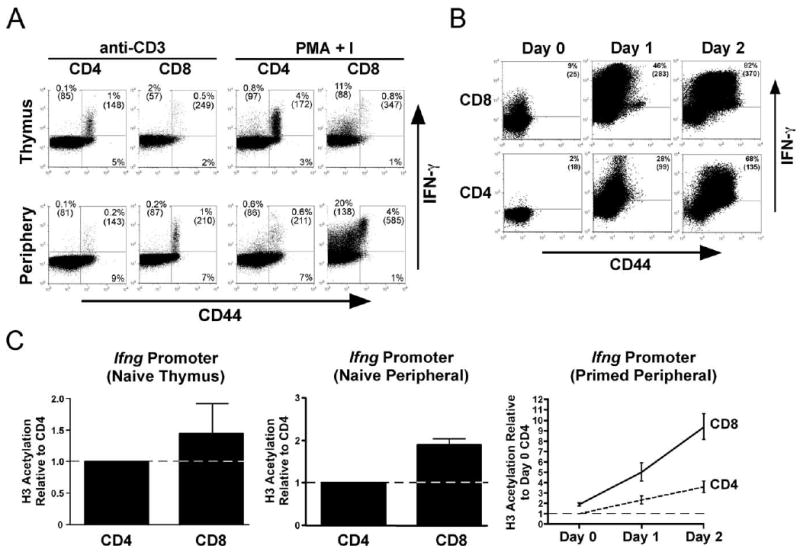
IFN-γ expression potential of naive and primed polyclonal CD8 vs CD4 T cells. A, LN T cells and single-positive thymocytes from NT mice were stimulated in vitro with either anti-CD3 mAb or PMA + I, and CD44 vs IFN-γ staining plots of CD4 or CD8-gated cells are shown. B, In vitro time course of IFN-γ expression potential development in naive polyclonal CD8 vs CD4 T cells. CD44-depleted NT LN T cells were primed with anti-CD3 plus IL-12, IL-2 and IFN-γ, and at days 0, 1, and 2 restimulated with PMA + I before intracellular IFN-γ staining. In both A and B, CD44 vs IFN-γ staining plots are representative of three mice per group, and the percentage of cytokine-expressing cells (determined using an isotype control Ab) as well as their mean fluorescence intensity of cytokine expression (shown in parentheses) are indicated. C, AcH3 within the Ifng proximal promoter in naive (CD44-depleted) thymic and peripheral and in vitro-primed peripheral polyclonal CD4 and CD8 cells. All data (expressed as the mean ± SEM) are normalized to naive (day 0) CD4 cells, which were assigned an arbitrary value of 1 (indicated by dotted line). n = 5 for paired samples.
To analyze the development of IFN-γ expression potential in naive polyclonal CD8 vs CD4 cells following antigenic priming, LN cells were depleted of CD44+ memory cells, primed in vitro with anti-CD3 under Th1 differentiation conditions, and IFN-γ expression was subsequently measured following restimulation with PMA plus I (Fig. 1B). At 24 h after priming, both CD4 and CD8 cells expressed enhanced IFN-γ levels compared with their non-primed (day 0) naive progenitors, although expression was severalfold greater in CD8 cells. IFN-γ expression was further enhanced in both CD4 and CD8 cells at day 2 after priming, although expression remained substantially higher in CD8 cells. IFN-γ mRNA expression measured by real-time RT-PCR in these same in vitro-primed T cell populations generally paralleled IFN-γ protein levels (data not shown).
To become capable of transcribing IFN-γ mRNA, T cells must first remodel the Ifng gene from a closed/repressed to an open epigenetic configuration (1, 3, 7–13, 50, 51). Thus, the differential abilities of naive and primed CD8 vs CD4 cells to express IFN-γ might be influenced by the degree to which this epigenetic transformation has occurred. Since acetylation of histone H3 (AcH3) at the Ifng proximal promoter is an early and well0characterized epigenetic event that correlates with the acquisition of transcriptional competence (8, 9), a real-time PCR-based ChIP assay (43) was used to compare the amount of AcH3 at this genomic site in naive and primed CD8 vs CD4 cells (Fig. 1C). Naive (CD44-negative) CD8+CD4− thymocytes exhibited a minimal increase in AcH3 relative to CD4+CD8− counterparts. Nevertheless, this difference increased to ∼2-fold in naive peripheral T cells (p = 0.0004 by paired t test), consistent with a previous study detecting a low amount of AcH3 at the Ifng promoter in naive CD8 cells (32). Given that only 10–20% of the naive CD8 cells expressed IFN-γ in response to PMA plus I (Fig. 1, A and B), it might be possible that the modest increase in AcH3 observed in naive CD8 relative to CD4 cells reflects the average between naive CD8 cells that have undergone extensive Ifng locus remodeling and those that have not. Nevertheless, although AcH3 at the Ifng proximal promoter progressively increased in both CD8 and CD4 cells following in vitro priming, it remained 2- to 3-fold greater in CD8 cells at each time point (Fig. 1C), paralleling the relative differences in PMA plus I-induced IFN-γ expression (Fig. 1B). Taken together, these data indicate that naive CD8 cells at the population level have a stronger tendency to undergo effector differentiation compared with CD4 cells.
IFN-γ expression potential in self-Ag-tolerized CD8 cells
Having compared the innate capacities of naive CD4 vs CD8 cells to differentiate to IFN-γ-expressing effectors, we wished to characterize IFN-γ expression potential in CD8 cells undergoing self-Ag-induced tolerization using a system in which tolerogenic CD4 cell responses were previously characterized. Thus, when naive 6.5 HA-specific TCR-transgenic CD4 cells are adoptively transferred into C3-HA-transgenic mice that express HA as a generic parenchymal self-Ag (self-HA), they initially proliferate and partially remodel their Ifng loci toward an open configuration, but ultimately develop an anergic phenotype. In contrast, when the same TCR-transgenic CD4 cells are transferred into NT mice infected with a recombinant vaccinia virus expressing HA (viral HA), they proliferate and differentiate into Th1 effectors (40, 52).
HA-specific clone 4 TCR-transgenic CD8 cells adoptively transferred into viral HA and self-HA recipients displayed equivalently robust CFSE dilution patterns (Fig. 2A); however, the frequency of accumulated TCR-transgenic CD8 cells was >10-fold lower in self-HA compared with viral HA recipients (Fig. 2B). Additionally, the self-HA-exposed TCR-transgenic CD8 cells that were present at days 1 through 4 after transfer expressed considerably lower amounts of both IFN-γ and TNF-α in response to HA peptide restimulation compared with their viral HA counterparts (Fig. 2, C and D). This self-Ag-induced tolerogenic response where CD8 cells proliferate but fail to accumulate and develop effector activities is consistent with previous work (53).
FIGURE 2.
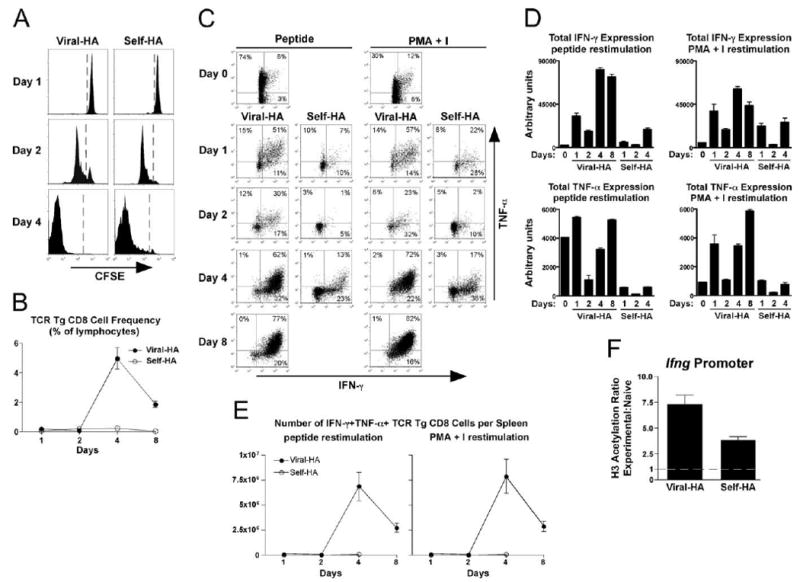
Functional response of naive TCR-transgenic CD8 cells exposed to cognate viral vs self-Ag. Naive wild-type CFSE-labeled Thy1.1+ TCR-transgenic CD8 cells (1 × 106) were adoptively transferred into Thy1.2+ viral HA or self-HA recipients and recovered from spleens at the indicated times. A, Representative histograms of CFSE-dilution. B, TCR-transgenic CD8 cell accumulation is presented as the percentage within total lymphocytes. C, Representative plots of intracellular IFN-γ vs TNF-α expression following in vitro restimulation with HA peptide or PMA + I. Quantitative analysis of total cytokine expression per TCR-transgenic CD8 cell (D) and the total number of IFN-γ+TNF-α+ TCR-transgenic CD8 cells per spleen (E) corresponding to C, n = 3/group. F, Naive Bcl-2-overexpressing Thy1.1+ TCR-transgenic CD8 cells (1 × 106) were adoptively transferred into viral HA and self-HA recipients and recovered from spleens on day 4 for ChIP analysis. AcH3 within the Ifng proximal promoter, n = 3/group.
We next asked whether Ifng locus remodeling occurs during CD8 T cell tolerance induction. First, IFN-γ expression was measured following stimulation with PMA plus I, which bypasses TCR-proximal signaling steps (16). At 4 days after transfer, PMA plus I-restimulated tolerized TCR-transgenic CD8 cells recovered from self-HA recipients expressed IFN-γ at levels that were only 2- to 3-fold lower than in viral HA-primed effectors (Fig. 2, C and D). These data suggested that the Ifng locus partially remodels during CD8 cell tolerance induction, although it was curious that PMA plus I-induced IFN-γ expression in both virally primed effector and self-Ag-tolerized cells increased at day 1, decreased at day 2, and then rebounded at day 4. The basis for this biphasic response is unclear, but given that chromatin remodeling generally progresses in a linear manner (Ref. 7 and Fig. 1), this result suggested that in contrast to CD4 cells (52) the level of PMA plus I-induced IFN-γ expression may not always reflect the extent of Ifng locus remodeling in tolerized CD8 cells.
It was not possible to perform ChIP analysis to directly assess the extent of Ifng locus remodeling in tolerized CD8 cells using this model due to the low frequency of TCR-transgenic CD8 cells recovered from self-HA-expressing recipients (Fig. 2B). To get around this issue, adoptive transfers were performed using TCR-transgenic CD8 cells overexpressing the anti-apoptotic molecule Bcl-2 since it has been reported in an analogous system that Bcl-2 overexpression increases TCR-transgenic CD8 cell recovery without altering functional activity (54). Consistent with this previous study, in our system TCR-transgenic CD8 cells overexpressing Bcl-2 proliferated to the same extent as their wild-type counterparts but accumulated to substantially higher frequencies following adoptive transfer into both viral HA and self-HA recipients. Additionally, Bcl-2-overexpressing TCR-transgenic CD8 cells developed similar capacities to express IFN-γ and TNF-α in response to either HA peptide or PMA plus I restimulation compared with their wild-type counterparts (data not shown).
When ChIP was performed on Bcl-2-overexpressing adoptively transferred TCR-transgenic CD8 cells recovered from self-HA and viral HA recipients 4 days after transfer, AcH3 at the Ifng proximal promoter was ∼2-fold greater (p = 0.01, this and all subsequent p values were calculated using the unpaired two-tailed t test) in viral HA compared with self-HA recipients (Fig. 2F), paralleling the relative abilities of these TCR-transgenic CD8 cells to express IFN-γ in response to PMA plus I (Fig. 2, C and D, and data not shown).
Enforced OX40 plus 4-1BB dual costimulation fails to redirect self-Ag-induced CD8 cell tolerization toward effector differentiation
We next asked whether self-Ag-induced CD8 cell tolerization could be redirected toward effector differentiation. Since the TNF/TNFR superfamily member costimulatory molecules OX40 (CD134) and 4-1BB (CD137) when ligated simultaneously have a powerful effect in boosting both expansion and effector differentiation in CD8 cells responding to exogenous soluble Ag (29, 31), agonistic anti-OX40 and anti-4-1BB mAbs were coadministered to self-HA and viral HA TCR-transgenic CD8 cell-adoptive transfer recipients (Fig. 3). Enforced OX40 plus 4-1BB dual costimulation substantially augmented TCR-transgenic CD8 cell accumulation in both recipients (Fig. 3A). The ability of virally primed CD8 cells to express IFN-γ and TNF-α was already near maximal and thus not surprisingly enforced dual costimulation had little impact on this robust effector activity (Fig. 3B). Strikingly, however, enforced dual costimulation failed to augment the weak IFN-γ and TNF-α expression potentials that develop in TCR-transgenic CD8 cells encountering self-HA (Fig. 3B). Thus, TCR-transgenic CD8 cells encountering cognate self-Ag clearly responded to enforced OX40 plus 4-1BB dual costimulation as shown by their 18-fold increase in expansion; however, their ability to produce effector cytokines was not enhanced.
FIGURE 3.
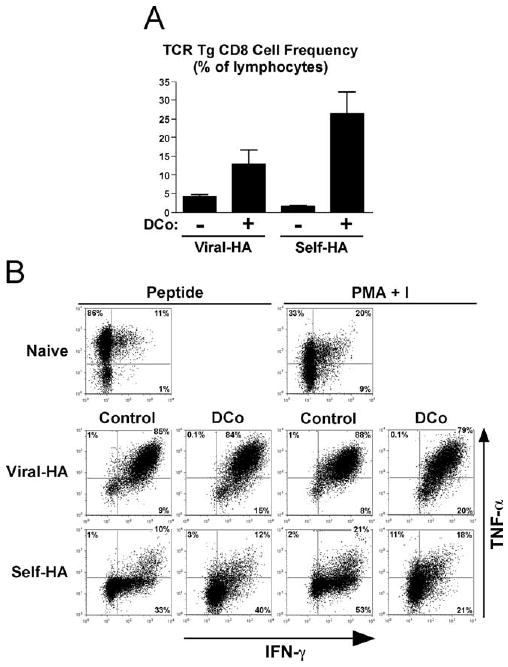
Enforced OX40 plus 4-1BB dual costimulation fails to augment effector differentiation in CD8 cells exposed to cognate self-Ag. Naive wild-type TCR-transgenic CD8 cells (1 × 106) were adoptively transferred into viral HA or self-HA recipients that were either untreated (control) or treated with anti-OX40 plus anti-4-1BB (DCo) and recovered from spleens at day 4. A, TCR-transgenic CD8 cell accumulations, n = 3/group. B, Representative FACS plots of intracellular IFN-γ vs TNF-α following restimulation with HA peptide or PMA + I.
The failure of enforced OX40 plus 4-1BB dual costimulation to redirect naive clone 4 HA-specific TCR-transgenic CD8 cells encountering cognate self-Ag to undergo effector differentiation was quite surprising, given that OT-I TCR-transgenic CD8 cells responding to exogenous cognate-soluble OVA peptide as well as Vβ3+ CD8 cells responding to bacterial superantigen can be redirected to develop robust effector functions (29, 31). To reconcile these differences, the effect of enforced OX40 plus 4-1BB dual costimulation on the response of clone 4 TCR-transgenic CD8 cells to exogenous soluble HA peptide was tested (Fig. 4). Soluble HA peptide boluses were either administered once or daily to mimic the persistence of self-Ag (a regimen that impairs subsequent recall responsiveness; data not shown). With both regimens, enforced dual costimulation markedly enhanced TCR-transgenic CD8 cell accumulation (Fig. 4A), just as it did in the OT-1 and superantigen systems (29, 31). However, in marked contrast to the failure of enforced dual costimulation to augment IFN-γ and TNF-α expression potentials in response to self-Ag (Fig. 3B), enforced dual costimulation substantially augmented IFN-γ and TNF-α expression capacities in response to both a single and daily boluses of HA peptide (Fig. 4B).
FIGURE 4.
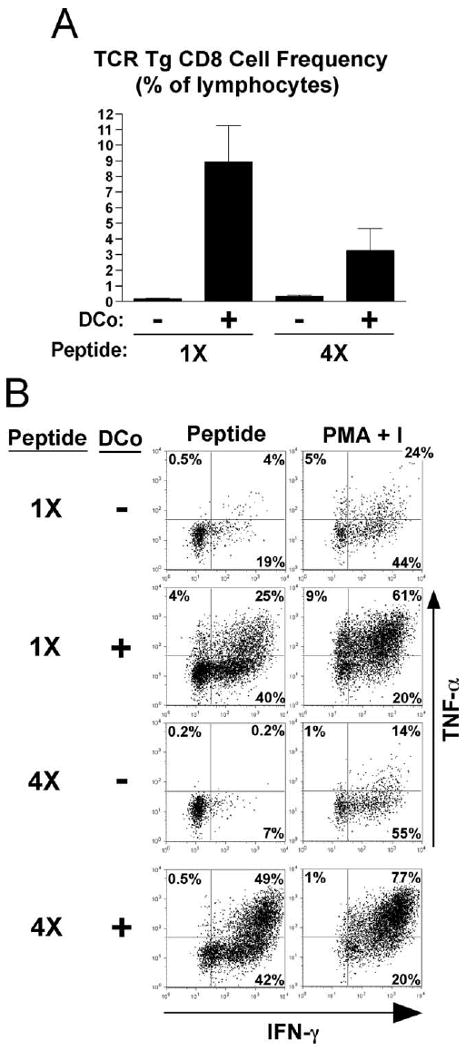
Enforced OX40 plus 4-1BB dual costimulation potently redirects soluble peptide-induced CD8 cell tolerance induction toward effector differentiation. Naive wild-type TCR-transgenic CD8 cells (1 × 106) were adoptively transferred into NT recipients that were treated with either a single bolus of soluble HA peptide on day 0 (1×) or daily peptide boluses on days 0–3 (4×) and that did or did not receive enforced dual costimulation (DCo). In all groups, the TCR-transgenic CD8 cells were recovered from spleens on day 4. A, TCR-transgenic CD8 cell accumulations, n = 3–4/group. B, Representative FACS plots of intracellular IFN-γ vs TNF-α following restimulation with HA peptide or PMA + I.
As summarized in Table I, enforced OX40 plus 4-1BB dual costimulation robustly redirected TCR-transgenic CD8 cell tolerance induction toward effector differentiation in response to soluble exogenous HA peptide but not parenchymal self-HA. Thus, with HA peptide, enforced dual costimulation increased the absolute magnitude of IFN-γ expression by 87-fold and the proportion of cells simultaneously producing both IFN-γ and TNF-α by 56-fold. In contrast, with self-HA, enforced dual costimulation only increased these values by 0.8- and 1.5-fold, respectively. Additionally, enforced OX40 plus 4-1BB dual costimulation elicited levels of IFN-γ expression with exogenous peptide that were 9-fold greater than with self-HA.
Table I. Effect of enforced OX40 plus 4-1BB dual costimulation on the ability of TCR-transgenic CD8 cells encountering soluble HA peptide vs self-HA to express effector cytokines.
The observation that enforced OX40 plus 4-1BB dual costimulation was able to redirect tolerogenic CD8 cell responses to exogenous peptide but not self-Ag stood in contrast to a previous study demonstrating that enforced OX40 costimulation redirects adoptively transferred AND TCR-transgenic CD4 cells to develop the capacity to express IFN-γ, rather than developing a fully tolerant phenotype in recipients that express the cognate pigeon cytochrome c epitope as a self-Ag (55). To thus assess whether the inability of enforced costimulation to redirect tolerogenic anti-self CD8 cell responses toward effector differentiation is the consequence of a specific peculiarity of our C3-HA-transgenic system that precludes effector T cell function, we tested the response of cognate CD4 cells in this system. As previously observed (52), adoptively transferred 6.5 TCR-transgenic HA-specific CD4 cells recovered from spleens of self-HA recipients only expressed minimal amounts of IFN-γ and TNF-α following restimulation with HA peptide (Fig. 5B). Notably, TCR-transgenic CD4 cells recovered from self-HA recipients receiving enforced OX40 costimulation not only accumulated to greater frequency (Fig. 5A) but also expressed both IFN-γ and TNF-α at levels that were even greater than in viral HA recipients (Fig. 5B). Additionally, enforced OX40 plus 4-1BB dual costimulation redirected TCR-transgenic CD4 cells encountered self-HA to undergo even more robust expansion (Fig. 5C) and effector differentiation (Fig. 5, D and E). Taken together, the combined results from Figs. 3–5 suggest that although both CD4 and CD8 cells can be redirected by enforced OX40 plus 4-1BB dual costimulation to undergo effector differentiation rather than tolerization, self-Ag prevents effector differentiation when enforced dual costimulation is provided to CD8 cells.
FIGURE 5.

Enforced OX40 plus 4-1BB dual costimulation redirects self-Ag-induced CD4 cell tolerance induction toward effector differentiation. Naive TCR-transgenic CD4 cells (2.5 × 106) were adoptively transferred into self-HA, viral HA. and anti-OX40-treated self-HA recipients and recovered from spleens on day 4. A, TCR-transgenic CD4 cell accumulations. B, FACS plots of intracellular IFN-γ vs TNF-α expression following restimulation with HA peptide are representative of n = 3/group. Naive TCR-transgenic CD4 cells (1 × 106) were adoptively transferred into self-HA recipients that were either treated with anti-OX40 plus anti-4-1BB or untreated (control) and then recovered from spleens on day 4. C, TCR-transgenic CD4 cell accumulations. D, Representative FACS plots of intracellular IFN-γ vs TNF-α expression following restimulation with HA peptide. E, Quantitative analysis of the percentage of TCR-transgenic CD4 cells simultaneously expressing both IFN-γ and TNF-α corresponding to D, n = 4/group.
Enforced OX40 plus 4-1BB dual costimulation fails to alter epigenetic remodeling of the Ifng locus in naive CD8 cells encountering cognate self-Ag
The failure of enforced OX40 plus 4-1BB dual costimulation to augment IFN-γ expression in TCR-transgenic CD8 cells exposed to cognate self-Ag might have resulted from an inability to program more complete remodeling of the Ifng gene locus. Alternatively, the Ifng locus might have fully remodeled, but the persistent presentation of self-Ag induced nonepigenetic lesions that dampen IFN-γ expression. To address this question, ChIP analysis was performed on adoptively transferred TCR-transgenic CD8 cells recovered from dual costimulation-treated viral HA, soluble HA peptide, and self-HA recipients. With enforced dual costimulation, AcH3 at the proximal promoter was 2-fold lower (p = 0.02) in self-HA compared with viral HA recipients (Fig. 6A), similar to the difference observed without enforced costimulation (Fig. 2F). Additionally, AcH3 at the proximal promoter was 1.5-fold lower (p = 0.02) in self-HA compared with soluble HA peptide recipients (Fig. 6A).
FIGURE 6.
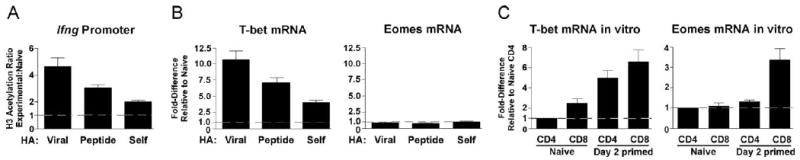
Enforced OX40 plus 4-1BB dual costimulation fails to alter epigenetic remodeling of the Ifng locus in naive CD8 cells encountering cognate self-Ag. Naive wild-type TCR-transgenic CD8 cells (1 × 106) were adoptively transferred into anti-OX40 plus anti-4-1BB-treated viral HA, 4× soluble HA peptide, and self-HA recipients and enriched from spleens 4 days after transfer. A, AcH3 within the Ifng proximal promoter, n = 3/group. B, Expression of T-bet and Eomes mRNAs were quantified by real-time RT-PCR. n = 4, 3, and 9 for viral HA, 4× soluble HA peptide, and self-HA, respectively. C, Expression of T-bet and Eomes mRNAs in in vitro-primed polyclonal CD4 and CD8 cells, n = 4/group.
Ifng locus remodeling in CD8 cells can be regulated through the combined actions of T-bet and Eomes (19). The failure of enforced OX40 plus 4-1BB dual costimulation to augment Ifng locus remodeling in CD8 cells encountering cognate self-Ag could thus be due to a failure to augment expression of one or both factors. With enforced dual costimulation, expression of T-bet mRNA in self-HA recipients was 3-fold lower (p < 0.0001) compared with viral HA recipients and 2-fold lower (p = 0.003) compared with soluble HA peptide recipients (Fig. 6B), paralleling the relative differences in AcH3 at the proximal promoter (Fig. 6A). Interestingly, Eomes mRNA expression in all three recipient groups remained unchanged compared with naive TCR-transgenic CD8 cells (Fig. 6B). Verifying the specificity of the T-bet and Eomes primers used in the real-time RT-PCR assay, T-bet mRNA was induced in both polyclonal naive CD8 and CD4 cells following in vitro priming, whereas Eomes mRNA was only induced in CD8 cells (Fig. 6C) as previously observed (19). Taken together, these data suggest that the in vivo regulation of Ifng locus remodeling in CD8 cells during enforced OX40 plus 4-1BB dual costimulation is regulated via a pathway that involves T-bet but not Eomes.
CD4 T cell help rescues the ability of enforced OX40 plus 4-1BB dual costimulation to redirect self-Ag-induced CD8 cell tolerization toward effector differentiation
Costimulatory agonists specific for TNF/TNFR family members such as OX40 and 4-1BB are currently being explored as cancer therapeutics (25–31). Because anti-OX40 plus anti-4-1BB selectively failed to push effector differentiation in CD8 cells encountering cognate self-Ag and tumor Ags are in and of themselves a form of self-Ag, we wished to understand the mechanistic basis of this result as well as how enforced OX40 plus 4-1BB dual co-stimulation-mediated CD8 cell effector differentiation might be rescued. It is well established that CD4 cell help can augment CD8 cell responses (56, 57) and, in particular, CD4 cell help has recently been shown to facilitate Ifng locus remodeling in CD8 cells primed in vitro or by viral infection in vivo (32, 33). Since enforced OX40 plus 4-1BB dual costimulation was able to redirect CD4 cells encountering cognate self-Ag to become robust effectors (Fig. 5), we therefore asked whether OX40 plus 4-1BB dual co-stimulation would be able to push CD8 cell effector differentiation if CD4 cells were simultaneously encountering cognate self-Ag (Fig. 7). Cotransfer of TCR-transgenic CD4 cells into anti-OX40 plus anti-4-1BB-treated self-HA recipients doubled both the frequency of TCR-transgenic CD8 cells that could express both IFN-γ and TNF-α (p = 0.02; Fig. 7A) as well as the total level of IFN-γ expression (p = 0.03; Fig. 7B). Even more strikingly, the inclusion of CD4 cell help increased granzyme B expression in CD8 cells by 61-fold (p = 0.0002; Fig. 7, C and D). Thus, CD4 cell help is selectively required to facilitate enforced OX40 plus 4-1BB dual costimulation-mediated effector differentiation when CD8 cells encounter cognate self-Ag (Fig. 7) but not soluble peptide (Fig. 4).
FIGURE 7.
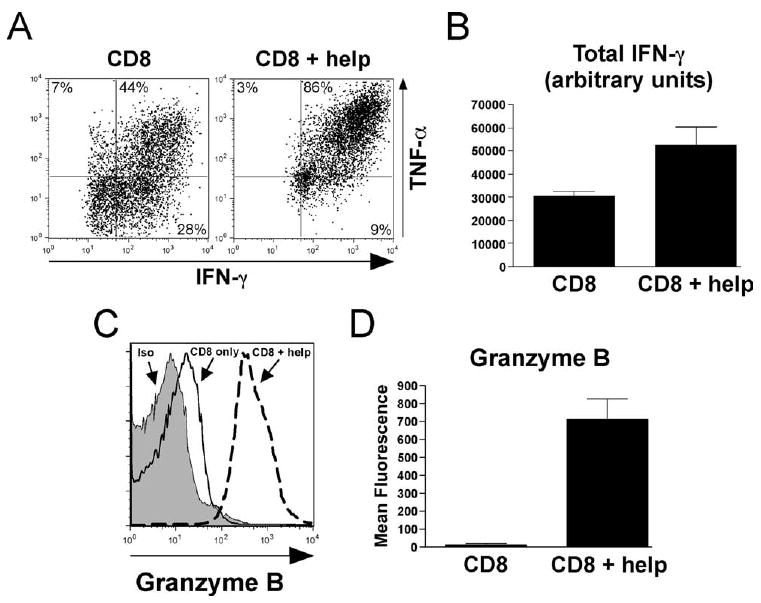
CD4 T cell help rescues the ability of enforced OX40 plus 4-1BB dual costimulation to redirect self-Ag-induced CD8 cell tolerization toward effector differentiation. Naive TCR-transgenic CD8 cells (2 × 105) were adoptively transferred into anti-OX40 plus anti-4-1BB-treated self-HA recipients either by themselves or with 1 × 106 TCR-transgenic CD4 cells (CD8 + help) and then recovered from spleens on day 4. A, Representative FACS plots of intracellular IFN-γ vs TNF-α expression following restimulation with HA peptide. B, Quantitative analysis of total IFN-γ expression corresponding to A, n = 5–6/group. C, Overlay of representative intracellular granzyme B expression histograms along with an isotype staining control (iso) from TCR-transgenic CD8 cells immediately following recovery from spleens. D, Quantitative analysis of the mean fluorescence intensity of granzyme B staining corresponding to C, n = 5/group.
Discussion
Owing to their specific roles in conferring immunity, CD8 and CD4 cells exhibit numerous functional differences. For instance, CD8 cells have greater proliferative capacity (58, 59) and develop a more stable memory following viral infection (59, 60). CD8 and CD4 cells also require different costimulatory signals for optimal activation (61, 62). Additionally, during in vitro priming, CD8 cells only require transient antigenic stimulation to differentiate into effectors (63, 64), whereas CD4 cells generally require longer periods of antigenic stimulation and/or extrinsic cytokines (65, 66). The ability to produce IFN-γ represents the major effector function that commonly develops in both CD8 and CD4 cells, although the precise contributions of trans-acting factors that program IFN-γ expression potential can differ (18, 19). Using in vivo TCR-transgenic adoptive transfer systems, we previously observed that in response to both strong and weak immunizing stimuli, naive TCR-transgenic CD8 cells develop the capacity to express IFN-γ more readily than naive TCR-transgenic CD4 cells (42). Based on our current study, this observation is likely explained by the stronger tendency of CD8 cells to differentiate into IFN-γ-expressing effectors.
The stronger tendency of CD8 relative to CD4 cells to express IFN-γ correlates with a partial remodeling of the Ifng gene locus that occurs during development and a more rapid remodeling toward a fully accessible configuration in response to antigenic priming. In lymphocytes such as NK, NKT, and γδ T cells that express invariant receptors specific for common pathogen-associated determinants, the Ifng locus is already fully remodeled and high-level IFN-γ expression is elicited in response to primary stimulation (67–69). Our current data thus indicate that CD8 cells occupy an intermediate position within a continuum where at the one extreme the Ifng locus undergoes complete remodeling in invariant receptor-expressing lymphocytes during development, but at the other extreme does not commence until antigenic priming in CD4 cells (7, 9, 13). Although the capacity of invariant receptor-expressing lymphocytes to rapidly express IFN-γ upon primary stimulation fits with their role in providing innate immune functions (70, 71), the reason for accelerated development of IFN-γ expression potential in CD8 cells is less apparent. Thus, since a limited number of pathogen-specific naive CD8 cells must first undergo expansion and further differentiation before gaining the capacity to directly participate in clearing infections, it is not clear how IFN-γ expression immediately following antigenic priming could facilitate pathogen clearance. One possibility is that the more rapid remodeling of the Ifng locus allows primed CD8 cells to express peak IFN-γ levels more rapidly. An alternate, but nonmutually exclusive, possibility is that because IFN-γ can enhance APC function (72), IFN-γ produced by naive CD8 cells during the initial phase of antigenic priming augments later stages of CD8 and/or CD4 cell priming.
Given the stronger tendency of naive CD8 cells to undergo effector differentiation compared with naive CD4 cells as well as the potent ability of enforced OX40 plus 4-1BB dual costimulation to redirect CD8 cells encountering soluble foreign Ag and CD4 cells encountering cognate self-Ag to undergo effector differentiation rather than tolerization, it was striking that enforced OX40 plus 4-1BB dual costimulation failed to push effector differentiation in naive CD8 cells encountering cognate self-Ag. This failure was not caused by a unique peculiarity of our C3-HA-transgenic self-Ag model system that precludes CD8 cell effector differentiation per se, since CD8 cells subjected to blockade of negative costimulatory pathways such as PD-1/B7-H1 (PD-L1) are redirected from tolerance induction toward effector differentiation (73). Additionally, this failure was not caused by a general inability of CD8 cells encountering self-Ag to respond to enforced OX40 plus 4-1BB dual costimulation since expansion was dramatically boosted. In fact, it appears that enforced OX40 plus 4-1BB dual costimulation-driven expansion and effector differentiation can be independently regulated because CD8 cells exposed to exogenous soluble peptide undergo weaker expansion but greater effector differentiation compared with self-Ag. An alternate explanation for this paradoxical result is that the particular combination of APC that present cognate Ag determines the outcome of CD8 cell responses during enforced OX40 plus 4-1BB dual costimulation. The antitumor effect of enforced OX40 plus 4-1BB dual costimulation in different models may therefore depend on the particular APC populations that present the relevant tumor Ags. It is nevertheless notable that in our system CD4 cell help can rescue the ability of enforced OX40 plus 4-1BB dual costimulation to facilitate effector differentiation in CD8 cells encountering cognate self-Ag, which should provide an important clue as to how costimulatory agonists can be translated into effective cancer therapeutics.
Acknowledgments
We thank Dr. Irving Goldschneider for helpful discussions.
Footnotes
This work was supported by National Institutes of Health Grants AI057441 and CA109339 (to A.J.A.) and AI052108 (to A.T.V.).
Abbreviations used in this paper: HA, hemagglutinin; AcH3, acetylated histone H3; ChIP, chromatin immunoprecipitation; LN, lymph node; NT, nontransgenic; PMA plus I, PMA plus ionomycin; CT, cycle threshold.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Murphy KM, Reiner SL. Decision making in the immune system: the lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 5.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 6.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 7.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 8.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 9.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 10.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable TH1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 11.Melvin AJ, McGurn ME, Bort SJ, Gibson C, Lewis DB. Hypomethylation of the interferon-γ gene correlates with its expression by primary T-lineage cells. Eur J Immunol. 1995;25:426–430. doi: 10.1002/eji.1830250218. [DOI] [PubMed] [Google Scholar]
- 12.Lee DU, Avni O, Chen L, Rao A. A distal enhancer in the interferon-γ (IFN-γ) locus revealed by genome sequence comparison. J Biol Chem. 2004;279:4802–4810. doi: 10.1074/jbc.M307904200. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 15.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 16.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 17.Cho JY, Grigura V, Murphy TL, Murphy K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-γ promoter. Int Immunol. 2003;15:1149–1160. doi: 10.1093/intimm/dxg113. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 20.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th) 1 and Th2 cells. J Exp Med. 2000;191:201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 22.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 23.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 24.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg AD. OX40: targeted immunotherapy–implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 26.Tamada K, Chen L. Renewed interest in cancer immunotherapy with the tumor necrosis factor superfamily molecules. Cancer Immunol Immunother. 2006;55:355–362. doi: 10.1007/s00262-005-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon B, Kim BS, Cho HR, Park JE, Kwon BS. Involvement of tumor necrosis factor receptor superfamily (TNFRSF) members in the pathogenesis of inflammatory diseases. Exp Mol Med. 2003;35:8–16. doi: 10.1038/emm.2003.2. [DOI] [PubMed] [Google Scholar]
- 28.Foell J, Mittler RS. Costimulatory molecules as immunotherapeutic targets in systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28:153–162. doi: 10.1007/s00281-006-0039-y. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, Mittler RS, Vella AT. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 30.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 Costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 32.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 33.Northrop JK, Wells AD, Shen H. Cutting edge: chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. J Immunol. 2008;181:865–868. doi: 10.4049/jimmunol.181.2.865. [DOI] [PubMed] [Google Scholar]
- 34.Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur J Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 35.Stavely-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 37.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan DJ, Liblau R, Scott S, Fleck HO, McDevitt N, Sarvetnick D, Lo D, Sherman LA. CD8+ cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 40.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 41.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 42.Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long M, Slaiby AM, Hagymasi AT, Mihalyo MA, Lichtler AC, Reiner SL, Adler AJ. T-bet down-modulation in tolerized Th1 effector CD4 cells confers a TCR-distal signaling defect that selectively impairs IFN-γ expression. J Immunol. 2006;176:1036–1045. doi: 10.4049/jimmunol.176.2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 45.Baguet A, Bix M. Chromatin landscape dynamics of the Il4-Il13 locus during T helper 1 and 2 development. Proc Natl Acad Sci USA. 2004;101:11410–11415. doi: 10.1073/pnas.0403334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SK, Reed DS, Heath WR, Carbone F, Lefrancois L. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 48.Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. Eur J Immunol. 1997;27:2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- 49.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–3698. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick DR, Shirley KM, McDonald LE, Bielefeldt-Ohmann H, Kay GF, Kelso A. Distinct methylation of the interferon γ (IFN-γ) and interleukin 3 (IL-3) genes in newly activated primary CD8+ T lymphocytes: regional IFN-γ promoter demethylation and mRNA expression are heritable in CD44highCD8+ T cells. J Exp Med. 1998;188:103–117. doi: 10.1084/jem.188.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W, Chang S, Aune TM. Long-range histone acetylation of the Ifng gene is an essential feature of T cell differentiation. Proc Natl Acad Sci USA. 2004;101:2440–2445. doi: 10.1073/pnas.0306002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long M, Slaiby AM, Wu S, Hagymasi AT, Mihalyo MA, Bandyopadhyay S, Vella AT, Adler AJ. Histone acetylation at the Ifng promoter in tolerized CD4 cells is associated with increased IFN-γ expression during subsequent immunization to the same antigen. J Immunol. 2007;179:5669–5677. doi: 10.4049/jimmunol.179.9.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8+ T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 56.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 57.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 59.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928–3935. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 60.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 61.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 62.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 63.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 65.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 66.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. Two distinct stages in the transition from naive CD4 T cells to effecters, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunol. 2000;165:5017–5026. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 67.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tato CM, Martins GA, High FA, DiCioccio CB, Reiner SL, Hunter CA. Cutting edge: innate production of IFN-γ by NK cells is independent of epigenetic modification of the IFN-γ promoter. J Immunol. 2004;173:1514–1517. doi: 10.4049/jimmunol.173.3.1514. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, He W, Kim ST, Tao J, Gao Y, Chi H, Intlekofer AM, Harvey B, Reiner SL, Yin Z, et al. Epigenetic and transcriptional programs lead to default IFN-γ production by γδ T cells. J Immunol. 2007;178:2730–2736. doi: 10.4049/jimmunol.178.5.2730. [DOI] [PubMed] [Google Scholar]
- 70.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 71.Hayday AC. γ δ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 72.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7–H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]


