Abstract
This article reviews the literature on the acute effects of Delta 9-tetrahydrocannabinol, the primary psychoactive component of marijuana, on working memory, and the implications for schizophrenia. Working memory deficits are a hallmark feature of schizophrenia, and have been implicated as an etiologic mechanism contributing to the onset of the disorder. Regular marijuana smokers may also exhibit subtle working memory impairment relative to healthy controls, and an association between marijuana abuse and subsequent development of schizophrenia, though controversial, has been reported in the literature. The causal role that marijuana plays in working memory impairment related to schizophrenia, however, remains unclear. Thus, this article specifically considers the acute effects of marijuana on working memory performance. The ecologic relevance and clinical significance of these findings will be examined, and directions for future research will be recommended.
FOCUS POINTS
Working memory deficits are a hallmark feature of schizophrenia.
Delta 9-tetrahydrocannabinol acutely impairs working memory in marijuana smokers.
An association between early marijuana abuse and adulthood schizophrenia has been reported.
To help clarify this relationship, the acute working memory effects of smoked marijuana should be examined in marijuana smokers who are already at heightened risk for schizophrenia.
INTRODUCTION
The purpose of this article is to examine the relationship between marijuana use, working memory, and schizophrenia. In young individuals at risk to develop schizophrenia, marijuana abuse has been reported to be associated with the onset of adulthood schizophrenia. Deficits in working memory are a hallmark feature of schizophrenia and have been implicated as an etiologic mechanism contributing to the onset of the disorder. Therefore, an examination of the effects of marijuana on working memory may shed light on the link between marijuana abuse and schizophrenia. In this article, working memory will first be defined, and theory and findings regarding working memory performance in schizophrenia patients and marijuana smokers will be briefly examined. Second, the association between marijuana smoking and schizophrenia will be considered. Third, selected acute effects of Delta 9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive component of marijuana, will be reviewed; findings from studies on working memory effects and findings from studies on other psychotomimetic effects will be examined. Finally, the relevance and implications of these effects on the development of schizophrenia as well as recommendations for future research will be discussed. Clinical issues related to prevention and treatment are discussed elsewhere in this issue of Primary Psychiatry.1
WORKING MEMORY
Working memory refers to the ability to mentally store and manipulate representations of stimuli over a short duration to execute a response.2 Working memory is distinguished from other forms of memory, such as immediate memory and delayed memory, both of which refer to the storage of information without manipulation. One prototypical working memory task is the Digits Backward condition of the Digit Span subtest. In its classic form,3 the participant is orally presented with increasingly long strings of digits (eg, 1–5–3–7) and is required to repeat them back to the examiner backwards (eg, 7–3–5–1). A more difficult variant of this task is the Letter-Number Sequencing task,3 which requires a reorganization of both numbers (in order) and letters (alphabetically). These tasks measure verbal working memory. Various digit recall tasks have been computerized and employ visually-presented digits, which retain the stimuli’s semantic but not auditory properties.
Another common working memory task is the spatial n-back task, during which the participant views an array on a computer screen that includes a fixation point and a dot set at one of a series of fixed points around the fixation point. The location of the dot changes on each trial, and the participant must decide if the position of the dot on the current trial (target stimulus) matches the position of the dot at a certain number of trials preceding the target (comparison stimulus). The number of trials preceding the target stimulus that the comparison stimulus resides at (n) can be altered, with a higher n-value indicating greater difficulty. This task measures visuospatial working memory.
These are two exemplars of primary working memory tasks since they both directly measure the storage and mental manipulation of stimuli representations. Many other tasks have been employed in neurocognitive studies that are primary measures of other cognitive functions, such as attention or executive functions, but require a significant contribution from working memory to perform them; these are considered secondary working memory tasks. These include the Wisconsin Card Sorting test,4 the Trailmaking test, the Tower of London task,5 and the Iowa Gambling task,6 which are described below.
Intact working memory—the ability to mentally hold and manipulate information—is necessary for the performance of many activities of daily life, including holding conversations, running errands, and performing academic and vocational tasks.2 Working memory is also highly correlated with measures of overall intelligence.7 As such, individuals whose working memory is impaired may appear distracted, impulsive, and forgetful, and may exhibit decreased academic, vocational, and interpersonal functioning. Thus, working memory is a clinically relevant cognitive function and may be impaired in individuals with a variety of neuropsychiatric conditions.
WORKING MEMORY AND SCHIZOPHRENIA
Individuals with schizophrenia have been found to exhibit impaired performance on tests of working memory in multiple modalities, including visuospatial,8,9 auditory verbal,10 and auditory nonverbal domains,11 relative to healthy control participants. Additionally, greater decrements in cognitive performance have been found in participants with schizophrenia, relative to control participants, as the response delay and size of the stimulus set increased.8,12 This suggests that the performance of schizophrenia patients is particularly susceptible to increased working memory requirements. Working memory performance has been found to be broadly correlated with performance on tests of other cognitive functions, such as delayed memory and motor functions, in participants with schizophrenia, but not in healthy control participants13; thus, working memory deficits have been hypothesized to play a central role in the other cognitive impairments commonly exhibited by schizophrenia patients. Visuospatial working memory impairments have been found to remain present even after psychotic symptoms have been stabilized, 9 indicating that working memory deficits in schizophrenia are stable across phases of the disorder.
In terms of clinical relevance, primary and secondary working memory performance has been reported to be predictive of aspects of functional outcome in schizophrenia14,15 and symptom formation,16 respectively. Additionally, in young individuals at heightened risk for schizophrenia, working memory performance has been found to be impaired17,18 and to be a sensitive predictor of the development of schizophrenia-related psychosis in adulthood.18 In sum, deficits in working memory represent a core feature of schizophrenic illness19 and, therefore, have been proposed to be a clinically relevant area to target for remediation.20
WORKING MEMORY AND MARIJUANA USE
The relationship between marijuana use and working memory deficits in the nonpsychiatric population is complex. Reviews21,22 of working memory function in psychiatrically healthy individuals who smoke marijuana regularly concluded that such individuals exhibit performance impairment on primary and secondary working memory tasks relative to healthy controls, and such impairments have been found to be associated with the self-reported frequency of marijuana smoking.23,24 However, such deficits have been found less consistently than in participants with schizophrenia only. When present in non-schizophrenic marijuana users, the impairment is generally of a less severe degree21,22 and is less stable25 than is typically seen in participants with schizophrenia only. Thus, regular marijuana use in the natural ecology may be associated with modest and short-term deficits in working memory performance when participants are not acutely intoxicated.
Of note, young marijuana smokers were found to exhibit alterations in activation of the prefrontal and parietal cortices in response to working memory tasks during functional magnetic resonance imaging (under conditions of short-term marijuana abstinence) relative to non-using controls.26,27 This indicates functional alteration in brain regions relevant to the development of psychopathology in young marijuana smokers while they engaged their working memory.
Although these studies of the residual sequelae of marijuana smoking suggest that marijuana smoking contributes to working memory deficits, the cross-sectional and correlational nature of the studies reviewed thus far do not allow an assessment of marijuana’s direct effects on working memory.
MARIJUANA USE AND SCHIZOPHRENIA
Marijuana is the most prevalent illicit substance used by schizophrenia patients, with lifetime marijuana use estimated at 30%, and lifetime marijuana dependence (according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition28) estimated at 28%.29 Marijuana is also the most commonly used illicit substance among youths identified as prodromal, or at heightened clinical risk, for psychosis.30,31 However, the link between marijuana smoking and the development of schizophrenia is highly controversial. An association between marijuana abuse and subsequent development of schizophrenia has been reported in epidemiologic and longitudinal observational studies,32,33 with several studies showing that the risk associated with marijuana smoking may be limited to young individuals predisposed towards schizophrenia.34,35 However, some studies have found no association between marijuana smoking and the onset of schizophrenia,36 and past and recent marijuana use may actually be associated with enhanced neurocognitive performance on secondary measures of working memory in nonintoxicated schizophrenia patients.37,38 As such, the interaction between marijuana use, working memory, and schizophrenia remains unclear.
Studies employing real-time experience-sampling methodology may help to clarify these associations.39,40 However, knowledge of the direct effects of Δ9-THC on the component processes of schizophrenia, such as working memory, may help to clarify the causal relationships between these variables. For example, if Δ9-THC were found to acutely decrease working memory performance, this would be consistent with a causal link between regular marijuana smoking and impairment of a cognitive function centrally related to schizophrenia. However, a finding that Δ9-THC had a negligible or beneficial impact on working memory performance would be inconsistent with such a role. Therefore, this article primarily reviews studies of the acute effects of smoked marijuana, conducted under controlled laboratory conditions, on working memory.
ACUTE EFFECTS OF Δ9-THC ON WORKING MEMORY
Acute effects refer to those effects that occur while the participant is directly intoxicated from Δ9-THC administration (ie, within 4 hours of drug administration). Ranganathan and D’Souza41 conducted a comprehensive review of studies of the acute Δ9-THC effects on memory and concluded that Δ9-THC acutely impaired immediate, delayed, and working memory in psychiatrically healthy marijuana smokers. Given the hypothesized centrality of working memory to schizophrenia, this article focuses more narrowly on working memory, considering both primary and secondary measures. Additionally, since smoking is the method by which marijuana is typically used in the natural ecology, this article primarily focuses on studies of the effects of smoked marijuana. Given the overall similarity of time course effects between smoked marijuana and intravenous (IV) Δ9-THC on subjective ratings (eg, “I feel high”) and physiologic measures (eg, heart rate),42,43 studies that employed an IV route of administration were also considered. Since oral Δ9-THC does not share this similarity,44 studies that employed this route were not reviewed.
There are many factors to consider when examining the literature on acute effects of Δ9-THC on working memory performance. One variable that may moderate the acute effects of Δ9-THC is tolerance, with less frequent users thought to be more susceptible to cognitive effects than more frequent users.45 Thus, attempts were made to examine studies in daily and near-daily marijuana smokers separately from studies in relatively less frequent marijuana smokers, although this was difficult since marijuana use frequency was characterized differently across studies, and some studies employed participants with a wide range of use frequency. One similarity between these studies is that they were generally conducted in relatively young marijuana smokers (≤30 years of age), potentially a useful feature when considering the implications for the emergence of schizophrenia in youths.
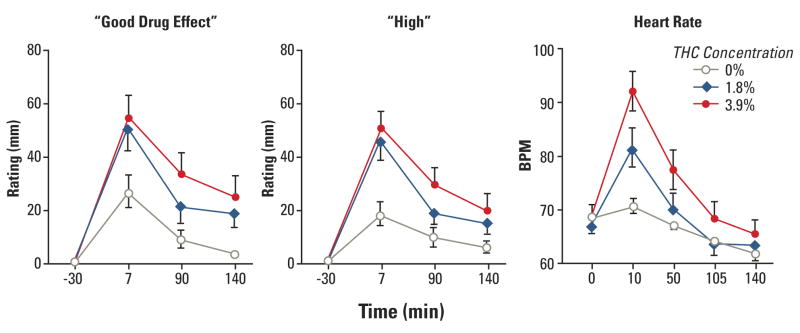
In the studies reviewed, only the effects of single marijuana cigarettes or deliveries of Δ9-THC, with varying concentrations of Δ9-THC across sessions, were examined, unless otherwise noted. In such studies, it is considered ideal to employ at least two active Δ9-THC concentrations and a placebo, since this allows for the analysis of Δ9-THC concentration-dependent functions.42 However, not all studies relevant to working memory employed this methodology, so studies that compared at least one concentration of Δ9-THC to placebo were also reviewed. However, caution must be exercised in interpreting the results of these studies. All studies that were reviewed incorporated counterbalancing of Δ9-THC concentrations across sessions, unless otherwise noted, and provided independent verification of participants’ Δ9-THC intoxication via subjective- effect ratings and/or physiologic measures. All smoked marijuana studies employed some form of standardized marijuana smoking (eg, paced puffing).46 The Figure 47 shows typical acute subjective and cardiovascular effects of single active marijuana cigarettes (closed symbols) relative to placebo marijuana cigarettes (open circles), produced under these controlled laboratory conditions. Based on this figure, it can be seen that subjective and cardiovascular effects peak within 7–10 minutes after marijuana smoking, and are Δ9-THC concentration dependent (eg, 3.9%>1.8%>placebo).
FIGURE. MEAN SUBJECTIVE-EFFECTS RATINGS (LEFT PANEL) AND MEAN HEART RATE (RIGHT PANEL) AS A FUNCTION OF Δ9-THC CONCENTRATION AND TIME*47.
* Error bars represent one SEM.
Δ9-THC=Delta 9-tetrahydrocannabinol; min=minutes; BPM=beats per minute; SEM= standard error of measurement.
Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25(5):757–765. Adapted with permission from Nature Publishing Group. Copyright 2001.
While most of these studies employed task batteries that measured a wide range of cognitive functions, this article focuses on the working memory performance data from those studies. Regarding measurement of working memory, there are several caveats to this review. First, since all of the studies that were reviewed employed working memory tasks that were visuomotor in nature (including digit recall tasks), this review cannot speak to Δ9-THC effects on auditory working memory. Second, the amount of training on the working memory tasks prior to the experimental sessions, which may influence the baseline level of performance during the sessions, differed across studies. Third, since the motivation for participants to perform cognitive tasks effortfully while intoxicated from Δ9-THC is unclear,48 some investigators provided monetary payment that was performance-contingent (noted below), while others did not. These studies are summarized in the Table and reviewed below.43,47,49–58
TABLE.
| WM Domain | Study | N | MJ Use Frequency | Laboratory Admin. Route | Active Δ9-THC Concentration/Dose | Task(s) | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy/strategy | Response time | ||||||||
| Less frequent users | Visuospatial | Ilan et al49 | 10 | 1–4 times/month | Smoked | 3.5% | n-back | ↓ | ↑ |
| Ilan et al50 | 23 | 15–17 joints/month | Smoked | 1.8, 3.6% | n-back | ↓ | ↑ | ||
| D’Souza et al43 | 22 | Not reported | IV | 2.5, 5.0 mg | DMTS | ↓ | ↑ (trend) | ||
| Lane et al51 | 5 | 2–10 times/month | Smoked | 2.2, 3.9% | DMTS | ↓ | ↑ | ||
| Verbal | Fant et al52 | 10 | 0.5–3 times/week | Smoked | 1.8, 3.6% | Digit Recall | – | – | |
| Serial Addition/Subtraction | – | ||||||||
| Chait and Perry53 | 14 | 4 times/month | Smoked | 3.6% | Digit Recall | – | NM | ||
| Heishman et al54 | 3 | 4.7 joints/month | Smoked | 2.6% (2 cigarettes) | Digit Recall | – | – | ||
| Serial Addition/Subtraction | ↑ | ||||||||
| Secondary | Raemakers et al55 | 20 | 3.4 times/month | Smoked | 13.0% (250, 500 mg cigarettes) | TOL | ↓ | ↑ | |
| IGT | – | NM | |||||||
| Lane et al56 | 10 | 7.4 times/month | Smoked | 1.8 (1/2 cigarette), 1.8, 3.6% | Risk-taking | ↓ | – | ||
| More frequent users | Verbal | Hart et al47 | 18 | 6 times/week | Smoked | 1.8, 3.9% | Digit Recall | – | ↑ |
| Arithmetic | – | ↑ | |||||||
| Secondary | Vadhan et al57 | 36 | 6 times/week | Smoked | 1.8, 3.9% | IGT | – | ↑ | |
| Weinstein et al58 | 14 | 7 times/week | Smoked | 13, 17 mg | IGT | – | – | ||
| WCST | ↓ | NM | |||||||
Δ9-THC=Delta 9-tetrahydrocannabinol; WM=working memory; MJ=marijuana; Admin.=administration; ↓=decrease; ↑=increase; IV=intravenous; DMTS=delayed matching to sample task; – =no effect; TOL=Tower of London task; NM=not measured; IGT=Iowa Gambling task; WCST=Wisconsin Card Sorting task.
VISUOSPATIAL WORKING MEMORY
Ilan and colleagues49 investigated the effects of smoked marijuana on working memory performance in infrequent marijuana smokers (1–4 times/month). In this study, the effects of active marijuana (3.5% Δ9-THC) on spatial n-back task performance (n=2 trials) were compared to placebo marijuana in 10 college-aged individuals. Relative to baseline performance, active marijuana significantly reduced the accuracy and increased the response time on this task, compared to placebo. For example, participants performed within a 96% to 97% accuracy range at baseline, but performed at ~94% accuracy 20 minutes after active marijuana, relative to 97% accuracy 20 minutes after placebo marijuana. After 1 more hour, performance under the active marijuana condition began to trend back towards baseline performance. This pattern of results was replicated when two Δ9-THC concentrations (1.8 and 3.6%) were tested in more frequent marijuana smokers (15–17 marijuana cigarettes/month).50 Therefore, these two studies indicated that smoking a single marijuana cigarette, relative to placebo, acutely impaired spatial n-back performance to a mild degree in non-daily marijuana smokers.
D’Souza and colleagues43 examined cognitive performance after administration of IV Δ9-THC (2.5 or 5.0 mg Δ9-THC) in participants with varied reported lifetime exposures to marijuana (<5 uses [n=7], 11–100 uses [n=9], >100 times [n=6]); current use frequency was not reported. Working memory was assessed using a computerized delayed matching-to-sample task, during which a series of geometric shapes were presented consecutively, and participants were required to indicate when a repeated shape was shown. Performance on this task was assessed before and after Δ9-THC administration. The results indicated that active Δ9-THC decreased accuracy on this task, with a trend towards increased response time. Consistent with these results, it has also been found that smoked marijuana decreased performance accuracy and increased response time on a simpler delayed matching-to-sample task in infrequent marijuana smokers (2–10 days/month), despite performance accuracy being reinforced with monetary earnings.51
Thus, it appears that single administrations of marijuana or Δ9-THC acutely impaired visuospatial working memory performance in relatively infrequent marijuana smokers. Further, since this impairment occurred whether or not participants were being reinforced for accuracy with monetary payment, performance motivation did not appear to play a role.
VERBAL WORKING MEMORY
Marijuana-related effects on verbally based working memory have also been studied. The effects of single marijuana cigarettes were tested in marijuana smokers (n=10) who reported infrequent weekly use of marijuana (0.5–3.0 times/week).52 Working memory was primarily measured by a digit recall task, during which participants were first presented with a string of nine digits, and then a string of eight of the same digits in a random order after a delay. Participants were asked to identify the missing digit, thus requiring encoding and storage of the initial string, a mental reorganization of the new digit string, and a comparison of the two strings. Performance was reinforced with money earnings. The results indicated that, relative to placebo, marijuana (1.8 or 3.6% Δ9-THC) had no effect on accuracy or response time on digit recall, or on serial addition/subtraction, a task that requires a significant contribution from working memory. Consistent with these results, another study53 found that single marijuana cigarettes (3.6% Δ9-THC) had no impact on the accuracy of backwards digit recall in a comparable group of 14 marijuana users. However, in a smaller study54 of similar marijuana smokers (n=3), smoking two consecutive marijuana cigarettes (2.6% Δ9-THC) did impair accuracy of digit recall and accuracy and response time on serial addition/subtraction, despite task performance being reinforced with monetary earnings.
These studies suggest that smoking a single marijuana cigarette had no impact on accuracy or speed of verbal working memory when measured by digit storage/manipulation and mental arithmetic, regardless of the incentive to perform, whereas smoking two marijuana cigarettes did have a negative effect. Also, one of the studies with negative results52 employed forced-randomization for session order (ie, the lower-strength marijuana always preceded the higher-strength marijuana), which may have produced some state-dependent practice effects under the higher strength marijuana.
SECONDARY WORKING MEMORY MEASURES
In addition to the studies that have directly assessed working memory in infrequent smokers, investigators have employed executive function tasks that are secondary measures of working memory.59–61 The computerized version of the Tower of London task62 displays both starting and ending arrangements of colored balls on sticks, and requires participants to judge the fewest movements of the balls it would take to arrive at the end arrangement without violation of certain rules. The Iowa Gambling task requires participants to repeatedly select cards from four decks, each associated with a different pattern of winnings and losses in hypothetical money, which must be learned and kept in mind while choosing cards. In terms of overall winnings and losses, two of the decks are considered disadvantageous (“risky”), and two of the decks are considered advantageous.
Performance on these tasks during intoxication from single Δ9-THC-infused nicotine cigarettes (250 or 500 mg/kg cigarettes with 13.0% Δ9-THC) was examined in 20 participants who reported ~3.4 marijuana uses/month.55 The results indicated that the number of correct choices on the Tower task was decreased and response time was increased to a mild degree following active marijuana relative to placebo. Marijuana did not alter card selection on the Gambling task. However, single marijuana cigarettes (3.6% Δ9-THC) increased “risky” selections for real money on a different decision-making task, relative to placebo, in participants with comparable marijuana use.56 In sum, it appears that single marijuana cigarettes generally impaired performance on secondary measures of working memory in relatively infrequent marijuana smokers.
Generally, the studies reviewed above indicate that single administrations of smoked marijuana and IV Δ9-THC acutely impaired visually-based working memory function in marijuana smokers whose reported use frequency ranged from a handful of lifetime exposures to multiple times per month or week, but not near-daily or greater. Performance accuracy or strategy as well as response time (when measured) were affected, and these effects did not seem to be due to decreased performance motivation. However, impairments were temporary and generally of a mild degree. Accuracy and speed of verbal working memory were not affected by single marijuana cigarettes but were impaired by two cigarettes, suggesting that verbal working memory was more resistant than visuospatial working memory to marijuana-related impairments. Though not reviewed, other functions such as immediate memory and attention were also acutely impaired by Δ9-THC in these studies. Although this indicated that Δ9-THC did not selectively impact working memory, it should be noted that deficits in these other cognitive domains have been widely observed in schizophrenia patients.63
Relatively fewer studies have been conducted to examine Δ9-THC’s effects on working memory in more experienced marijuana smokers, such as those who smoke on a daily or near-daily basis. Hart and colleagues47 investigated the effects of single marijuana cigarettes (1.8 or 3.9% Δ9-THC) on a broad range of cognitive functions in 18 participants who reported smoking marijuana 6 days/week. In this study, working memory was assessed with a computerized backwards Digit Span test from the MicroCog test battery.64 The results showed that while participants required greater amounts of time to complete the task after active marijuana, relative to placebo, accuracy was not altered. The same pattern of effects was seen on a test of mental arithmetic. Thus, consistent with studies in less experienced marijuana smokers, marijuana slowed performance on tests of working memory; however, in contrast, it did not disrupt accuracy in frequent smokers.
This was the only study found that directly measured working memory performance under conditions of marijuana intoxication in near-daily marijuana smokers. However, similar to studies in less-frequent smokers, investigators have also examined performance on secondary measures of working memory. Employing a comparable group of participants (n=36) and similar marijuana administration procedures as the study by Hart and colleagues,47 another study57 found that smoked marijuana had no effect on Gambling task performance for real money in terms of card selection or money earned, but did increase the time required to complete the Gambling task, relative to placebo. Δ9-THC-infused nicotine cigarettes (13 and 17 mg Δ9-THC) were also found to have no effect on Gambling task card selection for hypothetical earnings, nor on performance speed, in daily marijuana smokers (n=14).58 However, in this study, Δ9-THC increased errors on the Wisconsin Card Sorting Task, which requires participants to sort cards according to implicit rules that covertly shift, relative to placebo. In sum, data from studies in relatively frequent marijuana smokers indicate that accuracy or strategy on primary and secondary tests of working memory was not disrupted during Δ9-THC intoxication, except on a measure of categorization and cognitive flexibility. Most studies did indicate a slowing effect of Δ9-THC on working memory tasks in this population.
The studies reviewed here varied considerably in participant characteristics, route of drug administration, and the tasks used to measure working memory. Nevertheless, this review indicated, although not unequivocally, that Δ9-THC acutely impaired accuracy and response time on tests of working memory function in occasional marijuana smokers. Acute marijuana-related impairment was generally limited to response time in relatively more frequent smokers. None of the studies revealed any beneficial impact of marijuana smoking on working memory. Although there is some controversy over the potency of the marijuana administered in the smoked marijuana studies, as compared to the marijuana smoked in naturalistic settings,65 several lines of evidence are inconsistent with this concern.45 For example, the marijuana cigarettes employed in these studies produced robust Δ9-THC concentration-dependent changes in mood and cardiovascular measures, and the upper levels of Δ9-THC were within the range of Δ9-THC found in American street marijuana.66 Therefore, the marijuana employed in these studies appears to be relevant and meaningful. In conclusion, if one considers both accuracy and response time as meaningful components of working memory function, it appears that Δ9-THC acutely decreases working memory function in marijuana smokers. As such, this review is consistent with the conclusions of Ranganathan and D’Souza.41
ACUTE Δ9-THC EFFECTS AND SCHIZOPHRENIA
The conclusion that Δ9-THC acutely impairs working memory in psychiatrically healthy participants may suggest that marijuana smoking is a mechanism by which individuals already vulnerable to schizophrenia may further impair this critical function, albeit acutely. However, the working memory deficits acutely induced by Δ9-THC in psychiatrically healthy marijuana smokers appear to be fairly mild49 relative to those reported in nonintoxicated schizophrenia patients.67 Additionally, schizophrenia is a disorder with multiple classes of symptoms, some of which appear to be related to working memory deficits,19,68,69 but some of which may not be.13,70,71 In other words, the presence of co-occurring working memory impairment, while suggestive, does not necessarily indicate an etiologic relationship. Therefore, the relevance of marijuana-related effects on working memory to the development of schizophrenia may become clearer still when studied in concert with other aspects of psychosis.72
Accordingly, studies have been conducted specifically to investigate the effects of Δ9-THC on cognitive functions simultaneously with other psychotomimetic experiences, such as positive and negative psychiatric symptoms and perceptual disturbances.41,73 For example, in addition to the working memory effects in psychiatrically healthy individuals described above, IV Δ9-THC also acutely increased global clinician ratings of positive and negative symptoms and perceptual alterations, as well as participant-rated anxiety, and decreased performance on other neurocognitive measures.43 These findings suggest that Δ9-THC acutely produces numerous effects qualitatively similar to psychiatric symptoms of schizophrenia, in addition to working memory deficits.
Expanding the clinical relevance of this work, IV Δ9-THC was administered to participants diagnosed with schizophrenia who reported a minimum of one lifetime exposure to marijuana.74 The results of this study essentially replicated the findings of these investigators’ earlier study43: Δ9-THC acutely increased global ratings of positive, negative, and general symptoms; perceptual disturbances; and global ratings of extrapyramidal symptoms in schizophrenia patients, although the magnitude of these increases did not differ from those seen in the healthy participants from D’Souza and colleagues.43 Working memory was not directly assessed in the schizophrenia patients, but verbal list-learning was found to be disrupted to a greater extent by Δ9-THC in the schizophrenia patients than in the controls. Additionally, Δ9-THC did not increase subjective ratings of euphoria in the schizophrenia patients, as it had in the healthy participants. Thus, Δ9-THC did not have a unique impact on participants with fully developed schizophrenia, except for verbal learning and possibly euphoria.
A different study75 examined the role of genetic factors in Δ9-THC’s acute effects. Δ9-THC-infused nicotine cigarettes were administered to psychotic participants, first-degree relatives of psychotic patients, and healthy controls, and psychiatric and cognitive measures were taken. Results indicated that acute effects in these areas were genetically moderated by the same functional polymorphism in the catechol-O-methyltransferase gene that was found in an epidemiologic study35 to have moderated the relationship between reported early onset marijuana smoking and subsequent emergence of adult psychosis. This suggests a common mechanism for both the acute and long-term psychosis-related responses to marijuana, highlighting the value of studying the acute psychotomimetic effects of marijuana in the laboratory with respect to the broader question of the development of schizophrenia.
Both of these studies represent significant progress in terms of expanding the clinical relevance of Δ9-THC administration studies. However, interpretations of the data are constrained by several methodologic limitations. While D’Souza and colleagues’43 study compared two doses of Δ9-THC to placebo, as is ideal, the investigators administered Δ9-THC intravenously, which may be limited in terms of its ecologic relevance. Specifically, it does not allow participants the opportunity to titrate their intake of Δ9-THC to produce desired effects, as smoked marijuana does.76 This may help explain why this study consistently found increases in psychotic symptoms and aversive feeling states in both the psychiatric and nonpsychiatric groups, but did not find similarly consistent increases in euphoria. In other words, this methodology may have concealed any acutely positive or beneficial effects of marijuana in the schizophrenia patients, such as has been shown for nicotine and working memory.77 Another concern was the use of clinician-rated measures of symptomatology to measure acute drug effects, which have unknown sensitivity and reliability for this purpose. The study by Henquet and colleagues75 employed a more ecologically relevant route of drug administration, but only compared one Δ9-THC dose to placebo. Further, the participants in both of these studies appeared to have a wide range of previous experience with marijuana, and neither study examined working memory directly.
ACUTE Δ9-THC EFFECTS IN INDIVIDUALS AT RISK FOR SCHIZOPHRENIA?
Since the clinical impact of Δ9-THC is of the most concern in relatively young individuals who smoke marijuana regularly and are at risk for schizophrenia, acute studies of Δ9-THC in individuals already diagnosed with schizophrenia may have limited relevance to the broader question of marijuana’s relationship to the development of schizophrenia. Testing smoked marijuana’s acute effects in marijuana smokers who are at risk to develop schizophrenia would address this concern. Given that first-degree relatives of schizophrenia patients share some latent liability for schizophrenia, they constitute one potential group for examination.75 However, the group with perhaps the most clinical relevance would be those identified as prodromal for schizophrenia, eg, those individuals who experience subthreshold psychotic symptoms such as suspiciousness, overvalued ideation, and illusions.78 Marijuana use disorders have been found to be one of the most common comorbid psychiatric diagnoses in these individuals,30,31 and naturalistic studies31,79 have demonstrated an association between marijuana smoking and psychotic-like experiences in psychosis-prone participants. Yet, no controlled laboratory studies have been conducted to date to test the acute effects of smoked marijuana on working memory and other psychotomimetic experiences in a group of psychosis-prone marijuana smokers, an empirically, clinically, and ecologically meaningful endeavor.
CONCLUSION
This article argues for the centrality of working memory function in schizophrenia, examines the association between marijuana smoking and schizophrenia, and reviews studies of the acute effects of Δ9-THC on working memory in psychiatrically healthy participants. The authors generally found that in psychiatrically healthy marijuana smokers, Δ9-THC acutely decreased working memory performance, including speed and/or accuracy, regardless of route of Δ9-THC administration (smoked or IV), with more prominent effects on visuospatial working memory. Thus, Δ9-THC acutely impairs a critical cognitive function that is associated with the development of schizophrenia. The authors have also reviewed studies that specifically targeted the psychiatric features of schizophrenia, which suggested that Δ9-THC may acutely produce or exacerbate these features in healthy participants and schizophrenia patients, respectively, although these studies were constrained by methodologic limitations. Lastly, the authors proposed future directions that may improve the ecologic and clinical relevance of such research.
Acknowledgments
The authors thank Cheryl M. Corcoran, MD, for comments on an earlier version of this manuscript, and Elysia Michaels and Eliezer Pickholtz for assistance with the literature search.
Footnotes
Disclosure: The authors report no affiliation with or financial interest in any organization that may pose a conflict of interest.
References
- 1.Raby WN. Comorbid cannabis misuse in psychotic disorders: treatment strategies. Primary Psychiatry. 2009;16(4):29–34. [Google Scholar]
- 2.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 3.Wechsler D. The Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 4.Heaton RK. Wisconsin Card Sorting Test—Computer Version 4—Research Edition—“English”. 4.21. San Antonio, TX: Psychological Corporation; 2005. [Google Scholar]
- 5.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 6.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 7.Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity? ! Intelligence. 1990;14(4):389–433. [Google Scholar]
- 8.Snitz BE, Curtis CE, Zald DH, Katsanis J, Iacono WG. Neuropsychological and oculomotor correlates of spatial working memory performance in schizophrenia patients and controls. Schizophr Res. 1999;38(1):37–50. doi: 10.1016/s0920-9964(98)00178-9. [DOI] [PubMed] [Google Scholar]
- 9.Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biol Psychiatry. 1999;46(3):392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 10.Brebion G, Amador X, Smith MJ, Gorman JM. Memory impairment and schizophrenia: the role of processing speed. Schizophr Res. 1998;30(1):31–39. doi: 10.1016/s0920-9964(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 11.Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Arch Gen Psychiatry. 1998;55(12):1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- 12.Dreher JC, Banquet JP, Allilaire JF, Paillere-Martinot ML, Dubois B, Burnod Y. Temporal order and spatial memory in schizophrenia: a parametric study. Schizophr Res. 2001;51(2–3):137–147. doi: 10.1016/s0920-9964(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 13.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160(10):1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 14.McClure MM, Bowie CR, Patterson TL, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. 2007;89(1–3):330–338. doi: 10.1016/j.schres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Williams LM, Whitford TJ, Flynn G, et al. General and social cognition in first episode schizophrenia: identification of separable factors and prediction of functional outcome using the IntegNeuro test battery. Schizophr Res. 2008;99(1–3):182–191. doi: 10.1016/j.schres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Serper MR, Beech DR, Harvey PD, Dill C. Neuropsychological and symptom predictors of aggression on the psychiatric inpatient service. J Clin Exp Neuropsychol. 2008;30:700–709. doi: 10.1080/13803390701684554. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran C, Whitaker A, Coleman E, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80(2–3):283–293. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlenmeyer-Kimling L, Rock D, Roberts SA, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157(9):1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 19.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 20.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Carey C, Grant I. Nonacute (residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. J Clin Pharmacol. 2002;42(11 suppl):48S–57S. doi: 10.1002/j.1552-4604.2002.tb06003.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81(2):319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 24.Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 26.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 2006;185(3):358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- 28.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Fowler IL, Carr VJ, Carter NT, Lewin TJ. Patterns of current and lifetime substance use in schizophrenia. Schizophr Bull. 1998;24(3):443–455. doi: 10.1093/oxfordjournals.schbul.a033339. [DOI] [PubMed] [Google Scholar]
- 30.Rosen JL, Miller TJ, D’Andrea JT, McGlashan TH, Woods SW. Comorbid diagnoses in patients meeting criteria for the schizophrenia prodrome. Schizophr Res. 2006;85(1–3):124–131. doi: 10.1016/j.schres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Corcoran C, Kimhy D, Stanford A, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr Res. 2008;106(2–3):286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental heath outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 33.Bodkin LL, Singh A, Corcoran C. Cannabis as a risk factor for psychosis in vulnerable teens: implications for treatment. Primary Psychiatry. 2008;15(6):51–57. [Google Scholar]
- 34.Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend. 2003;71(1):37–48. doi: 10.1016/s0376-8716(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 35.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Phillips LJ, Curry C, Yung AR, Yuen HP, Adlard S, McGorry PD. Cannabis use is not associated with the development of psychosis in an ultra high-risk group. Aust N Z J Psychiatry. 2002;36(6):800–806. doi: 10.1046/j.1440-1614.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 37.Coulston CM, Perdices M, Tennant CC. The neuropsychology of cannabis and other substance use in schizophrenia: review of the literature and critical evaluation of methodological issues. Aust N Z J Psychiatry. 2007;41(11):869–884. doi: 10.1080/00048670701634952. [DOI] [PubMed] [Google Scholar]
- 38.Coulston CM, Perdices M, Tennant CC. The neuropsychological correlates of cannabis use in schizophrenia: lifetime abuse/dependence, frequency of use, and recency of use. Schizophr Res. 2007;96(1–3):169–184. doi: 10.1016/j.schres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Verdoux H, Gindre C, Sorbara F, Tournier M, Swendsen JD. Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Psychol Med. 2003;33(1):23–32. doi: 10.1017/s0033291702006384. [DOI] [PubMed] [Google Scholar]
- 40.Kimhy D, Durbin K, Corcoran CM. Cannabis and psychosis: what can daily diaries tell us about who is vulnerable? Primary Psychiatry. 2009;16(4):44–48. [PMC free article] [PubMed] [Google Scholar]
- 41.Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188(4):425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- 42.Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8(2–3):101–112. [PubMed] [Google Scholar]
- 43.D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 44.Haney M, Gunderson EW, Rabkin J, et al. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45(5):545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- 45.Nordstrom BR, Hart CL. Assessing cognitive functioning in cannabis users: cannabis use history an important consideration. Neuropsychopharmacology. 2006;31(12):2798–2799. doi: 10.1038/sj.npp.1301210. [DOI] [PubMed] [Google Scholar]
- 46.Foltin RW, Capriotti RM, McEntee MA, Fischman MW, Brady JV, Pedroso JJ. Effects of marijuana, cocaine, and task performance on cardiovascular responsivity. NIDA Res Monogr. 1987;76:259–265. [PubMed] [Google Scholar]
- 47.Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25(5):757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 48.Cherek DR, Lane SD, Dougherty DM. Possible amotivational effects following marijuana smoking under laboratory conditions. Exp Clin Psychopharmacol. 2002;10(1):26–38. doi: 10.1037//1064-1297.10.1.26. [DOI] [PubMed] [Google Scholar]
- 49.Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology (Berl) 2004;176(2):214–222. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16(5–6):487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- 51.Lane SD, Cherek DR, Lieving LM, Tcheremissine OV. Marijuana effects on human forgetting functions. J Exp Anal Behav. 2005;83(1):67–83. doi: 10.1901/jeab.2005.22-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60(4):777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- 53.Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115(3):340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- 54.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37(3):561–565. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- 55.Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31(10):2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- 56.Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30(4):800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- 57.Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. J Clin Exp Neuropsychol. 2007;29(4):357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein A, Brickner O, Lerman H, et al. A study investigating the acute dose - response effects of 13 mg and 17 mg {Delta} 9- tetrahydrocannabinol on cognitive - motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol. 2008;22(4):441–451. doi: 10.1177/0269881108088194. [DOI] [PubMed] [Google Scholar]
- 59.Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- 60.Phillips LH, Wynn V, Gilhooly KJ, Della Sala S, Logie RH. The role of memory in the Tower of London task. Memory. 1999;7(2):209–231. doi: 10.1080/741944066. [DOI] [PubMed] [Google Scholar]
- 61.Lane SD, Yechiam E, Busemeyer JR. Application of a computational decision model to examine acute drug effects on human risk taking. Exp Clin Psychopharmacol. 2006;14(2):254–264. doi: 10.1037/1064-1297.14.2.254. [DOI] [PubMed] [Google Scholar]
- 62.Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26(6):1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- 63.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 64.Powell DH. Comment on Computerized assessment of arithmetic computation skills with MicroCog. J Int Neuropsychol Soc. 1997;3(2):200. [PubMed] [Google Scholar]
- 65.Ramaekers JG, Kauert G, Theunissen EL, Moeller MR. Up in smoke: comparability of THC dosing across performance studies. Neuropsychopharmacology. 2006;31(12):2800–2801. [Google Scholar]
- 66.ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF., 3rd Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forensic Sci. 2000;45(1):24–30. [PubMed] [Google Scholar]
- 67.Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Res. 2007;150(2):111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 68.Harvey PD, Serper MR, White L, et al. The convergence of neuropsychological testing and clinical ratings of cognitive impairment in patients with schizophrenia. Compr Psychiatry. 2001;42(4):306–313. doi: 10.1053/comp.2001.24587a. [DOI] [PubMed] [Google Scholar]
- 69.Vadhan NP, Serper MR, Harvey PD, Chou JC, Cancro R. Convergent validity and neuropsychological correlates of the schedule for the assessment of negative symptoms (SANS) attention subscale. J Nerv Ment Dis. 2001;189(9):637–641. doi: 10.1097/00005053-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Neuropsychological correlates of negative, disorganized and psychotic symptoms in schizophrenia. Schizophr Res. 1998;31(2–3):99–111. doi: 10.1016/s0920-9964(98)00023-1. [DOI] [PubMed] [Google Scholar]
- 71.Bell MD, Lysaker PH, Milstein RM, Beam-Goulet JL. Concurrent validity of the cognitive component of schizophrenia: relationship of PANSS scores to neuropsychological assessments. Psychiatry Res. 1994;54(1):51–58. doi: 10.1016/0165-1781(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 72.D’Souza DC. Cannabinoids and psychosis. Int Rev Neurobiol. 2007;78:289–326. doi: 10.1016/S0074-7742(06)78010-2. [DOI] [PubMed] [Google Scholar]
- 73.Koethe D, Gerth CW, Neatby MA, et al. Disturbances of visual information processing in early states of psychosis and experimental delta-9-tetrahydrocannabinol altered states of consciousness. Schizophr Res. 2006;88(1–3):142–150. doi: 10.1016/j.schres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 74.D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31(12):2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- 76.Cooper ZD, Haney M. Comparison of the subjective, pharmacokinetic and physiologic effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2009.01.023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.George TP, Vessicchio JC, Termine A, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 78.McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L, editors. Instrument for the Assessment of Prodromal Symptoms and States. Amsterdam, Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 79.Verdoux H, Husky M, Tournier M, Sorbara F, Swendsen JD. Social environments and daily life occurrence of psychotic symptoms--an experience sampling test in a non-clinical population. Soc Psychiatry Psychiatr Epidemiol. 2003;38(11):654–661. doi: 10.1007/s00127-003-0702-8. [DOI] [PubMed] [Google Scholar]