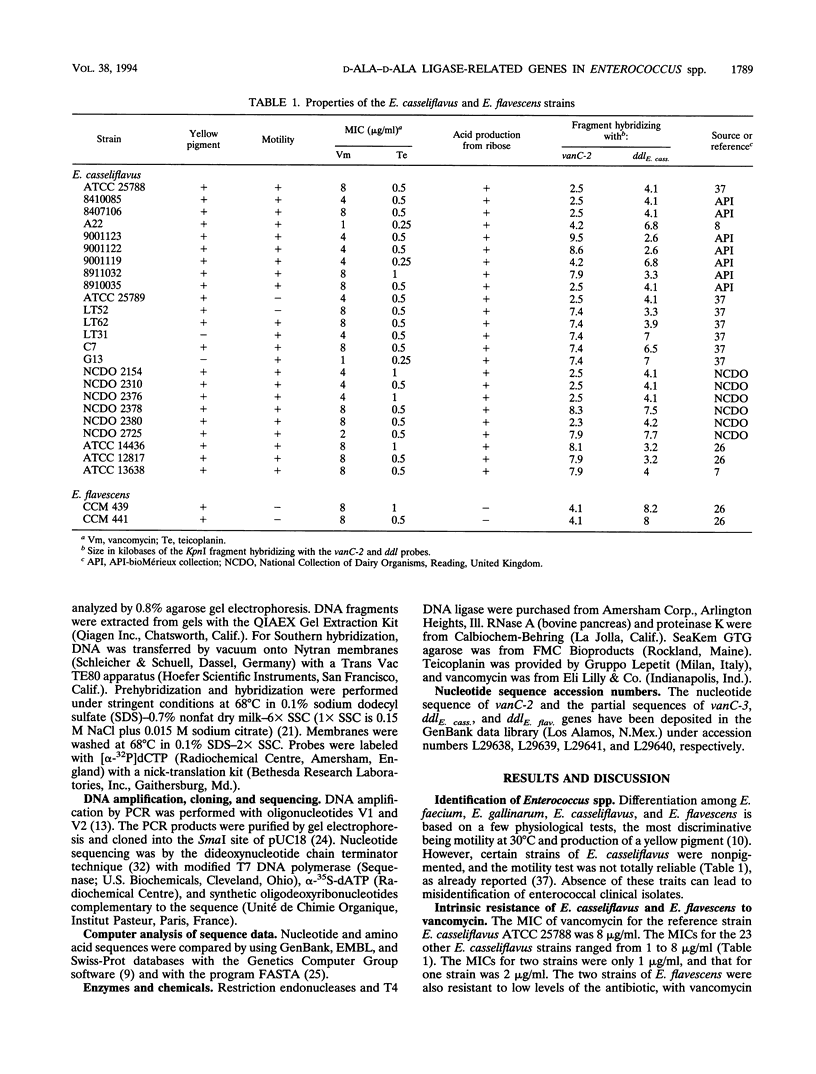
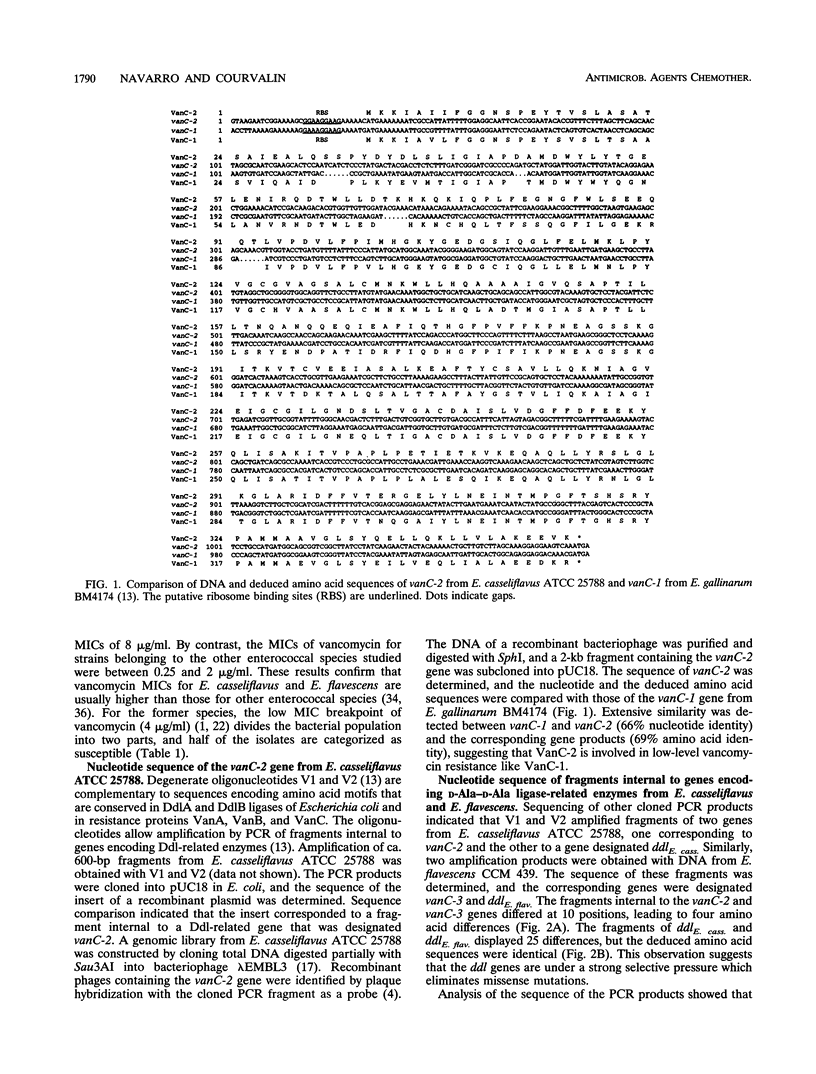
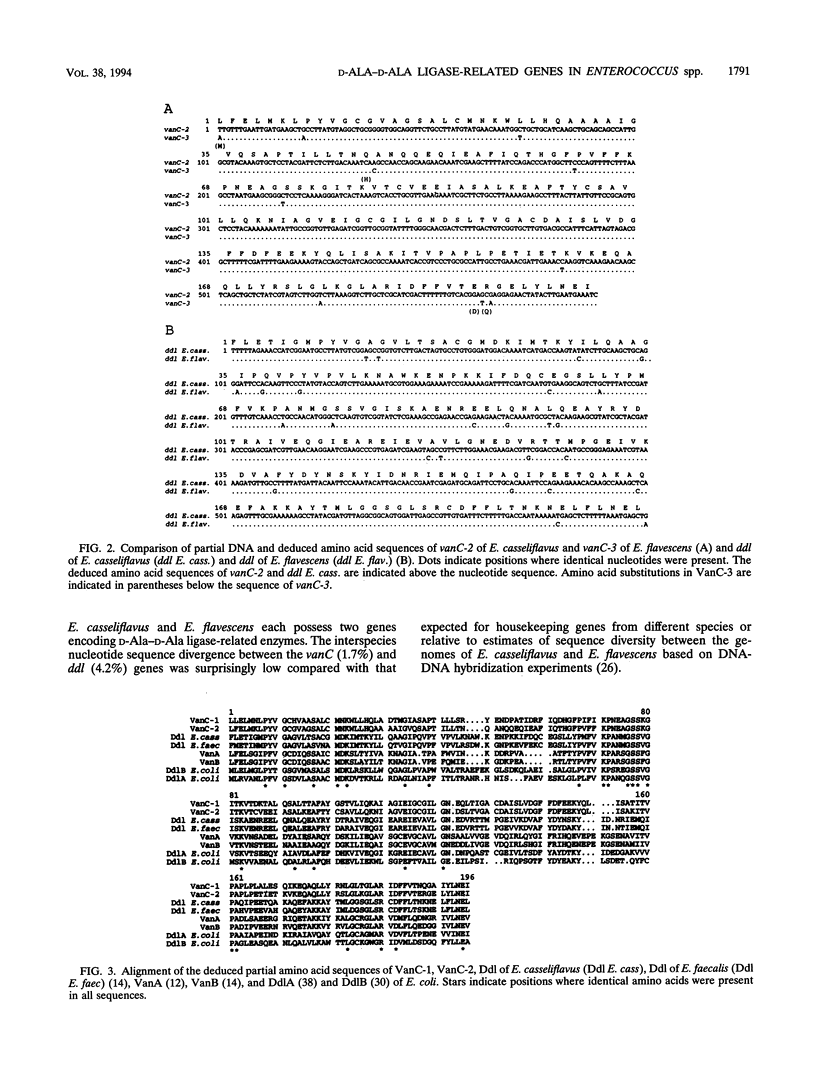
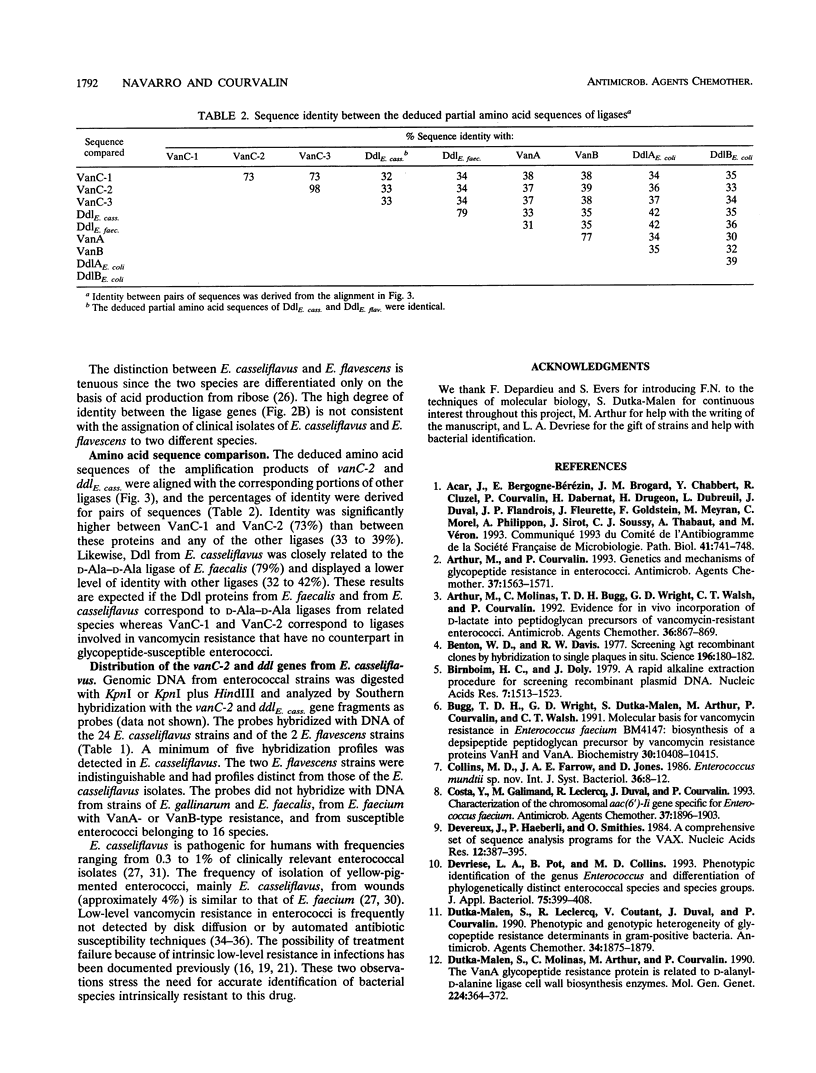
Abstract
Using degenerate oligonucleotides complementary to sequences encoding conserved amino acid motifs in D-alanine-D-alanine (Ddl) ligases, we have amplified ca. 600-bp fragments from Enterococcus casseliflavus ATCC 25788 and Enterococcus flavescens CCM439. Sequence analysis of the amplification products indicated that each strain possessed two genes, ddlE. cass. and vanC-2, and ddlE. flav. and vanC-3, respectively, encoding Ddl-related enzymes. The fragments internal to the vanC genes were 98.3% identical. The vanC-2 gene was cloned into Escherichia coli and sequenced. Extensive similarity (66% nucleotide identity) was detected between this gene and vanC-1 from Enterococcus gallinarum (S. Dutka-Malen, C. Molinas, M. Arthur, and P. Courvalin, Gene 112:53-58, 1992), suggesting that the vanC genes are required for intrinsic low-level resistance to vancomycin. The partial deduced amino acid sequences of ddlE. cass. and ddlE. flav. were identical and closely related to that of the Ddl ligase of Enterococcus faecalis (79% identity). In Southern hybridization experiments, only DNA from E. casseliflavus and E. flavescens hybridized to probes internal to the vanC-2 and ddlE. cass. genes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Bugg T. D., Wright G. D., Walsh C. T., Courvalin P. Evidence for in vivo incorporation of D-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Apr;36(4):867–869. doi: 10.1128/aac.36.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Wright G. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991 Oct 29;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Costa Y., Galimand M., Leclercq R., Duval J., Courvalin P. Characterization of the chromosomal aac(6')-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993 Sep;37(9):1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriese L. A., Pot B., Collins M. D. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J Appl Bacteriol. 1993 Nov;75(5):399–408. doi: 10.1111/j.1365-2672.1993.tb02794.x. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992 Mar 1;112(1):53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. The VANA glycopeptide resistance protein is related to D-alanyl-D-alanine ligase cell wall biosynthesis enzymes. Mol Gen Genet. 1990 Dec;224(3):364–372. doi: 10.1007/BF00262430. [DOI] [PubMed] [Google Scholar]
- Evers S., Reynolds P. E., Courvalin P. Sequence of the vanB and ddl genes encoding D-alanine:D-lactate and D-alanine:D-alanine ligases in vancomycin-resistant Enterococcus faecalis V583. Gene. 1994 Mar 11;140(1):97–102. doi: 10.1016/0378-1119(94)90737-4. [DOI] [PubMed] [Google Scholar]
- Evers S., Sahm D. F., Courvalin P. The vanB gene of vancomycin-resistant Enterococcus faecalis V583 is structurally related to genes encoding D-Ala:D-Ala ligases and glycopeptide-resistance proteins VanA and VanC. Gene. 1993 Feb 14;124(1):143–144. doi: 10.1016/0378-1119(93)90779-3. [DOI] [PubMed] [Google Scholar]
- Fantin B., Leclercq R., Arthur M., Duval J., Carbon C. Influence of low-level resistance to vancomycin on efficacy of teicoplanin and vancomycin for treatment of experimental endocarditis due to Enterococcus faecium. Antimicrob Agents Chemother. 1991 Aug;35(8):1570–1575. doi: 10.1128/aac.35.8.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Volk K. J., Liu J., Lee M. S. The cytoplasmic peptidoglycan precursor of vancomycin-resistant Enterococcus faecalis terminates in lactate. J Bacteriol. 1992 Sep;174(18):5982–5984. doi: 10.1128/jb.174.18.5982-5984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J Bacteriol. 1990 Feb;172(2):727–734. doi: 10.1128/jb.172.2.727-734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Duval J., Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Sep;36(9):2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei R., Berlutti F., Thaller M. C., Ingianni A., Cortis G., Dainelli B. Enterococcus flavescens sp. nov., a new species of enterococci of clinical origin. Int J Syst Bacteriol. 1992 Jul;42(3):365–369. doi: 10.1099/00207713-42-3-365. [DOI] [PubMed] [Google Scholar]
- Pompei R., Lampis G., Berlutti F., Thaller M. C. Characterization of yellow-pigmented enterococci from severe human infections. J Clin Microbiol. 1991 Dec;29(12):2884–2886. doi: 10.1128/jcm.29.12.2884-2886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani R., Jr, Evers S., Courvalin P. The vanB gene confers various levels of self-transferable resistance to vancomycin in enterococci. J Infect Dis. 1993 May;167(5):1220–1223. doi: 10.1093/infdis/167.5.1220. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Snaith H. A., Maguire A. J., Dutka-Malen S., Courvalin P. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem J. 1994 Jul 1;301(Pt 1):5–8. doi: 10.1042/bj3010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Kenan D. J., Sweeney J., Donachie W. D. Further evidence for overlapping transcriptional units in an Escherichia coli cell envelope-cell division gene cluster: DNA sequence and transcriptional organization of the ddl ftsQ region. J Bacteriol. 1986 Sep;167(3):809–817. doi: 10.1128/jb.167.3.809-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoff K. L., de la Maza L., Murtagh M. J., Spargo J. D., Ferraro M. J. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol. 1990 Mar;28(3):435–437. doi: 10.1128/jcm.28.3.435-437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Ferraro M. J., Sahm D. F., Charache P., Tenover F. C. New vancomycin disk diffusion breakpoints for enterococci. The National Committee for Clinical Laboratory Standards Working Group on Enterococci. J Clin Microbiol. 1992 Oct;30(10):2525–2528. doi: 10.1128/jcm.30.10.2525-2528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Hill B. C., Thornsberry C. Problems with the disk diffusion test for detection of vancomycin resistance in enterococci. J Clin Microbiol. 1989 Sep;27(9):2140–2142. doi: 10.1128/jcm.27.9.2140-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Tokars J., Swenson J., Paul S., Spitalny K., Jarvis W. Ability of clinical laboratories to detect antimicrobial agent-resistant enterococci. J Clin Microbiol. 1993 Jul;31(7):1695–1699. doi: 10.1128/jcm.31.7.1695-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Knight R. G., Green M., Sahm D. F., Shlaes D. M. Vancomycin susceptibility and identification of motile enterococci. J Clin Microbiol. 1991 Oct;29(10):2335–2337. doi: 10.1128/jcm.29.10.2335-2337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzke L. E., Bugg T. D., Walsh C. T. Existence of two D-alanine:D-alanine ligases in Escherichia coli: cloning and sequencing of the ddlA gene and purification and characterization of the DdlA and DdlB enzymes. Biochemistry. 1991 Feb 12;30(6):1673–1682. doi: 10.1021/bi00220a033. [DOI] [PubMed] [Google Scholar]



