Abstract
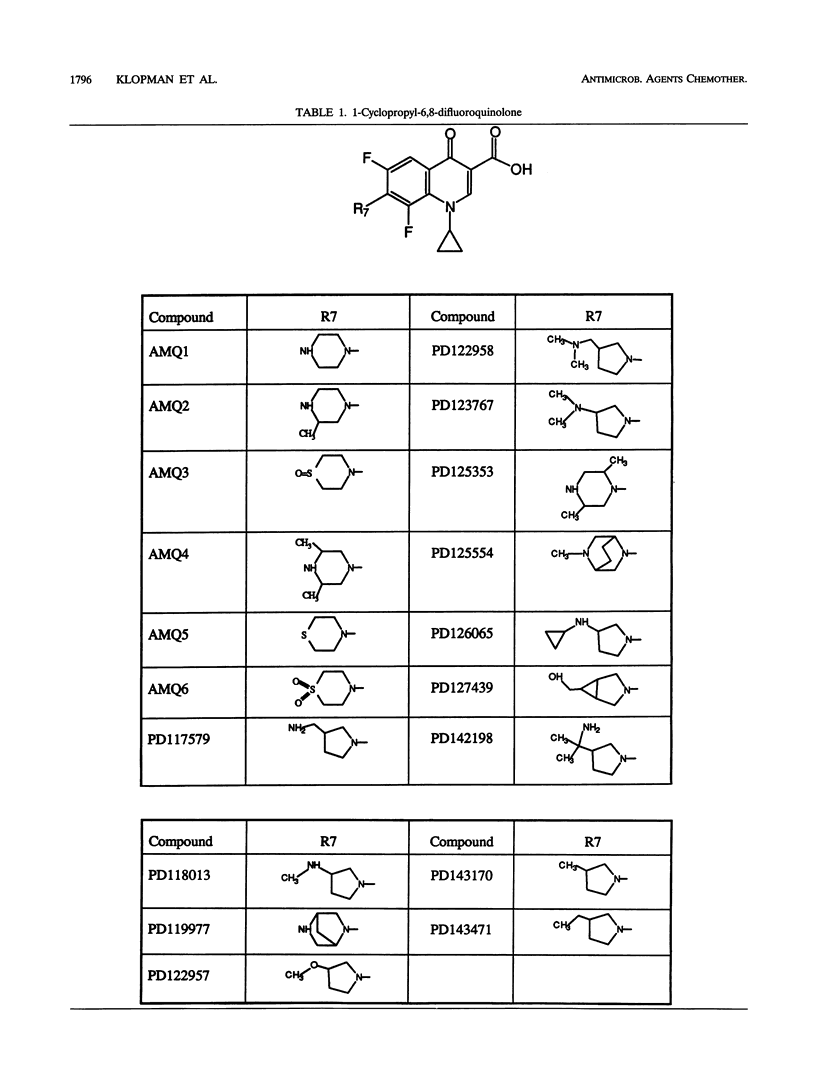
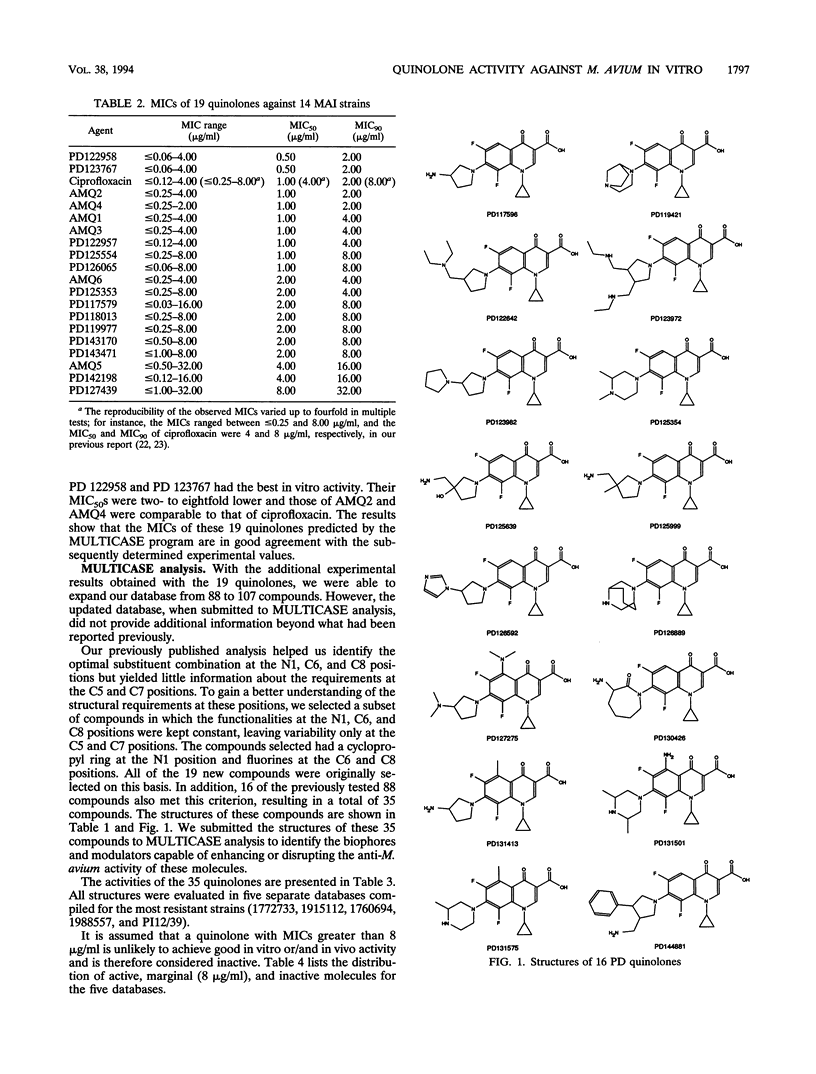
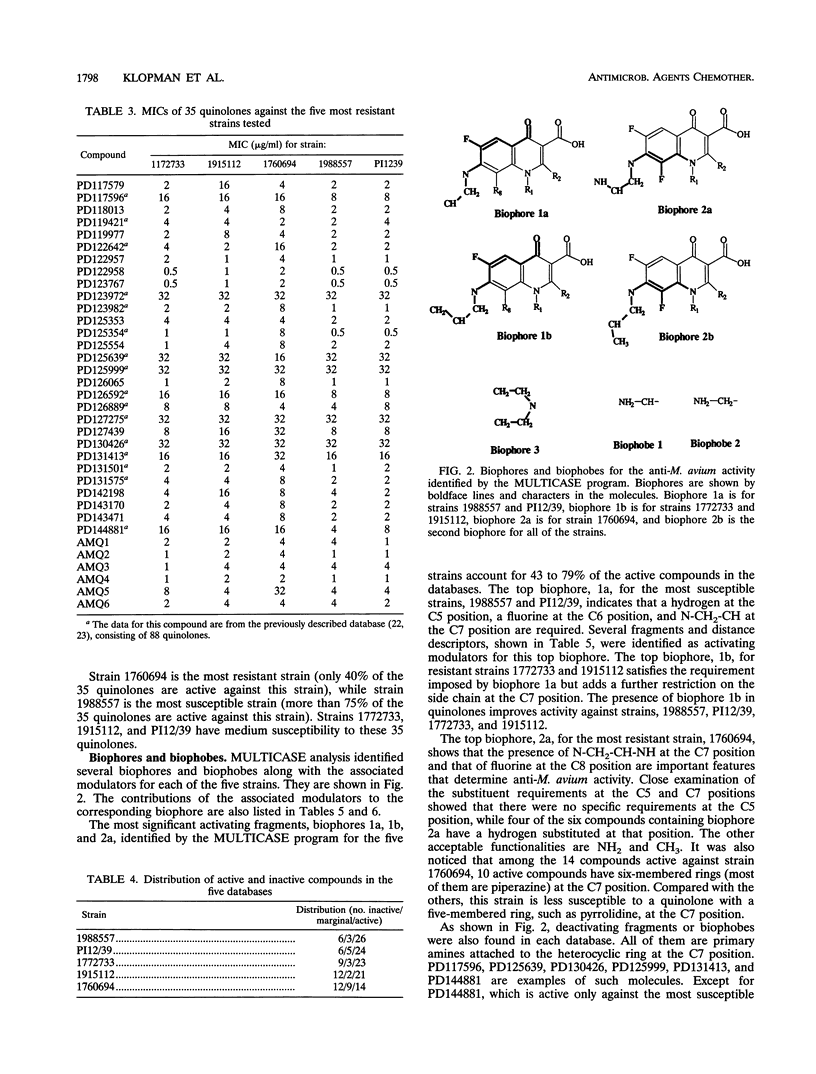
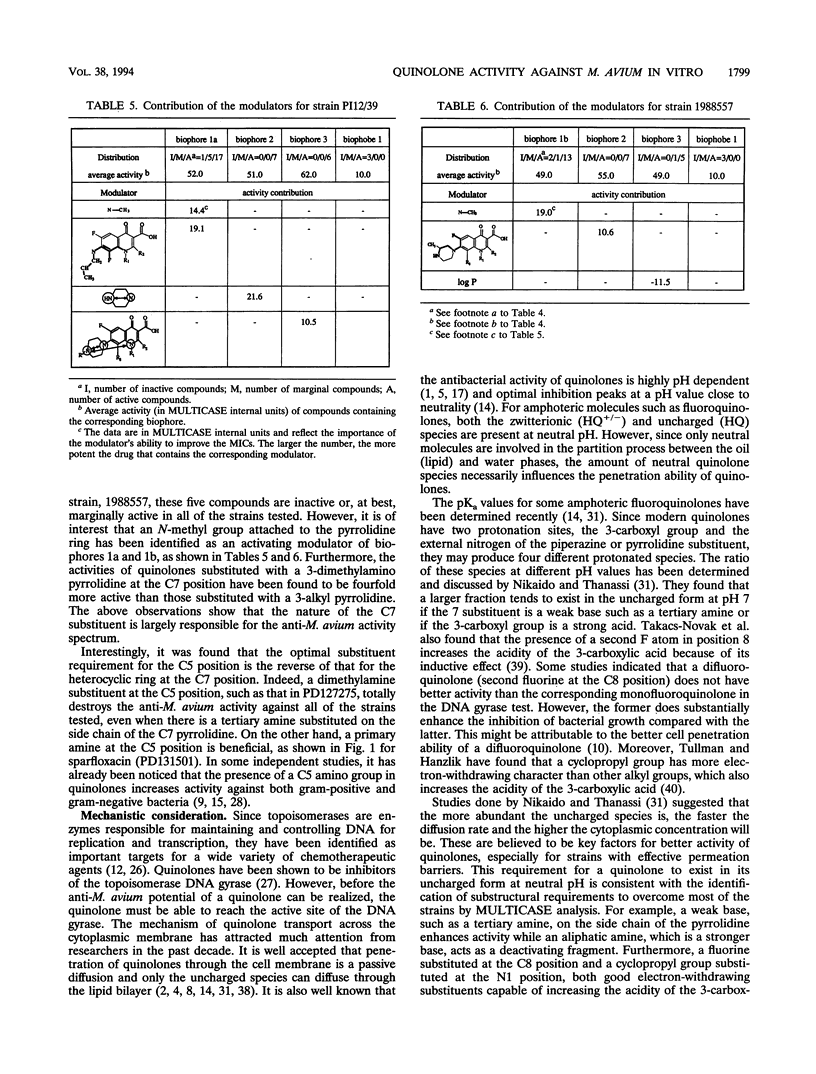
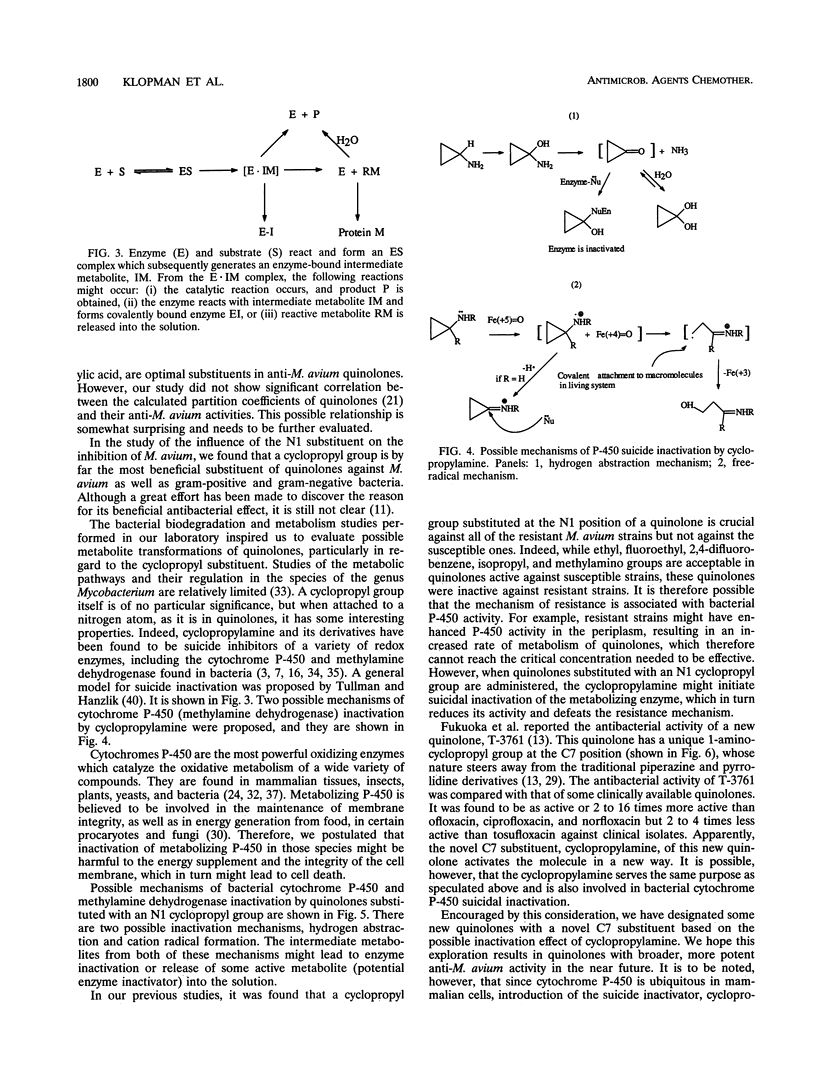
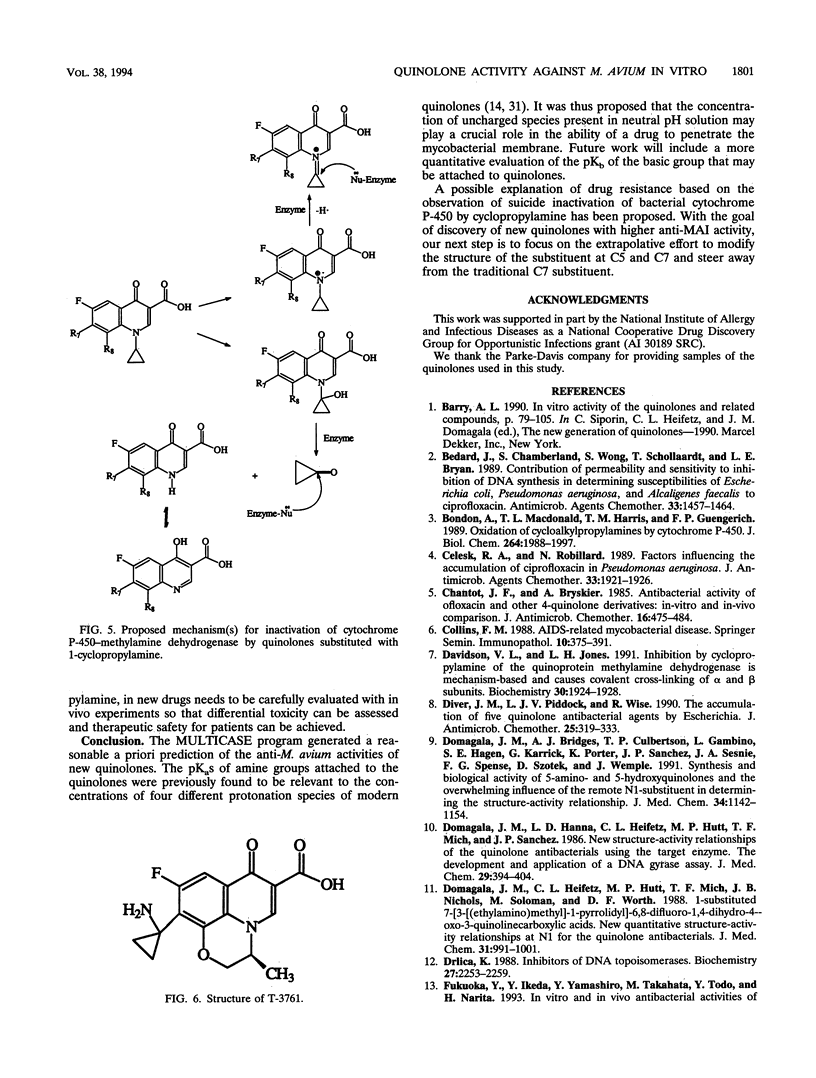
The relationship between the structures of quinolones and their anti-Mycobacterium avium activities has been previously derived by using the Multiple Computer-Automated Structure Evaluation program. A number of substructural constraints required to overcome the resistance of most of the strains have been identified. Nineteen new quinolones which qualify under these substructural requirements were identified by the program and subsequently tested. The results show that the substructural attributes identified by the program produced a successful a priori prediction of the anti-M. avium activities of the new quinolones. All 19 quinolones were found to be active, and 4 of them are as active or better than ciprofloxacin. With these new quinolones, the updated multiple computer-automated structure evaluation program structure-activity relationship analysis has helped to uncover additional information about the nature of the substituents at the C5 and C7 positions needed for optimal inhibitory activity. A possible explanation of drug resistance based on the observation of suicide inactivation of bacterial cytochrome P-450 by the cyclopropylamine moiety has also been proposed and is discussed in this report. Furthermore, we confirm the view that the amount of the uncharged form present in a neutral pH solution plays a crucial role in the drug's penetration ability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondon A., Macdonald T. L., Harris T. M., Guengerich F. P. Oxidation of cycloalkylamines by cytochrome P-450. Mechanism-based inactivation, adduct formation, ring expansion, and nitrone formation. J Biol Chem. 1989 Feb 5;264(4):1988–1997. [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantot J. F., Bryskier A. Antibacterial activity of ofloxacin and other 4-quinolone derivatives: in-vitro and in-vivo comparison. J Antimicrob Chemother. 1985 Oct;16(4):475–484. doi: 10.1093/jac/16.4.475. [DOI] [PubMed] [Google Scholar]
- Collins F. M. AIDS-related mycobacterial disease. Springer Semin Immunopathol. 1988;10(4):375–391. doi: 10.1007/BF02053847. [DOI] [PubMed] [Google Scholar]
- Davidson V. L., Jones L. H. Inhibition by cyclopropylamine of the quinoprotein methylamine dehydrogenase is mechanism-based and causes covalent cross-linking of alpha and beta subunits. Biochemistry. 1991 Feb 19;30(7):1924–1928. doi: 10.1021/bi00221a027. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Piddock L. J., Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990 Mar;25(3):319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Bridges A. J., Culbertson T. P., Gambino L., Hagen S. E., Karrick G., Porter K., Sanchez J. P., Sesnie J. A., Spense F. G. Synthesis and biological activity of 5-amino- and 5-hydroxyquinolones, and the overwhelming influence of the remote N1-substituent in determining the structure-activity relationship. J Med Chem. 1991 Mar;34(3):1142–1154. doi: 10.1021/jm00107a039. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Heifetz C. L., Hutt M. P., Mich T. F., Nichols J. B., Solomon M., Worth D. F. 1-Substituted 7-[3-[(ethylamino)methyl]-1-pyrrolidinyl]-6,8- difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acids. New quantitative structure-activity relationships at N1 for the quinolone antibacterials. J Med Chem. 1988 May;31(5):991–1001. doi: 10.1021/jm00400a017. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Furet Y. X., Deshusses J., Pechère J. C. Transport of pefloxacin across the bacterial cytoplasmic membrane in quinolone-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Nov;36(11):2506–2511. doi: 10.1128/aac.36.11.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen S. E., Domagala J. M., Heifetz C. L., Johnson J. Synthesis and biological activity of 5-alkyl-1,7,8-trisubstituted-6-fluoroquinoline-3-carboxylic acids. J Med Chem. 1991 Mar;34(3):1155–1161. doi: 10.1021/jm00107a040. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J. MICs and MBCs of Win 57273 against Mycobacterium avium and M. tuberculosis. Antimicrob Agents Chemother. 1990 May;34(5):770–774. doi: 10.1128/aac.34.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Cohn D. L., Roberts R. B., Masur H., Miller R. A., Tsang A. Y., Iseman M. D. Mycobacterium avium-M. intracellulare isolates from patients with or without acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1986 Dec;30(6):955–957. doi: 10.1128/aac.30.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Bajaksouzian S., Edmonds K., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: in vitro activities. Antimicrob Agents Chemother. 1993 Sep;37(9):1799–1806. doi: 10.1128/aac.37.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: structure-activity relationship studies. Antimicrob Agents Chemother. 1993 Sep;37(9):1807–1815. doi: 10.1128/aac.37.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koymans L., Donné-op den Kelder G. M., Koppele Te J. M., Vermeulen N. P. Cytochromes P450: their active-site structure and mechanism of oxidation. Drug Metab Rev. 1993;25(3):325–387. doi: 10.3109/03602539308993979. [DOI] [PubMed] [Google Scholar]
- Leysen D. C., Haemers A., Pattyn S. R. Mycobacteria and the new quinolones. Antimicrob Agents Chemother. 1989 Jan;33(1):1–5. doi: 10.1128/aac.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Moran D. B., Ziegler C. B., Jr, Dunne T. S., Kuck N. A., Lin Y. I. Synthesis of novel 5-fluoro analogues of norfloxacin and ciprofloxacin. J Med Chem. 1989 Jun;32(6):1313–1318. doi: 10.1021/jm00126a028. [DOI] [PubMed] [Google Scholar]
- Muratani T., Inoue M., Mitsuhashi S. In vitro activity of T-3761, a new fluoroquinolone. Antimicrob Agents Chemother. 1992 Oct;36(10):2293–2303. doi: 10.1128/aac.36.10.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Thanassi D. G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob Agents Chemother. 1993 Jul;37(7):1393–1399. doi: 10.1128/aac.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács-Novák K., Noszál B., Hermecz I., Keresztúri G., Podányi B., Szász G. Protonation equilibria of quinolone antibacterials. J Pharm Sci. 1990 Nov;79(11):1023–1028. doi: 10.1002/jps.2600791116. [DOI] [PubMed] [Google Scholar]
- Tullman R. H., Hanzlik R. P. Inactivation of cytochrome P-450 and monoamine oxidase by cyclopropylamines. Drug Metab Rev. 1984;15(5-6):1163–1182. doi: 10.3109/03602538409033560. [DOI] [PubMed] [Google Scholar]
- Uotila J. S., Kitunen V. H., Saastamoinen T., Coote T., Häggblom M. M., Salkinoja-Salonen M. S. Characterization of aromatic dehalogenases of Mycobacterium fortuitum CG-2. J Bacteriol. 1992 Sep;174(17):5669–5675. doi: 10.1128/jb.174.17.5669-5675.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]



