Abstract
Preparative separation of elatine in Delphinium shawurense was achieved for the first time using high-speed countercurrent chromatography (HSCCC). The separation was performed with a solvent system composed of ethyl acetate-chloroform-methanol-water (3:0.1:2:3, v/v) using the lower organic phase as a mobile phase under a revolution speed of 800 rpm. This yielded 72 mg of elatine at over 97% purity with an approximately 95% recovery. The chemical structure was identified by MS and NMR.
Keywords: High-speed countercurrent chromatography (HSCCC), Delphinium Shawurense, elatine
INTRODUCTION
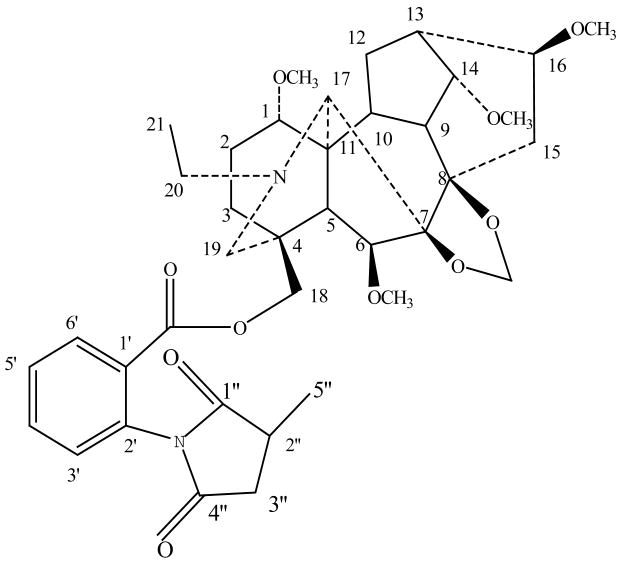
Delphinium species have been employed in analgesic balms, sedatives, emetics and antihelminthics in folk medicine [1]. Diterpenoid alkaloids are the major active constituents of this plant. Delphinium shawurense is a unique plant resource in Xinjiang, China. However, few phytochemical investigations about this plant have been described in the literature up to now. In this paper, we report a diterpenoid alkaloid from the ethanol extract of Delphinium Shawurense which has been shown to have muscle relaxing activity [2, 3]. The chemical structure of this diterpenoid alkaloid was shown in Fig. 1.
Fig. 1.
Chemical structure of elatine from Delphinium shawurense by HSCCC
High-speed countercurrent chromatography (HSCCC), being a support-free liquid–liquid partition chromatographic technique, eliminates irreversible adsorption of the sample onto the solid support [4], and has been widely used in preparative separation of natural products [5], especially for alkaloids [6, 7].
The present paper describes the successful preparative separation of elatine from Delphinium shawurense by HSCCC.
EXPERIMENTAL
Apparatus
The preparative HSCCC instrument employed in this study is a Model GS 10A2 multiplayer coil of 110 m × 1.6 mm I.D. with a total capacity of 200 ml designed and constructed at Beijing Institute of New Technology Application, Beijing, China. The β values of this preparative column range from 0.5 to 0.8. The revolution speed of the apparatus could be regulated with a speed controller in a range between 0 to 1000 rpm. An optimum speed of 800 rpm was used in this study.
The solvent was pumped into the column with a Model NS-1007 constant-flow pump (Beijing Institute of New Technology Application, Beijing, China). Continuous monitoring of the effluent was achieved with a Model 8823A-UV monitor (Beijing Institute of New Technology Application) at 254 nm. A manual sample injection valve with a 20-ml loop for the preparative HSCCC (Tianjin High New Science Technology, Tianjin, China) was used to introduce the sample into the column, respectively. A portable recorder (Yokogawa Model 3057, Sichuan Instrument Factory, Chongqing, China) was used to draw the chromatogram.
The high-performance liquid chromatography equipment (DIONEX, USA) used was a DIONEX system including a P680 pump, a ASI-100 automated sample injector, a TCC-100 temperature-controlled column compartment, a UVD170U detector. The analysis was carried out with a Luna reversed-phase C18 column (4.6 mm I.D. × 250 mm, 5 μm; PHENOMENEX, USA). Evaluation and quantification were made on a Chromeleon WorkStation.
Reagents
All organic solutions used for HSCCC were of analytical grade and purchased from Tianjing Chemical Factory (Tianjing, China). Methanol used for HPLC was of HPLC-grade and purchased from Fisher Scientific Company (Fair Lawn, NJ, USA).
The whole plants of D. shawurense were collected from Buerjin County, Xinjiang Province, People’s Republic of China in June, 2005 and were identified by Prof. Shiming Duan of the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, People’s Republic of China.
Preparation of Crude Sample
Air-dried and powdered plants (2.9 kg) were percolated with 80% ethanol (7 times) at room temperature. The extracts were concentrated in vacuum to syrup, which was acidified with 0.5% H2SO4 to pH 2 and filtered. The acid solution was extracted six times with chloroform to give crude material (6.3 g) which was dissolved in 70 ml of acetone and filtered. The acetone extracts were concentrated to 20ml at room temperature to precipitate the crude sample (1.1 g), which was used for further HSCCC separation.
Preparation of Two-Phase Solvent System
A 10 ml volume of the solvent system to be examined was delivered into a test tube to which 5 mg of the crude sample was added. After the tube was shaken for 5 min to distribute the sample, two layers formed within 30 s. The two phases were separated manually, each transferred to a glass vial, dried and dissolved in 1 ml methanol for HPLC analysis. Partition coefficient (Kupper/lower) of elatine was obtained from the peak area of the upper phase divided by that in the lower phase (Table 1).
Table 1.
The partition coefficient (Kupper/lower) of elatine in ten solvent systems
| Solvent system | Volume ratio | Kelatine | |
|---|---|---|---|
| 1 | n-hexane-ethyl acetate-methanol-water | 1:2:1:2 | 3.98 |
| 2 | chloroform-ethyl acetate-methanol-water | 3:1:2:3 | 0.05 |
| 3 | ethyl acetate-chloroform-methanol-water | 3:0.5:2:3 | 0.10 |
| 4 | chloroform-1-butanol-methanol-water | 3:2:2:3 | 0.02 |
| 5 | chloroform-1-butanol-methanol-water | 1.5:1.5:2:3 | 6.05 |
| 6 | n-butanol-chloroform-methanol-water | 4:0.5:1:4 | 41.74 |
| 7 | n-butanol-dichloromethane -methanol-water | 4:0.5:1:4 | 139.42 |
| 8 | ethyl acetate-chloroform-methanol-water | 3:0.2:2:3 | 16.54 |
| 9 | ethyl acetate-chloroform-methanol-water | 3:0.1:2:3 | 1.28 |
| 10 | chloroform-1-butanol-isopropanol -water | 2:3:1:4 | 0.003 |
HSCCC Separation
The preparative HSCCC was similarly performed with a Model GS-10A2 HSCCC instrument as follows: the multiplayer coiled column was first entirely filled with the upper aqueous stationary phase. The lower organic mobile phase was then pumped into the head end of the column at a flow-rate of 2.0 ml/min, while the apparatus was run at a revolution speed of 800 rpm. After hydrodynamic equilibrium was reached, the sample solution (100 mg crude sample dissolved in 15 ml of the lower phase) was injected through the sample port. The effluent from the outlet of the column was continuously monitored with a UV detector at 254 nm. Peak fractions were manually collected according to the chromatogram.
HPLC Analysis and Identification of Crude Sample and Peak Fractions from HSCCC
The crude sample and the peak fraction from HSCCC were analyzed by HPLC. The analyses were performed with a C18 column (4.6 mm I.D. × 250 mm, 5 μm) at column temperature of 35°. The mobile phase was a linear gradient of methanol (A) and 0.4% phosphate acid (B) as follows: A–B (25:75, v/v) to A–B (35:65, v/v) in 15 min, then to A–B (40:60, v/v) in 5 min, then to A–B (40:60, v/v) in 10 min, and to A–B (60:40, v/v) in 5 min. The flow-rate was 1.0 ml min−1 and the effluent was monitored at 235 nm by a UV detector.
Identification of the HSCCC peak fraction was carried out by mass spectrometry (MS), 1H nuclear magnetic resonance (1H NMR), and 13C nuclear magnetic resonance (13C NMR).
RESULTS AND DISCUSSION
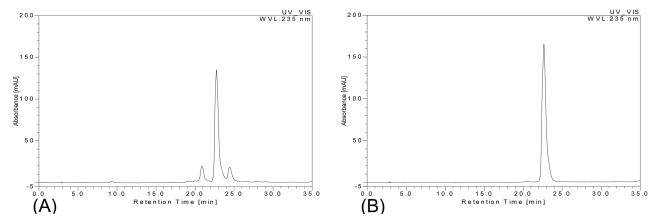
The crude sample was first analyzed by HPLC. The result indicated that it contained several compounds including elatine (retention time: 22.68 min) and some unknown compounds as shown in Fig. 2A.
Fig. 2.
HPLC analysis of the crude sample and elatine from HSCCC separation. Separation column: a C18 column (4.6 mm I.D. × 250 mm, 5 μm); column temperature: 35°C; detection wavelength: 235 nm; The mobile phase: a linear gradient of methanol (A) and 0.4% phosphoric acid (B) as follows: A–B (25:75, v/v) to A–B (35:65, v/v) in 15 min, then to A–B (40:60, v/v) in 5 min, then to A–B (40:60, v/v) in 10 min, then to A–B (60:40, v/v) in 5 min. The flow-rate was 1.0 ml min−1. (A) Crude sample; (B) elatine
In HSCCC, successful separation depends upon the selection of a suitable two-phase solvent system, which requires the following considerations: retention of the stationary phase should be satisfactory which is attained with the short settling time of the solvent system (<25 sec) [8] and the partition coefficient of the target compound is between 0.4–2.5 [9]. Small K values result in a loss of peak resolution, while large K values tend to produce excessive sample band broadening and long run times [10]. Elatine is freely soluble in chloroform and to some extents soluble in methanol and ethyl acetate, but it is hardly soluble in water. According to these properties of elatine, several two-phase solvent systems were tested and their K values are summarized in Table 1. Among them, the two-phase solvent systems, including systems 2, 3, 4, and 10, had small K values (Table 1). When they were used for HSCCC, elatine would be eluted together with other non-polar compounds near the solvent front. So these two-phase solvent systems were not suitable for separation of elatine from the crude sample. The two-phase solvent systems, systems 1, 5, 6, 7, and 8 had large K values (Table 1), which tend to produce excessive sample band broadening and long run times. Thus, these two-phase solvent systems were not suitable for separation of elatine from crude sample. Finally, the two-phase solvent system of ethyl acetate-chloroform-methanol-water at a volume ratio of 3:0.1:2:3 was found to be satisfactory for the separation of elatine from the crude sample (Table 1).
Fig. 3 shows the chromatogram obtained from 100 mg of the crude sample by preparative HSCCC using a two-phase solvent system composed of ethyl acetate-chloroform-methanol-water (3:0.1:2:3, v/v). The fraction from peak 2 of this separation yielded 72 mg of elatine at over 97% purity with approximately 95% recovery as determined by HPLC (Fig. 2B).
Fig. 3.
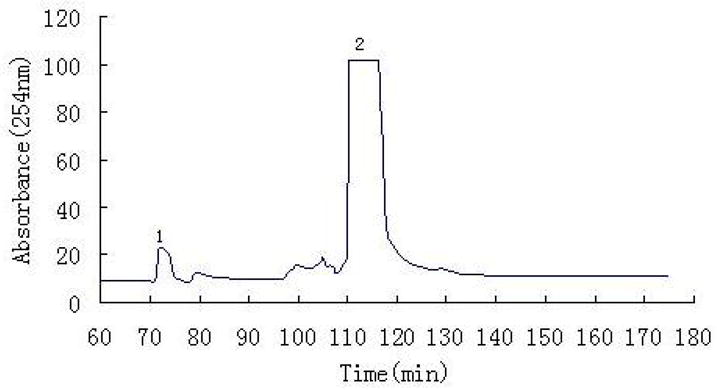
HSCCC chromatogram of crude sample from Delphinium shawurense.
Solvent system: ethyl acetate-chloroform-methanol-water (3:0.1:2:3, v/v); stationary phase: upper aqueous phase; mobile phase: lower organic phase; flow rate: 2.0 ml/min; revolution speed: 800rpm; sample size: 100 mg; detection wavelength: 254 nm.
The structural identification of elatine was carried out by MS, 1H- and 13C-NMR as follow: m.p.225°C–227°C(acetone). The FAB-MS of the fraction showed a molecular ion at m/z 695 [M+H]+ corresponding to the formula C38H50N2O10. 1H NMR (400 MHz, CDCl3): δ 1.07 (3H, t, J=7.2 Hz, NCH2CH3), 3.26, 3.32, 3.34, 3.43 (3H, s, 4×OCH3), 5.07 (2H, s, OCH2O), 7.26 ~ 8.06 (4H, m, Ar-H) 13C- NMR (CDC13): 83.3(C-1), 27.8(C-2), 31.7(C-3), 37.1(C-4), 52.7(C-5), 89.3(C-6), 92.1(C-7), 83.3(C-8), 48.4(C-9), 39.9(C-10), 49.9(C-11), 26.4(C-12), 38.6(C-13), 81.4(C-14), 34.8(C-15), 81.6(C-16), 64.1(C-17), 69.6(C-18), 52.8(C-19), 50.5(C-20), 13.9(C-21), 58.9(OCH3), 56.2(OCH3), 55.2(OCH3), 57.8(OCH3), 93.5(OCH2O), 164.1(O=C-O), 127.2(C-1′), 133.0(C-2′), 129.9(C-3′), 133.6(C-4′), 129.4(C-5′), 131.2(C-6′ 175.9(C-1″), 36.9(C-2″), 35.3(C-3″), 179.9(C-4″), 16.3(C-5″), 13.9(NCH2CH3) [2].
CONCLUSION
The above results of present study demonstrate for the first time the successful separation and purification of elatine in Delphinium shawurense by HSCCC. The present study indicates that HSCCC is a valuable method in isolating various alkaloid components from Chinese herbal medicinal products.
Acknowledgments
This work was financially supported by grant (code:KGCX2-SW-213-08) from Key Project of Knowledge Innovation Program of Chinese Academy of Sciences and grant (code: 200515123) from the program for development and research of high technologies in Xinjiang.
References
- 1.Bisset NG. J Ethnopharmacol. 1981;4:247. doi: 10.1016/0378-8741(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Hardick DJ, Blagbrough IS, Cooper G, Potter BVL, Critchley T, Wonnacott S. J Med Chem. 1996;39:4860. doi: 10.1021/jm9604991. [DOI] [PubMed] [Google Scholar]
- 3.Doblis P, Madl JE, Pfister JA, Manners GD, Walrond JP. J Pharm Exper Therap. 1999;291:538. [PubMed] [Google Scholar]
- 4.Ito Y. CRC Crit Rev Anal Chem. 1986;17:65. [Google Scholar]
- 5.Ma YM, Aisa HA, Liao LX, Aibai S, Zhang TY, Ito Y. J Chromatogr A. 2005;1076:198. doi: 10.1016/j.chroma.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Yang FQ, Zhang TY, Zhnag R, Ito Y. J Chromatogr A. 2000;829:137. doi: 10.1016/s0021-9673(98)00776-6. [DOI] [PubMed] [Google Scholar]
- 7.Yang FQ, Ito Y. J Chromatogr A. 2002;943:219. doi: 10.1016/s0021-9673(01)01464-9. [DOI] [PubMed] [Google Scholar]
- 8.Oka H, Goto Y, Ito Y, Hashimoto H, Harada K, Suzuki M, Iwaya M, Fuji K, Ito Y. J Chromatogr A. 2002;946:157. doi: 10.1016/s0021-9673(01)01548-5. [DOI] [PubMed] [Google Scholar]
- 9.Froesen JB, Pauli GF. J Liq Chrom & Rel Technol. 2005;28:2777. [Google Scholar]
- 10.Oka H, Harada K, Ito Y. J Chromatogr A. 1998;812:35. doi: 10.1016/s0021-9673(97)01277-6. [DOI] [PubMed] [Google Scholar]