Abstract
Helicobacter pylori is etiologically related to peptic ulcer disease and gastric adenocarcinomas. Reports of geographical enigmas (African, Asian, Indian and Costa Rican enigmas) are based on perceptions that clinical presentations in a population or region are not as the authors expected. We discuss the background for these enigmas and examine the evidence whether they are real or are medical myths. The African enigma was challenged almost as soon as it was proposed and recent analyses of endoscopic data have confirmed it is a myth, as H. pylori-related diseases occur in Africa at the expected frequencies. The Asian and Indian enigmas relate to gastric cancer and peptic ulcers, respectively, and when one takes the patterns of gastritis in the different regions, these enigmas disappear. The pattern of gastritis underlies and predicts the clinical outcome and the predominant pattern of gastritis has been observed to change much more rapidly than can be accounted for by changes in host genetics. There is also no evidence that these changes relate to changes in the predominant H. pylori strain. The factors that link most closely to preventing an atrophic corpus are environmental, with food preservation and diet currently assuming the most prominent roles. This focus on diseases (cancer vs duodenal ulcers) instead of the underlying patterns of gastritis has fostered, and possibly helped to perpetuate, these mythical enigmas. We suggest that a better strategy would be to focus on the pathogenesis of underlying histopathologic differences which could also lead to the identification of specific chemoprevention strategies.
Keywords: African enigma, Asian enigma, gastric cancer, gastritis, Helicobacter pylori, Indian enigma, peptic ulcer
INTRODUCTION
Helicobacter pylori is a gastric bacterial pathogen that causes ongoing gastric inflammation (gastritis). The infection is etiologically associated with iron deficiency anemia, gastric atrophy, duodenal and gastric ulcer disease, primary gastric B-cell lymphomas and gastric adenocarcinomas.1–5 The pattern and severity of the gastritis governs the possible outcomes of a particular infection (e.g., duodenal ulcer vs gastric cancer). As a general rule, if one knows the predominant pattern of gastritis in a population, they can predict the predominant clinical manifestations of H. pylori infections in that population and vice versa. A number of investigators have reported geographical ‘enigmas’ (i.e., African, Asian, Indian, Costa Rican enigmas) based on their perceptions that the outcomes they expected were not achieved in a particular population or region.6–10
H. pylori-related enigmas are not a new phenomena. Early in the 20th century it was noted that gastric cancer and peptic ulcer, now known to be caused by H. pylori infections, were virtually absent in Malays although this was never given the status of an enigma.11 The first enigma related to H. pylori infections was the African enigma, which described the apparent lack of peptic ulcers and gastric cancer in Africa.6,11–13
Exceptions often provide insights leading to an enriched understanding of a problem. Enigmas, whether real or mythological, often result in serious investigations and are used as straw men to justify possibly related studies, sometimes resulting in circular logic where studies support myths and vice versa. This article addresses the background of the H. pylori-related enigmas and examines the evidence showing whether they are real or whether they are medical myths.
ENIGMAS
First and foremost, H. pylori are bacteria that cause an infectious disease. The clinical course of the infection is characterized by a variable and often lengthy latent period eventually leading to variable clinical expressions. This pattern is not unique, as it is similar to the other classical chronic infectious diseases, syphilis and tuberculosis.14 Different expressions of infectious diseases are common, for example, while streptococcal infections are common, the incidence of rheumatic fever varies widely among regions and in different time periods. Other examples include the fact that the incidence of clinical tuberculosis in the developed world in the late 19th and early 20th centuries fell dramatically, despite the high prevalence of latent infections.15–17 However, even today tuberculosis remains a significant problem in some areas. Dramatic and rapid changes in the incidence of disease occurring in different geographical regions often stimulates speculations leading to scientific investigations. However, rarely have such differences been afforded the status of an enigma. It seems that there is more interest in new experimental studies trying to explain H. pylori enigmas than in trying to discern whether the enigma itself is real.
Factors predicting H. pylori clinical outcomes
Gastric cancer and duodenal ulcers occupy opposite ends of the spectrum of H. pylori-related disease and are mutually exclusive, as the one protects against the other. For almost 100 years it has been recognized that there are fundamental differences in the gastric physiology and patterns of gastritis that underlie these different outcome (i.e., gastric cancer is related to atrophic gastritis and duodenal ulcers to nonatrophic corpus-sparing gastritis).18,19 In addition, gastric cancer and duodenal ulcer have different latent periods; gastric cancer is typically a disease of the elderly whereas duodenal ulcers are a disease of middle life. It follows that gastric cancer is expected to be rare in regions where duodenal ulcers (surrogate for nonatrophic gastritis) are common and in regions where the average life expectancy is low, and that the incidence of gastric cancer is expected to be increased in regions where atrophic gastritis is common (especially in areas where the age of acquisition of atrophic gastritis is low).20
H. pylori-related enigmas relate to perceived differences between populations whereas the pathogenesis of H. pylori-related diseases focuses on individuals and interactions of the host, bacteria and the environment. Differences in the pathogenesis should be capable of explaining variations in the outcomes of H. pylori infections among populations. The relation between H. pylori and gastric cancer hinges on the factors that determine the severity and rate of progression of atrophic gastritis. The basic underlying condition is mucosal inflammation, which actually causes the damage. Thus, the questions regarding pathogenesis can be further defined in terms of factors that determine the degree and location of the inflammatory response to the infection. This again comes down to interactions between the host, the bacteria and the environment.21
Host genetics
Host genetics play an important role in any inflammatory process and in the interactions between the host and H. pylori.22 For example, polymorphisms in genes controlling the host’s response to inflammation can either accentuate or attenuate the inflammatory response and thus the outcome of an infection. Based on the ground-breaking studies of host–bacterial interaction leading to periodontal disease, one can generalize that host factors that enhance the inflammatory response are likely to increase the incidence of clinically significant outcomes.23,24 Any number of host factors might contribute to the severity of inflammation by a wide variety of mechanisms, such as providing or denying sites for binding the bacteria to the host. While the possibilities seem endless, unless the presence or absence of a factor is unique or of high incidence in a particular population, the effects are likely to be more patient specific and unlikely to affect overall outcome in terms of which disease is predominant.25
Bacterial factors
H. pylori strains differ in terms of their ability to stimulate an inflammatory response. For example, it has long been noted that patients infected with strains with an intact cytotoxin-associated gene (cag) pathogenicity island have a more intense inflammatory response and this was also associated with an increased chance of developing gastric cancer or peptic ulcer disease.26 Increased inflammation has also been associated with the presence of the bacterial factor outer inflammatory protein A (OipA).27,28 However, an absence of these putative virulence factors does not equate with the absence of mucosal inflammation and strains lacking these factors are also found in patients with duodenal ulcer or gastric cancer. Currently, there is no evidence to support the notion that there may be non-pathogenic H. pylori; all infections cause inflammation and any can lead to significant clinical outcomes. The coexistence of a more virulent H. pylori strain (e.g., containing a cag pathogenicity island) and genetic polymorphisms (e.g., in interleukin 1β leads to a pronounced inflammatory response and results in a further enhancement of the risk of a clinical outcome (such as being assessed to be at risk of gastric cancer).29–31 Just as there are no non-pathogenic H. pylori, there are no disease-specific strains such as gastric cancer strains (i.e., that are exclusively associated with one or another clinical outcome). For example, the presence of the cag pathogenicity island is associated with an enhanced risk of both gastric cancer and of duodenal ulcer disease, despite the fact that clinically each disease seem to protect against development of the other. Some other factor must trump the effects of these virulence factors. Those factors group under the heading of environmental factors.
Environmental factors
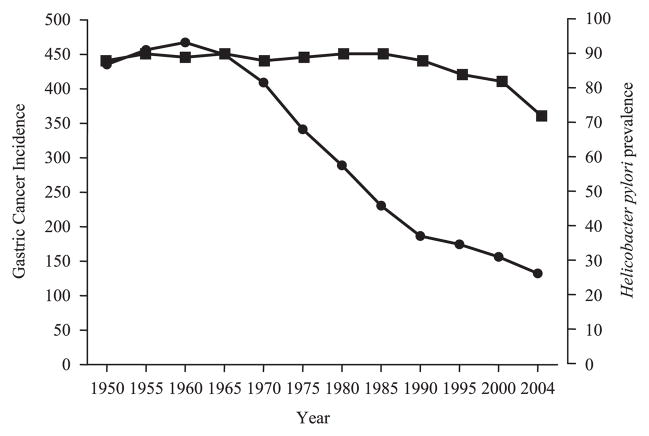
Rapid changes in the incidence of different H. pylori diseases have been repeatedly documented. Notable examples are the changes in relate to immigration, such as the Japanese immigrants to Hawaii, or even within countries, such as the rise in duodenal ulcers and fall in gastric cancer seen in the USA in the 20th century.25 More recently there has been a dramatic fall in the incidence of gastric cancer in both Japan and Shanghai, despite there being no significant change in the incidence of H. pylori in the population at risk (Fig. 1).32,33 These changes occurred without changes in the predominant H. pylori strain or in host genetics.
Figure 1.
Changes in the incidence of (●) gastric cancer and (■) Helicobacter pylori infection among Japanese men age 65–69 during the latter half of the 20th century (Constance Wang and David Y. Graham, unpublished observations).
Such changes, as noted previously, actually reflect changes in the patterns of gastritis (i.e., from prevalent atrophic gastritis to nonatrophic gastritis) and should be thought of in terms of such changes. The interpretation of the changes in the younger population is complicated by the fact that two events are occurring simultaneously. One is a marked reduction in the acquisition of the infection among children, which prevents the development of H. pylori-related diseases and eventually will result in a loss of such diseases from the population. The other is changes in the pattern of gastritis, despite there being no change in the prevalence of infection among the older population. Factors known to protect against gastric cancer (which are actually factors that promote nonatrophic gastritis) include the presence of a refrigerator (a surrogate for a method of food preservation and a reduction in eating a seasonal diet) and the increased use of fresh fruit and vegetables. Correlates with gastric cancer (which arises from atrophic gastritis) include a high salt intake, seasonal diets and nitrate consumption.20,21,25 Any claims for the presence of an H. pylori-related enigma must be evaluated in light of the natural history of H. pylori-related diseases and the predominant pattern of gastritis in the population thought to represent the enigma.
The African enigma
The African enigma predated the discovery of H. pylori by about a half a century and was commandeered by those studying H. pylori and H. pylori-related diseases (see review in Agha and Graham, 200534). In fact, the concept of an African enigma was challenged almost as soon as it was proposed. For example, in 1961 Watkinson wrote, ‘In a carefully planned prospective necropsy survey performed in Kampala between 1951 and 1956, where particular care was taken to record all ulcers and scars present in 647 male and 129 female African subjects of varying tribal origins, Raper (1958) found ulcers in 15.3% of the men and 4.7% of the women examined. Duodenal ulcers were found six times more frequently than gastric, and ulcers became progressively more common in successive decades in men increasing from 6.3% in the second, to 15.3% in the fourth and reaching 27% in the sixth, figures which do not differ materially from similar prospective necropsy surveys in England and Scotland (Watkinson, 1959). Considerable intertribal variation in ulcer occurrence was apparent reflecting varying dietary and environmental conditions. This careful survey demonstrates such a striking disparity between clinical and pathological estimates of ulcer frequency that one is forced to the conclusion that ulcer must frequently pass unrecognized in the African’.12
The primary reservation was that the data relating to the incidence of peptic ulcers and gastric cancer were considered inadequate because they were obtained from populations with extremely limited access to health care and relatively short life expectancies.12 More recent publications in which objective data were available (e.g., obtained by endoscopy) have confirmed that the original reservations were correct and that the prevalence of peptic ulcer disease was not unusually or unexpectedly low (i.e., there is no justification for claiming the presence of an African enigma34,35) and, until proved otherwise, one can consider the African enigma a medical myth.
There are nevertheless still unanswered questions about Africa. One example is whether there is a specific pattern of gastritis associated with archetype strains largely restricted to South Africa. One of these is the so-called hpAfrica2 type strain identified based on the multi-locus sequence typing of seven housekeeping genes which lack the cag pathogenicity island.36 Other questions include whether co-infection with parasites might alter the expression of the infection and result in a change in the pattern and/or severity of gastritis and thus in the prevalence of clinical disease.37,38 Currently, there are little or no good human data to support this hypothesis and the few data that are available can be explained by alternative mechanisms. It is easier to make hypotheses than to test them rigorously using studies that control for diet and the pattern of gastritis. Such studies are expected to be forthcoming. The African enigma, as described, is currently considered a medical myth.
Indian enigma
The Indian enigma was described recently in relation to differences in the prevalence of gastric cancer among different ethnic groups residing in Malaysia.10 In reality, there is considerable existing information about H. pylori and its primary manifestation in India and among immigrants from India to Africa and Malaysia. The primary manifestation of H. pylori in India is the duodenal ulcer and the duodenal ulcer has long been considered a major problem in the Indian subcontinent and among migrants from India. This was recognized early in the 20th century and has been repeatedly confirmed.39 An example is a study done in the 1980s in rural Bangladesh in which 2675 individuals, 85% of whom were over the age of 15 years, were interviewed.40 Almost half (41.4% or 1106 participants) reported symptoms of ‘ulcer-like dyspepsia’ and 313 were invited for an endoscopy. Among the 283 who received an upper gastrointestinal endoscopy 27% had ulcers, almost all of which were duodenal ulcers: gastric ulcers were rare.40
This long and continuing association of the duodenal ulcer and the rarity of gastric ulcers in Indian and Indian migrants is, as noted above, actually indicative of a high prevalence of corpus-sparing gastritis as well as the rarity of gastric atrophy in this population.39 It follows that gastric cancer was not to be expected. Wisely, the authors describing the Indian enigma related the observed differences to differences in the traditional environmental factors known to affect the pattern of gastritis (i.e., the intake of salt and fresh fruit and vegetables) and it is not clear from reading their article whether their description of an ‘Indian enigma’ was not tongue in cheek.10 However, the Indian enigma did not go unnoticed and resulted in letters criticizing their statistical analyses,41 and others which provided alternative explanations, such as differences in host genetics.42,43 Probably the most amazing44 of these was a reference to the Indian enigma to justify why one might not wish to eradicate all H. pylori and thus eradicate gastric cancer from the world.45 Those whose primary focus is on gastric cancer may need to be reminded that H. pylori is etiologically associated with other important diseases. For example, H. pylori-related peptic ulcers in adults and an iron deficiency in children are major problems in India. In addition, in the H. pylori era, approximately 10% of North Americans experienced peptic ulcers, of whom 25% also suffered from life-threatening ulcer complications. Sometime myths can be dangerous.
Indian and Asian enigmas
The Indian enigma is actually a subset of the previously described Asian enigma, which referred to the observation that there were areas where H. pylori infection was common such as India, Thailand, Bangladesh and Pakistan, and gastric cancer was rare, whereas in other areas where H. pylori was prevalent gastric cancer was frequent (i.e., China, Japan and Korea).7,8 The basis of this enigma rests on epidemiological observations taken out of their historical context.
Historically, the fact that gastric cancer was closely associated with atrophic gastritis has led to numerous studies of the epidemiology of gastritis in the pre-H. pylori era (e.g., see Cheli et al.46). Unfortunately, this large body of information was, and still is, often underutilized by H. pylori researchers. In reality, when H. pylori was confirmed as being one cause, if not the cause, of gastritis, one question should have been how much of what had been learned before could be directly transferred to H. pylori. Studies asking that question soon showed that most of the information could be validly transferred.47,48
The link between patterns of gastritis and clinical presentation were also well known, yet many investigators focus on the outcome (e.g., cancer) instead of the underlying condition. For example, the Indian and Asian enigma, as well as the potential importance of a particular strain (e.g., the so-called East Asian strain) often rely on differences in the prevalence of gastric cancer among populations as the basis for their studies (i.e., gastric cancer is common in some parts of East Asia and uncommon in others or in the developed world). Such an approach focuses on the present while ignoring the fact that recent findings often reflect a rapid change in the prevalent disease.
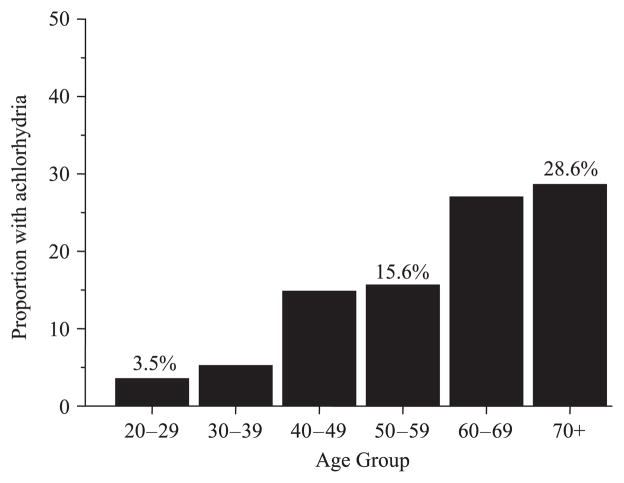
For example, at the turn beginning of the 20th century, gastric cancer was more common in the western world than in Japan.49 In 1930 gastric cancer was the most common cancer in the USA and more than 25% of adults over the age of 60 were found to have gastric atrophy (histamine fast achlorhydria) (Fig. 2).50 Duodenal ulcers were also uncommon in the western world until the late 19th century but by the mid-20th century they had became an important clinical problem (Fig. 3).33
Figure 2.
Proportion of US adults with histamine-fast achlorhydria in the first quarter of the 20th century.44
Figure 3.
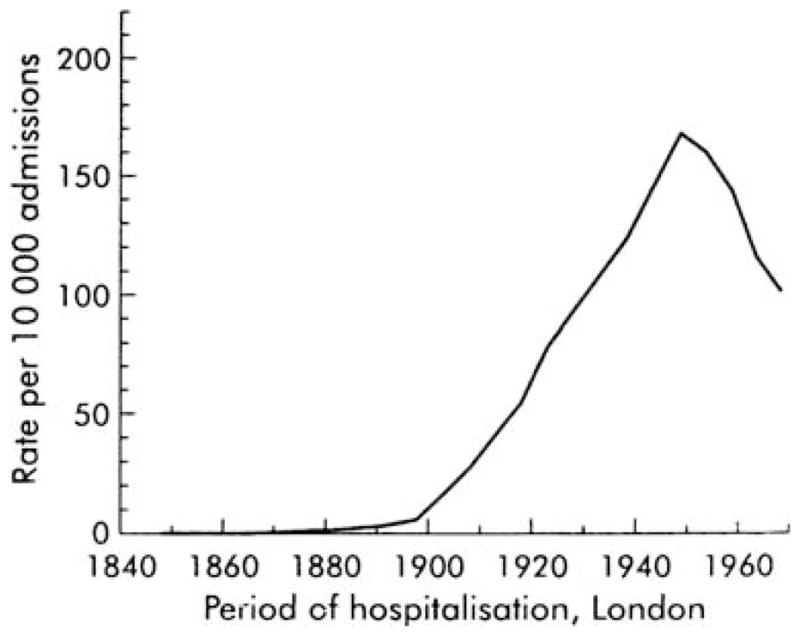
Hospital admissions for patients with duodenal ulcers in London in the early 20th centuries (adapted with permission from Sharma45).
The change in the pattern of disease actually reflected a change in the underlying dominant patterns of gastritis (from atrophic to nonatrophic). These factors related to changes in the pattern of the disease (i.e., the fall in the incidence of cancer) also reflect changes in the pattern of gastritis (i.e., identifying what potentially protects the gastric corpus or reduces the rate of development of its atrophy).25,47
As noted above, host genetics affect individuals and generally cannot explain widespread changes such as the fall in gastric cancer incidence among many populations.25 Similarly, because the infection is acquired in childhood and is often lifelong, one can, by comparing strains from the young and old, investigate whether the predominant strain has changed coincident with marked changes in outcome (here cancer incidence). The answer is no. What changed was the environment; the most likely candidate is the diet, such as changes in food storage and perseveration, a change from seasonal diets to a year-round availability of fruit and vegetables and the fortification of food with vitamins.25,47 Long ago, we named the pattern of diet and food presentation associated with corpus-sparing gastritis the banana hypothesis.47 While the details remain to be worked out, a hypothesis such as an East Asian strain promotes cancer, or a genetic difference in the host is responsible for preventing gastric cancer in a population are probably best described as wishful thinking. The real H. pylori enigma is why so many H. pylori investigators seem to put hard work before hard thinking.
East Asian strain and gastric cancer and the Asian enigma
There has been considerable interest in the role of H. pylori virulence factors in cancer pathogenesis. The dominant genotype in Japan is the cag pathogenicity island positive/vacA s1c subtype.51 CagA from patients with gastric cancer was noted to have a greater molecular weight generally compared to that from patients with simple gastritis or with duodenal ulcers. This difference was found to be related to a different number of repeats in the 3′ region.52 CagA is known to be injected into epithelial cells and undergoes tyrosine phosphorylation leading to the activation of multiple signaling factors. CagA is tyrosine-phosphorylated at Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs located in the 3′ region of cagA gene, result in changes in membrane dynamics, actin cytoskeletal rearrangements, inflammation, proliferation and anti-apoptotic nuclear responses based on in vitro studies using gastric cancer cell lines (see Backert and Selbach 53).
The structure of the 3′ region of the cagA gene varies between strains from East Asian and western countries.54,55 In East Asian strains two types of repeats are found: 57 bp repeats followed by 162 bp repeats (East Asian type cagA or type 1a). Strains in western countries also have 57 bp repeats, but they are followed by a repeat region consisting of 102 bp repeats, which are completely different from those of East Asian strains (western type cagA or type 2a). Recently another classification of the repeat region has been proposed based on the sequences surrounding EPIYA motifs; EPIYA-A, -B, -C, and -D.56 EPIYA-A and -B are conserved in both western and East Asian strains and correspond to the first repeat region, whereas EPIYA-C is specific to western strains and EPIYA-D is specific to East Asian strains and corresponds to the second repeat region. Overall, western and East Asian strains contain AnBnCn or AnBnDn, respectively, where ‘n’ is the number of repeating motifs, but ‘n’ does not have to be equal for A, B, C and D types (e.g., ABBCCC type [A1B2C3]).
CagA has been reported to interact with various target molecules in host epithelial cells. The best studied of these is SHP-2, an oncoprotein, cytoplasmic Src homology 2 domain of Src homology 2 phosphatase. Due to structural differences between EPIYA-C and -D motifs, the East Asian type CagA exhibits a stronger binding affinity for SHP-2 than does the western type CagA.57,58 It has been hypothesized that this difference in the cagA gene structure might be related to the high incidence of gastric cancer in East Asian countries compared to many other countries.
This hypothesis ignores the data discussed above relating to the marked change in gastric cancer incidence since 1900, and the fact that there are high-incidence western countries. The incidence of gastric cancer in Costa Rica, a western country, is also currently higher than it is in Japan. It appears more likely that the presence of the East Asian strain simply describes the predominant strain in a high cancer region. By definition, the prevalence of atrophic gastritis is also high, so this association is assured. A comparison with the predominant strain in a region with a low cancer incidence would expect to find a different strain and thus appear to confirm the original hypothesis, when in fact it only confirms the fact that the predominant strain often differs among regions. To test the hypothesis, one would need to compare the strains from a low risk subpopulation, such as the offspring of migrants from the Japanese mainland or young Japanese now that the incidence of cancer is fallen. To date the logic supporting a special role for the East Asian strain in gastric cancer is circular and, considering the rapid changes that have recently occurred in gastric cancer as well as the changes occurring in the developed world over the last 100 years, is most likely to be incorrect. However, as noted, it is testable.
In summary, there are very little data to support the premise that any of the so-called H. pylori enigmas are other than medical myths. Better data served to slay the African enigma. The only enigmatic feature of its children, the Asian and Indian enigmas, is that they were called enigmas. The Costa Rican enigma related to CagA and gastric cancer and the author who defined it also seemed to think it was a nonentity.9 It is clear that the predominant clinical manifestation of H. pylori infection in different regions varies and the details as to why this is true remain unclear. The rapid change in the incidence of gastric cancer that occurred in the developed world and that is currently occurring in Japan illustrates that hypotheses based on differences in disease using a contemporary snapshot are likely to be misleading. The predominant pattern of gastritis has been observed to change much more rapidly than can be accounted for by changes in host genetics and there is no evidence that these changes are related to changes in the predominant H. pylori strain. The factors that link most closely to preventing atrophic gastric corpus are environmental with food preservation and diet currently assuming the most prominent roles. A focus on disease (i.e., cancer vs duodenal ulcer) instead of the underlying patterns of gastritis fostered, and possibly helped to perpetuate, these mythical enigmas. We suggest that a better strategy is to focus on the pathogenesis of underlying histopathological differences which could also lead to the identification of better and specific chemoprevention strategies.
Acknowledgments
In the last 2 years Dr Graham has received small amounts of grant support and/or free drugs or urea breath tests from Meretek, BioHit for completely investigator-controlled research. Dr Yamaoka is supported in part by a grant from the NIH DK62813. Dr Lu is supported in part by a grant from the National Natural Science Foundation of China (30670940).
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs or National Institutes of Health. Dr. Graham is a consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr Graham is a also paid consultant for Otsuka Pharmaceuticals and until July 2007 was member of the Board of Directors of Meretek, Diagnostics, the manufacturer of the 13C-urea breath test. Dr Graham also receives royalties on the Baylor College of Medicine patent covering materials related to the 13C-urea breath test.
References
- 1.Graham DY, Sung JY. Helicobacter pylori. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Pathophysiology, Diagnosis, Management. 7. Philadelphia: W.B. Saunders Co; 2006. pp. 1049–66. [Google Scholar]
- 2.Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163:127–34. doi: 10.1093/aje/kwj018. [DOI] [PubMed] [Google Scholar]
- 3.Dholakia KR, Dharmarajan TS, Yadav D, Oiseth S, Norkus EP, Pitchumoni CS. Vitamin B12 deficiency and gastric histo pathology in older patients. World J Gastroenterol. 2005;11:7078–83. doi: 10.3748/wjg.v11.i45.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol. 2005;100:453–9. doi: 10.1111/j.1572-0241.2005.30252.x. [DOI] [PubMed] [Google Scholar]
- 5.Hershko C, Lahad A, Kereth D. Gastropathic sideropenia. Best Pract Res Clin Haematol. 2005;18:363–80. doi: 10.1016/j.beha.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Holcombe C, Omotara BA, Eldridge J, Jones DM. H. pylori, the most common bacterial infection in Africa: a random serological study. Am J Gastroenterol. 1992;87:28–30. [PubMed] [Google Scholar]
- 7.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106–12. doi: 10.1111/j.1572-0241.2002.05663.x. [DOI] [PubMed] [Google Scholar]
- 8.Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346–51. doi: 10.3748/wjg.v12.i9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji S. The ‘Costa Rican enigma’ of Helicobacter pylori CagA and gastric cancer. J Gastroenterol. 2006;41:716–17. doi: 10.1007/s00535-006-1824-z. [DOI] [PubMed] [Google Scholar]
- 10.Goh KL, Cheah PL, Md N, Quek KF, Parasakthi N. Ethnicity and H. pylori as risk factors for gastric cancer in Malaysia: a prospective case control study. Am J Gastroenterol. 2007;102:40–5. doi: 10.1111/j.1572-0241.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 11.Doll R. Peptic ulcer epidemiology. In: Jones FA, editor. Modern Trends in Gastro-enterology. Bell Yard: Butterworth & Co; 1952. pp. 361–76. [Google Scholar]
- 12.Watkinson G. Geographic aspects of peptic ulcer. In: Card WI, editor. Modern Trends in Gastro-enterology. 3. Washington: Butterworths; 1961. pp. 23–48. [Google Scholar]
- 13.Doll R. Cancer of the alimentary tract I. Aetiology of cancer of the stomach. In: Jones FA, editor. Modern Trends in Gastro-enterology. 2. New York: Paul B. Hoeber; 1958. pp. 53–65. [Google Scholar]
- 14.Graham DY. Can therapy ever be denied for Helicobacter pylori infection? Gastroenterology. 1997;113:S113–17. doi: 10.1016/s0016-5085(97)80023-6. [DOI] [PubMed] [Google Scholar]
- 15.Feldbert GD. Tuberculosis and the Shaping of Modern North American Society. New Brunswick: Rutgers University Press; 1995. Disease and Class. [Google Scholar]
- 16.McKeown T. The Role of Medicine: Dream, Mirage or Nemesis. London: Nuffield Provincial Hospital Trust; 1976. [Google Scholar]
- 17.Fletcher R, Fletcher SW, Wagner EH. Clinical Epidemiology: the Essentials. Baltimore, NJ: Williams & Wilkins; 2008. [Google Scholar]
- 18.Faber K. Chronic gastritis. its relation to achylia and ulcer. Lancet. 1927;2:902–7. [Google Scholar]
- 19.Faber K. Gastritis and its Consequences. Paris: Oxford University Press; 1935. [Google Scholar]
- 20.Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35 (Suppl 12):90–7. [PubMed] [Google Scholar]
- 21.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–91. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 22.El-Omar EM. Role of host genes in sporadic gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:675–86. doi: 10.1016/j.bpg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Kornman K, Duff G, Reilly P,Re. A critical assessment of interleukin-1 (IL-1) genotyping when used in a genetic susceptibility test for severe chronic periodontitis. Greenstein G, Hart TC (2002; 73: 231–247) J Periodontol. 2002;73:1553–6. doi: 10.1902/jop.2002.73.2.231. [DOI] [PubMed] [Google Scholar]
- 24.Takashiba S, Naruishi K. Gene polymorphisms in periodontal health and disease. Periodontol 2000. 2006;40:94–106. doi: 10.1111/j.1600-0757.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 26.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–44. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34 000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533–8. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka Y, Kikuchi S, El Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–24. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo C, Machado JC, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 30.Rad R, Prinz C, Neu B, et al. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J Infect Dis. 2003;188:272–81. doi: 10.1086/376458. [DOI] [PubMed] [Google Scholar]
- 31.Sicinschi LA, Lopez-Carrillo L, Camargo MC, et al. Gastric cancer risk in a Mexican population. role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118:649–57. doi: 10.1002/ijc.21364. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Wu CX, Zheng Y, Wang JJ. Time trends and characteristics of gastric cancer incidence in urban Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28:875–80. [PubMed] [Google Scholar]
- 33.Baron JH, Sonnenberg A. Hospital admissions for peptic ulcer and indigestion in London and New York in the 19th and early 20th centuries. Gut. 2002;50:568–70. doi: 10.1136/gut.50.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agha A, Graham DY. Evidence-based examination of the African enigma in relation to Helicobacter pylori infection. Scand J Gastroenterol. 2005;40:523–9. doi: 10.1080/00365520510012280. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen TH. Peptic ulcer disease is strongly associated with Helicobacter pylori in east, west, central and South Africa. Scand J Gastroenterol. 2001;36:561–4. [PubMed] [Google Scholar]
- 36.Gressmann H, Linz B, Ghai R, et al. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 2005;1:419–28. doi: 10.1371/journal.pgen.0010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox JG, Wang TC, Nagler-Anderson C. The African enigma: the parasite’s perspective. Gut. 2001;49:156–7. doi: 10.1136/gut.49.1.156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon D. Solving the African enigma: parasites may have their place. Gastroenterology. 2000;119:611. doi: 10.1016/s0016-5085(00)70104-1. [DOI] [PubMed] [Google Scholar]
- 39.Tovey F. Peptic ulcer in India and Bangladesh. Gut. 1979;20:329–47. doi: 10.1136/gut.20.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan AKA, Hasan M, Roy PK, et al. Peptic ulcer in Bangladesh. BMRC Bull. 1987;8:29–42. [PubMed] [Google Scholar]
- 41.Katz AR. Indian enigma? Reanalyzed data are less than supportive. Am J Gastroenterol. 2007;102:2114–15. doi: 10.1111/j.1572-0241.2007.01324_14.x. [DOI] [PubMed] [Google Scholar]
- 42.Ghoshal UC, Tripathi S, Ghoshal U. The Indian enigma of frequent H. pylori infection but infrequent gastric cancer: is the magic key in Indian diet, host’s genetic make up, or friendly bug? Am J Gastroenterol. 2007;102:2113–14. doi: 10.1111/j.1572-0241.2007.01324_13.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghoshal UC, Tiwari S, Dhingra S, et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215–22. doi: 10.1007/s10620-008-0229-7. [DOI] [PubMed] [Google Scholar]
- 44.Beatty WK. Searching the literature comes before writing the literature. How clinicians can use printed bibliographies, tapes, and computers. Ann Intern Med. 1973;79:917–24. doi: 10.7326/0003-4819-79-6-917. [DOI] [PubMed] [Google Scholar]
- 45.Sharma SP. H. pylori and gastric cancer in Asia: enigma, or a play on words? Lancet Oncol. 2008;9:827. doi: 10.1016/s1470-2045(08)70226-2. [DOI] [PubMed] [Google Scholar]
- 46.Cheli R, Perasso A, Giacosa A. Gastritis: a Clinical Review. Berlin: Springer-Verlag; 1987. [Google Scholar]
- 47.Graham DY, Adam E, Klein PD, et al. Epidemiology of Campylobacter pylori infection. Gastroenterol Clin Biol. 1989;13:84B–8B. [PubMed] [Google Scholar]
- 48.Graham DY, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman FL. The Mortality from Cancer Throughout the World. Newark: Prudential Press; 1915. [Google Scholar]
- 50.Polland WS. Histamine test meals: an analysis of nine hundred and eighty-eight consecutive tests. Arch Intern Med. 1933;51:903–19. [Google Scholar]
- 51.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–9. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaoka Y, El-Zimaity HM, Gutierrez O, et al. Relationship between the cagA-3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342–9. doi: 10.1053/gast.1999.0029900342. [DOI] [PubMed] [Google Scholar]
- 53.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 54.Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998;36:2258–63. doi: 10.1128/jcm.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–4. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 56.Hatakeyama M. The role of Helicobacter pylori CagA in gastric carcinogenesis. Int J Hematol. 2006;84:301–8. doi: 10.1532/IJH97.06166. [DOI] [PubMed] [Google Scholar]
- 57.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99:14428–33. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naito M, Yamazaki T, Tsutsumi R, et al. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006;130:1181–90. doi: 10.1053/j.gastro.2005.12.038. [DOI] [PubMed] [Google Scholar]