Abstract
Despite the importance of the subject, the effects of nicotine on the interplay between affect and attentional bias are not clear. This interplay was assessed with a novel design of the Primed Attentional Competition Task (PACT). It included a 200 ms duration emotional priming picture (negative, positive, or neutral) followed by a dual-target picture of two emotional faces side-by-side. A second task included an emotional priming picture followed by a single emotional target picture in a classic affective priming (CAP) task, assessing RT to identify the valence. Smokers completed the tasks in a double-blind repeated measures design wearing a nicotine patch on one day and a placebo patch on the other day. Consistent with hypotheses, nicotine enhanced the effectiveness of positive primes to bias first gaze-fixations (FGFs) toward positive pictures relative to negative pictures and attenuated the effectiveness of negative primes on FGFs towards negative pictures, but did not bias performance in the CAP task where competing target stimuli were not present. These effects of nicotine on affective priming and attentional bias towards competing reinforcers may contribute to smoking motivation.
Keywords: Nicotine, Smoking, Affect, Priming, Attentional Bias, Eye Tracking
With regard to tobacco smoking motivation, the roles of attention and positive and negative affect appear important, but are far from clear (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Gilbert, 1995; Stewart, de Wit, & Eikelboom, 1984; Kassel, Stroud, & Paronis, 2003). Smokers report that their predominant motive for smoking is to reduce stress and negative affect (Beckham et al. 2008; reviewed by Kassel et al. 2003; Spielberger, 1986). Recent experimental studies (Gilbert, Sugai, et al. 2007; Izetelny et al. 2008) support the view that nicotine can modulate reported positive and negative affect and attentional bias towards emotional stimuli. In addition, models of addictive drug use motivations suggest that nicotine alters the salience of stimuli (Stewart et al. 1984), making them more attractive or “attention grabbing” (Robinson & Berridge, 1993). Given the importance of both (positive and negative) affects and salience, tasks that can assess affect and attentional bias could prove useful for the characterization of competing motives (Asgaard, 2007).
Evidence suggests that nicotine promotes increased positive affect and enhances the salience of rewards and reinforcers. Gilbert (1995) proposed that nicotine activates positive affect-related brain mechanisms that promote attentional bias to positive emotional stimuli and thereby increases positive affect and reduces negative affect. Gilbert (1995) referred to these effects of nicotine as a “priming effect” on positive affect. Consistent with this hypothesis, nicotine has been found to increase reported positive affect and to decrease negative affect and to decrease attentional gaze directed to emotionally negative pictures presented in combination with positive pictures (Gilbert, Rabinovich, et al. 2008). Supporting it’s putative lateralized affect-modulating and attention-biasing effect (Gilbert, 1995), nicotine has been reported (Gilbert, Carlson, et al. 2008) to enhance the detection of salient emotional words presented to the left hemisphere when presented side-by-side with non-salient (non-word) letter sequences, but to decrease the detection of such words presented to the right hemisphere. Robinson and Berridge (1993) suggested that nicotine alters the incentive salience of rewards and reinforcers and that of mediating neural systems, thereby making these stimuli more “attention grabbing.” Nicotine has been demonstrated to promote the enhancement of reinforcing value of environmental stimuli in rats (Caggiula et al. 2002, 2001; reviewed by Chaudhri et al. 2006; Donny et al., 2003; Olausson, Jentsch, & Taylor, 2004; Shram, Funk, Li, & Lê, 2007). Taken together, evidence suggests that nicotine may prime (enhance) positive affect and the salience of rewards and reinforcers, while little is know about nicotine’s effects on negative affect and negative reinforcement.
There is little experimental research on the effects of nicotine and abstinence on relationships of attention and negative affective processing or priming in humans (reviews by Buckley, Holohan, Mozley, Walsh, & Kassel, 2007; Gilbert, 1995). The small number of studies in the area suggests that situational mechanisms (emotional valence and salience of distracters and attractors) may be important in modulating the effects of nicotine on affect. Consistent with the view that attentional processes are important in the ability of nicotine to reduce negative affect, Kassel and Unrod, (2000) found that smokers reported less negative affect after smoking regular nicotine-delivery cigarettes (relative to low-nicotine ones) when engaged in a distracting activity, but tended to increase negative affect when emotionally benign distracters were not present. Gilbert, Riise, et al. (2008) found that the effects of nicotine depended on the nature of the emotional stimuli in the environment and the emotional state that was assessed. Further research is needed to better understand the contexts that promote when and how nicotine alters attention and affect. Priming is a fundamental experimental context that may promote better understanding of nicotine’s effects on affect and reinforcement.
In the classic affective priming paradigm, emotional priming stimuli alter the processing of immediately subsequent affective targets so that reaction time and accuracy of responses (reinforcement) are altered. The typical finding of such priming studies is that rapidly presented emotional primes increase the speed and accuracy of identification of target stimuli with similar valences (negative-negative and positive-positive prime-target pairs), while attenuating performance to identify dissimilar targets (reviewed by Musch & Klauer, 2003). These prime-target, match and mismatch, relations appear to reflect rapid emotional processing (Murphy & Zajonc, 1993) and attentional mechanisms (Posner, DiGirolamo, & Fernandez-Duque, 1997). Evidence suggests that affective priming may have the most potent effects on early attentional mechanisms during the first fraction of a second after the onset of the targets and offset of the emotional primes (Posner, 1975; Posner, et al. 1997). The priming effect typically decays rapidly in most experimental paradigms (Murphy & Zajonc, 1993; Klauer & Musch, 2003). In support of the importance of early affective processing and attention, Calvo and Lang, (2004) found that emotional stimuli biased the location of first gaze fixation (reinforcement) towards emotional stimuli paired with neutral stimuli, while the later location of fixation was not differentially altered. Taken together, affective priming may be used to alter affective processing and the reinforcing value (attentional bias) of competing salient stimuli. However, to our knowledge the effects of emotional priming stimuli on attentional bias towards competing salient targets have not been assessed directly in an affective priming paradigm.
The Affect-Priming hypothesis (Asgaard, 2007) suggests that nicotine functions in a similar manner to positive emotional priming stimuli by enhancing the reinforcing value of positive or neutral stimuli, while attenuating the value of negative stimuli. Similar to the hypothesized functioning of a positive prime, nicotine operates rapidly on the attentional bias towards competing stimuli. Thus, nicotine primes (enhances) first gaze-fixations towards salient positive stimuli, while attenuating bias towards salient negative stimuli competing for attention. This hypothesis builds on the above priming models and evidence by focusing on the importance of nicotine’s effects on affective priming and the attentional bias towards competing reinforcers, assessed with first gaze-fixations.
In sum, few studies have assessed the effects of nicotine on attentional bias to emotional stimuli using competing stimulus presentations (e.g., Gilbert, Rabinovich, et al. 2008; Gilbert, Riise, et al. 2008). However, to date no study has assessed the effects of nicotine on affective priming of first gaze-fixation towards competing emotional stimuli. The Affect-Priming hypothesis states that nicotine may enhance the reinforcing value of positive stimuli, while attenuates the value of negative stimuli, competing for attention. To test this hypothesis, two tasks were used. In both tasks, centrally presented emotional priming stimuli were used to promote rapid affective processing of emotional stimuli. In the Primed Attentional Competition Task (PACT), an emotional face in one visual field and a competing emotional or neutral valence face in the other field was presented followed the prime. The dependent variable in this task was the emotional valence of the picture toward which the first gaze-fixation was directed after the onset of the dual picture. To assess rapid affective processing without competing salient stimuli, a classic affective priming (CAP) task design was used to assess the reaction time (RT) of evaluative responding (positive or negative) toward a single positive or negative emotional target picture that was of similar or different emotional valence than the preceding prime. The dependent variable in the CAP task was the RT differences between various prime-target combinations, presented in the absence of competing emotional stimuli.
Based on the effects of nicotine on affect and attention, nicotine is expected to function as a positive emotional prime, promoting attention away from negative stimuli and towards positive or neutral stimuli, while the placebo condition promotes negative affect (because of withdrawal) and thus acts as a negative affective prime. However, our strongest predictions are based on the nicotine condition while the placebo condition serves primarily as a control condition. The primary hypothesis was that nicotine would enhance the effects of positive primes on attentional bias towards positive pictures relative to negative pictures and would attenuate the effectiveness of negative primes on attentional bias towards negative pictures. We expected minimal or no observable performance differences based on the valence of the prime and target in the CAP task due to the hypothesized importance of attentional bias competing alternative stimuli for nicotine to produce significant priming effects.
Method
Participants
Participants were 23 female and 23 male smokers. Participants had a mean age of 27.2 years (SD = 8.4), had smoked an average of 10.5 years (SD = 9.1), with a current average of 16.9 (SD = 6.1) cigarettes per day. Three participants were multi-racial, two African American, and the remaining were Caucasian. Participants received $175 monetary compensation upon completion of study. The study was reviewed and approved by the Southern Illinois University institutional review board (IRB) and participants signed an IRB-approved written consent form.
Participants were recruited from newspaper ads and flyers posted in the university community and campus. Exclusionary criteria included smoking fewer than 10 cigarettes per day for the past two years; habitual cigarette Federal Trade Commission nicotine estimated deliveries of less than 0.6 mg; reported use of psychoactive drugs or medications other than alcohol, marijuana, and caffeine; alcohol use in excess of 30 or more drinks per week; age less than 18 and more than 46; history of brain injury, and non-fluent reading of English. Participants were instructed not to smoke tobacco or drink alcohol for the 12 hr preceding each of the experimental sessions and not to smoke marijuana for at least 72 hr prior to the session.
Equipment and Materials
The pictures were presented on an 18-inch LCD color computer monitor with a SuperLab™ 2.0 software (Cedrus®, Phoenix, AZ) connected to an infrared eye-tracking system (Arrington Research, Inc., Scottsdale, AZ). Gaze direction was measured at 30 samples/second. A Cedrus® RB-530 response pad was used to record RT responses.
Questionnaire
The Positive and Negative Affect Schedule (PANAS: Watson, Clark, & Tellegen, 1988). The PANAS was administered during each experimental session.
Procedure
Primed Attentional Competition Task (PACT)
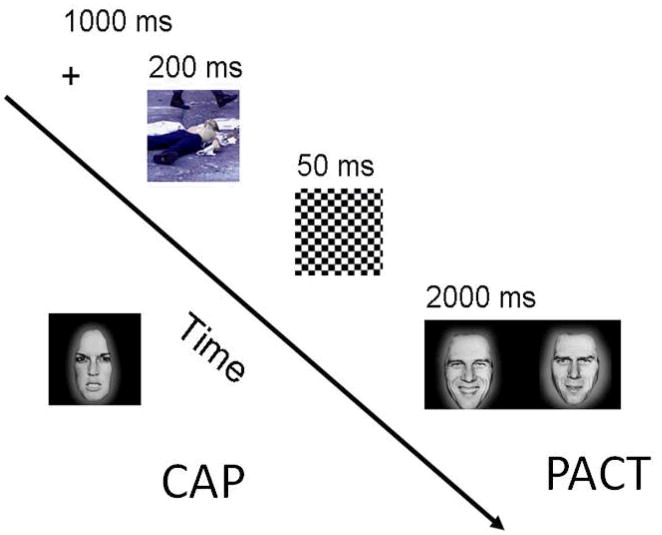
The PACT was designed for the first authors dissertation research (Asgaard, 2007) by combining a dual-picture viewing task similar to that used by Gilbert, Rabinovich, et al. (2008) with a classic affective priming (CAP) task paradigm. The CAP task with single rather than dual-pictures was included (See Figure 1). Three blocks of trials for both the PACT and the CAP tasks (6 total blocks) lasted approximately 43.2 min (6 blocks x 7.2 min/block = 43.2 min) of total picture viewing time during both experimental sessions. Each participant completed one block of each task followed by the other type of task, with the order of task sequence counterbalanced so that an equal number of participants completed each of the two tasks in all 6 possible sequence positions. These two tasks were completed while intermixed with additional tasks during the 3-hour session. At the beginning and end of each block, participants completed the PANAS.
Figure 1.
The affective priming task sequence of events. A fixation cross oriented attending toward the center of the screen to provide a common starting point for the task. The priming stimulus was presented for 200 ms immediately followed by a masking stimulus for 50 ms and a dual-picture presented for 2000 ms. There was a 1500 ms inter-stimulus interval (not shown in Figure 1) that included a 500 ms black screen followed by a written ‘blink’ prompt presented for 1000 ms. Participants were instructed to look freely at one of the pictures during the PACT task. For the CAP task, participants were instructed to identify the emotional valence (positive or negative) of the faces as quickly as possible by pressing the correct button.
Materials and Apparatus
The priming stimuli were 108 color photos, each presented 8 times across the two sessions and tasks (PACT and CAP), while the 64 target stimuli consisted of black and white faces, each presented a total of 45 times. The positive, negative, and neutral primes (36 of each type) were 16 cm square pictures. The primes were selected from an in-house validated picture set that included photos from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1995) as well as pictures collected from the internet and other sources. The experimental stimuli (prime-target picture combinations) were counterbalanced to match an equal number of positive, negative, and neutral primes with an equal number of unique targets produced by each individual performer (actor and actress) portraying all combinations and permutations of emotions. In addition to the balanced pairing of stimuli, the picture selection process was constructed to produce the final set of stimuli to be balanced across as many dimensions (e.g., color) as possible.
The 48 target stimuli used in the PACT task consisted of three types of dual-photos (angry: neutral, happy: neutral, and angry: happy) presenting one face in the left visual-field (LVF) and another in the right visual-field (RVF). The reversals of each picture type in both (right and left) visual fields produced a total of six unique types of target stimuli. One picture was presented with the inner edge slightly (3 degrees) to the left of the center of the screen and the partner picture with the inner edge of equal distance to the right of center. Therefore, both stimuli were outside of the central visual field but within the peripheral visual field. The 48 unique dual-photos were created from the 24 individual facial expressions of eight actors (4 men) and actresses (4 women), each portrayed three types of emotions (positive, negative, and neutral) in the Ekman picture series (Ekman, 2003; Ekman & Friesen, 1977). In the CAP task, a total of 16 happy and angry target pictures were used while neutral faces were excluded to promote high levels of accuracy with two rather than three button-press options.
Picture selection process
The priming stimuli were chosen based on a sequence of statistical and rational procedures. The goal was to select biologically prepared (unconditioned) stimuli that appealed to the widest possible audience at equal levels across the three dimensions of emotional valence: positive, negative, and neutral. These three dimensions were balanced to have equal levels of valence by statistically grouping them into sets (triads) of three pictures. Within each triad, an equal level of emotional valence was created by grouping pictures with equal distance (within 2 points out of a 10 point scale) of mean valence ratings for each dimension. Each picture in each triad was balanced to have equal levels of luminosity, brightness, and color balance using quantitative procedures in Adobe Photoshop (Adobe Systems, Inc., 2007). In addition to quantitative procedures, each picture was manually matched in triads across as many possible dimensions including the contrast of color tones, location and relative size of objects, and complexity of background or scene. The pictures with highest levels of recognition speed and least amount of variability were selected into a potential stimulus pool. The mean recognition speed of content of emotional scenes was based on a sample of 12 participants (6 females and 6 males) who were asked to rate how quickly they could recognize the content of the pictures. Finally, pictures were chosen with the widest range of content of scenes (people, activities, and background), while the number of photos with strong negative content was balanced (e.g., equal number of scenes with and without blood). The Ekman picture series was selected because of the relatively uniform (black and white) stimulus properties. To create equal levels of visibility (salience) among the faces, the luminosity, brightness, and color contrast of black and white, were adjusted manually in Adobe Photoshop (Adobe Systems, Inc., 2007).
Orientation/Experimental Sessions
During the first orientation session each participant was given information about the study and signed an IRB-approved consent form. The two experimental sessions were separated by a minimum of 48 hours; a maximum of 7 days separated the practice sessions and each of the experimental sessions. Compliance with instructions for overnight smoking abstinence and habitual smoking status were verified with an expired breath carbon monoxide (CO) monitor (Vitalograph, Lenexa, KS) and by self-report at the start of each session. The required CO level for each individual, during experimental sessions, was less than 1/3 of the late-afternoon orientation session or an absolute value of 10 ppm at the time of patch placement. The CO level was less than or equal to the morning concentration for all individuals when they returned four hours later to begin the experimental session. Participants began sessions by practicing the experimental tasks for approximately 10 minutes. Both of the experimental sessions began between noon and 1:30 p.m. and lasted about 3 hours. Each participant sat alone in a small experimental room that was electronically connected to a central control room. The control room contained video display units for monitoring each participant’s computer and behavior for control of the experimental tasks.
Patch Administration
Patch administration was double blind with placement on the upper arm of smokers about 4 hours prior to the beginning of the experimental sessions by an individual not involved in data collection. Four hours prior to the beginning of the experimental session, a patch (14 mg Nicoderm® or an identically appearing placebo) was placed on the upper arm. The patches were counterbalanced so an equal number of participants received each patch type during the first and second sessions. In order to minimize the ability of participants to differentiate active and placebo patches by skin sensations, a cover bandage was used with a small amount (.05cc) of capsaicin 075% cream (Capzasin-HP7, Chattem, Inc) applied to the Teflon-coated surface of the cover bandage. This bandage covered an area of 5 mm wide immediately next to each of the patch edges. Pilot testing showed this procedure to reduce the ability to detect differences between the active and placebo patch.
First Gaze-Fixations (FGFs) of the PACT
Data processing
Eye-gaze data for each trial consisted of 70 data points (2100 ms) collected across the presentations of each dual-picture. Blink-related and other gaze-tracking artifacts were detected and replaced with estimates based on linear imputations. Data processing was conducted blind to experimental conditions.
The FGFs were defined by pupil fixation on one of the Ekman faces within a 450 millisecond (ms) window of opportunity set to occur between 300 ms to 750 ms post dual-picture onset. The 300 ms post-stimulus onset period was determined to be the earliest possible time when a valid response (FGFs) to the experimental stimuli could occur, while excluding any anticipatory movements and excessive delays for the onset of FGFs. The onset of this time window is consistent with the mean latency of shift of first fixations reported by Caseras, Garner, Bradley, and Mogg, (2007) while consistent with the timing of peak amygdala activity (Garolera et al. 2007) and significantly enhanced brain responses at 300 ms post stimulus onset, indicative of attending towards facial stimuli (Schuller & Rossion, 2001). The offset time is consistent with the latency of mean RT in a CAP task collected during another psychophysiology study (Garolera et al. 2007). For each trial, the duration of first eye gaze-fixations were defined as pupils fixated on part (edge) of one of the two faces for 150 ms or longer; this minimum required duration is consistent with the average duration of first fixations that enables facial recognition (Hsiao & Cottrell, 2008). The beginning and end of fixations were marked by data where the pupils crossed the edge of one of the faces. Thus, valid FGFs were defined as five consecutive data points (150 ms) or more directed towards one of the lateralized pictures within the time window between 300 ms and 750 ms post dual-picture onset. Each valid fixation was assigned a value of 1 or -1. The mean proportion of the total number of assigned code values, of pupil fixations, was computed for each of the 18 types of trials (3 primes × 3 dual-picture types for 2 VFs) separately for the (nicotine and placebo) days.
Trials were rejected for not meeting the criteria for clearly defined FGFs. Trials were rejected for the following reasons: failure of the FGFs to occur within the 450 ms window, premature eye-movements, blink-related artifacts, and equipment recoding failure. A minimum total of 4 trials with good quality of data were required for each of the 18 trial types of each session (nicotine and placebo) in order to accept the participant’s data for inclusion in the final analyses. Two participants were rejected because of an insufficient number of good quality trials.
Variable descriptors
The variables used in statistical analyses were created by sorting trials based on the emotional valence of priming stimuli (i.e., positive, negative, or neutral) in combination with the type of dual-picture (angry: neutral, happy: neutral, and angry: happy) and visual field (i.e., emotional picture in the LVF vs. emotional picture in the RVF). The variables were (a) Nicotine (nicotine patch vs. placebo patch), (b) Priming Picture Valence (positive, negative and neutral), and (c) Dual-Picture Valence (Angry: Neutral vs. Happy: Neutral vs. Angry: Happy), while the visual field variable was insignificant in exploratory analyses and not retained in the results.
RT of the CAP Task
Data processing
The standard deviation of mean reaction times (RT) suggested that a value of 1200 ms was 3 standard deviations above the mean RT. Thus, trials with a value of 1200 or greater were rejected. Trials with incorrect responses were also rejected.
Variable descriptors
The variables were (a) Nicotine (nicotine patch vs. placebo patch), (b) Priming Picture Valence (positive, negative and neutral), and (c) Target Picture Valence (happy vs. angry).
Self Report Measures
Data processing
The Positive and Negative Affect Schedule (PANAS: Watson, Clark, & Tellegen, 1988) measured positive and negative affect. Separate indices of positive and negative affect for each experimental session were aggregated across time (pre and pos-task) and across both tasks (PACT and CAP) because no differences were expected across these variables.
Variable descriptors
The variables were: (a) Nicotine (nicotine patch vs. placebo patch), and (b) Affective Valence (positive vs. negative).
Statistical Normality
The normality (skewness and kurtosis) of dependent variables was assessed with Z scores computed in SPSS 13.0 and then evaluated in reference to a standardized table (Howell, 2002). Results for the PACT data (FGFs) suggested that the variables were all normally distributed. However, the RT for the CAP task was skewed, but corrected to be normally distributed using Log-10 transformations in SPSS 13.0. Despite the self-report data being non-normal, the data remained uncorrected because of the failure to normalize by commonly used procedures.
Analytic Strategy
Overall statistical model
The primary hypotheses were assessed with Repeated Measures Multivariate Analysis of Variance (MANOVA) in SPSS 13.0. The hypotheses were initially assessed with three-way interactions among Nicotine, Prime Valence, and either Dual-Picture Valence for the PACT task or Target-Picture Valence for the CAP task. The questionnaire data was assessed with a two way interaction between Nicotine and Affective Valence. These interactions were assessed using Pillai’s Trace Multivariate Test of each effect in the MANOVA that is appropriate for small sample sizes (Tabachnick & Fidell, 1996). The effect size for all analyses was based on Partial Eta Squared because it is more appropriate than the popular Eta Squared, for assessing higher order interactions (Tabachnick & Fidell, 1996). The specific hypothesized interaction effects were assessed using single degree-of-freedom contrasts to use the error term generated by the ANOVA, with increased power in this modest sample size, and to identify specific sources of the overall interaction effects, while controlling other levels of variables (Winer, Brown, & Michaels, 1991).
Results
First Gaze-Fixations (FGFs) Data of the PACT Task
Nicotine and positive priming of attending
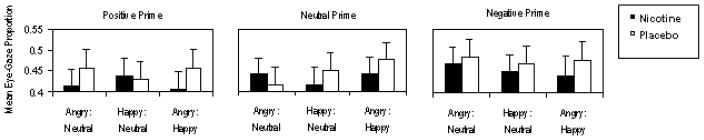
The hypothesized Nicotine × Prime Valence × Dual-Picture Valence interaction was significant, F(4, 42) = 2.641, p = .047, η2 = .201 (see Figure 2). The strongest effect size among our predicted-findings was the effects of nicotine and positive primes on reduced FGFs towards angry faces, while increased FGFs towards neutral faces in a significant interaction with Nicotine × Positive (vs. Neutral) Primes, F(1, 45) = 7.173, p = .010, η2 = .137.
Figure 2.
The mean proportions of trials (0 to 1) that individuals first looked at the emotional content of the pair labeled on the top row (angry, happy, or angry) compared to the paired picture type indicated on the bottom row (neutral, neutral, or happy). The proportion of trials that individuals first looked at the neutral or happy pictures (bottom row), can be computed by subtracting the plotted mean proportions from the value of one. The error bars are for standard errors. The hypothesized contrasts are based on subtracting the neutral prime condition from the negative and positive conditions, as illustrated in Figure 3 below.
Nicotine and negative priming of attending
There was a trend for nicotine to attenuate the effectiveness of negative primes; that is, nicotine (vs. placebo) and negative (vs. neutral) primes promoted the decreased FGFs towards angry faces (see middle and right panels of Figure 2), while increasing FGFs towards neutral faces, in an interaction that just failed to reach significance, F(1, 45) = 3.585, p = .065, η2 = .074 with a medium effect size. Contrary to expectations, nicotine did not significantly alter the affective priming of FGFs towards the other two dual-picture (happy: neutral and angry: happy) conditions.
Irrespective of the emotional primes condition, there was a nonsignificant trend for nicotine to decrease FGFs towards the angry faces, while increasing FGFs towards happy faces, in a two-way interaction for the angry and happy faces condition F(1, 45) = 2.377, p = .130, η2 = .050 with a near-medium effect size (see Figure 2). This trend was found across all three prime conditions.
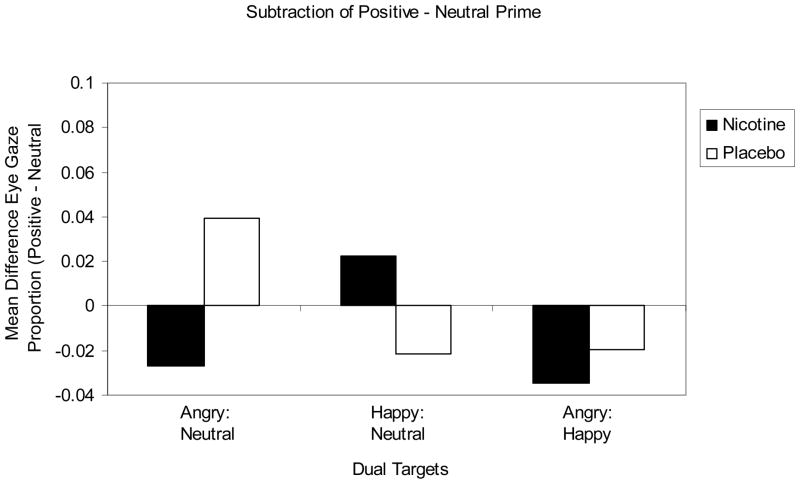
Affective priming of attending
The positive (vs. neutral) primes significantly increased FGFs towards happy (vs. angry) faces in a significant interaction, F(1, 45) = 4.347, p = .043, η2 = .088 (see Figure 3). Negative (vs. neutral) primes significantly increased FGFs towards angry (vs. neutral and happy) faces of the two combined dual-picture conditions (angry: neutral and angry: happy) in a significant interaction, F(1, 45) = 4.286, p = .044, η2 = .087. There was no other significant contrasts of the interaction, Positive and Negative (vs. Neutral) Prime Valence × Dual-Picture Valence.
Figure 3.
The mean difference between positive and neutral primes on the mean proportions of trials (0 to 1) that individuals first looked at the emotional content of the pair labeled on the top row (angry, happy, or angry) compared to the paired picture type indicated on the bottom row (neutral, neutral, or happy). For each dual-picture condition, a greater positive value indicates a higher frequency of FGFs between nicotine and placebo, while a lower negative value indicates a relatively lower number of FGFs for these conditions.
Reaction Time Data of the CAP Task
Nicotine and affective priming of reaction time performance
Consistent with hypotheses, the Nicotine × Prime Valence × Target Picture Valence interaction was not significant, F(2, 44) = .415, p = .663, η2 = .019. Nicotine (relative to placebo) significantly reduced the RT to correctly identify the valence of emotional faces F(1, 45) = 7.909, p = .007, η2 = .149. No other effects of the ANOVA were significant.
Affective priming of reaction time performance
A Prime Valence × Target Picture Valence interaction was significant, F(2, 44) = 3.897, p = .028, η2 = .150. Positive primes significantly reduced (sped) RT to correctly identify the happy (vs. angry) target faces, F(1, 45) = 25.652, p < .001, η2 = .363. However, reverse priming was found for the negative prime condition; that is, negative primes significantly reduced RT to identify the happy (vs. angry) target faces, F(1, 45) = 10.448, p = .002, η2 = .188. Neutral primes significantly reduced RT to correctly identify the happy (vs. angry) target faces, F(1, 45) = 22.234, p < .000, η2 = .331. No other effects of the ANOVA were significant.
Self-Reported Affective Data
Nicotine and reported affect
The hypothesized Nicotine × Affective Valence interaction was significant, F(1, 45) = 22.188, p <.000, η2 = .330 (Figure 4). Nicotine (vs. placebo) significantly increased levels of positive affect, F(1, 45) = 10.116, p = .003, η2 = .184. Nicotine significantly decreased levels of negative affect, F(1, 45) = 13.760, p = .001, η2 = .234.
Figure 4.
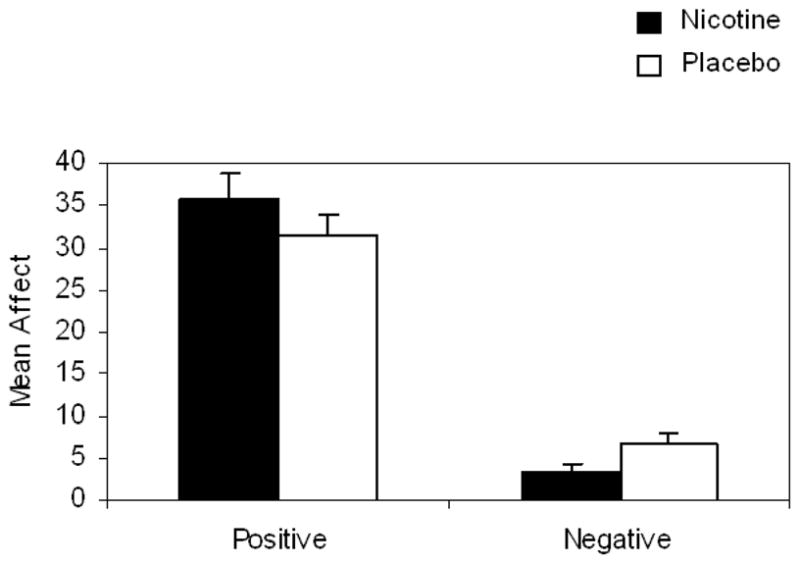
Mean and standard errors of positive and negative affect in nicotine rather placebo as measured by the PANAS.
Summary of Overall Results
There was mixed support for the primary hypothesis that nicotine and positive (relative to neutral) pictures primed (enhanced) attending towards neutral faces, while attenuated attending towards angry faces. While nicotine (relative to placebo) reduced the effect of the negative primes on attending towards angry faces, the statistical assessment of this hypothesis fell just short of significance. Consistent with hypotheses, nicotine had minimal effect on CAP performance. An assessment of reported affect demonstrated that nicotine significantly increased positive affect, while decreasing negative affect. However, the primary hypotheses were based on support for the affective priming effects on responding; that is, responding based on the match and mismatch of valences of the prime and target stimuli.
Support for the main hypotheses are strengthened by the secondary effects for the PACT, averaged across the nicotine and placebo conditions, revealed results that strengthened the conclusions of this study. Emotional primes promoted an increase in attending towards faces with similar emotional valence as the primes. Negative primes consistently increased attending towards negative (angry) faces; positive primes increased attending towards positive (happy) or neutral faces but only when negative faces were competing for attention. Results for the CAP task, independent of nicotine conditions, provided mixed support for the effects of prime-target match and mismatch relations on performance. That is, positive and neutral primes sped responding towards positive (relative to negative) faces while negative primes sped responding towards positive (relative to negative) faces. In summary, the overall pattern of results provided strong support for the hypothesized effects of nicotine on performance for the PACT and CAP tasks, and affective states. The interpretations of these data in the context of the literature and research questions are discussed in the next section.
Discussion
The most important findings of the current study are that nicotine functioned as a positive emotional prime, promoting attention away from negative stimuli and towards positive or neutral stimuli. These results are consistent with the Situation by Trait Adaptive Response (STAR) model (Gilbert, 1995) and Affect-Priming hypothesis (Asgaard, 2007) suggesting that nicotine and positive stimuli primed attentional bias to competing affective stimuli. The discussion below first addresses the importance of nicotine’s effects on competing reinforcers and affect, and then nicotine’s effects on salience by functioning as a positive emotional prime and finally alternative ways to conceptualize these findings.
Nicotine’s Effects on Competing Reinforcers
The results suggest possible explanations of the unreliable findings of the effects of nicotine on negative affect (reviewed by Buckley et al. 2007). Nicotine had different effect in the presence of competing affective stimuli (PACT performance) than it did without stimulus competition (CAP task). It has been suggested that nicotine reduces negative affect to a greater extent in situations that include attentional freedom and choice (Gilbert, 1995) when smokers can focus on emotionally positive stimuli that are in competition with emotionally negative stimuli (Asgaard, 2007; Gilbert, Rabinovich, et al., 2008). Consistent with the importance of competing motives, nicotine altered both positive and negative reported affect. These results are consistent with the STAR model (Gilbert, 1995). Thus, nicotine may have anxiolytic effects and promote negative reinforcement during the availability of competing reinforcers, but not without the presence of such stimuli.
Nicotine’s Effects on Salience by Functioning as a Positive Emotional Prime
Nicotine and positive emotional primes were functionally equivalent in that they both enhanced initial attention to positive (happy) stimuli and reduced initial attention to negative (angry) emotional stimuli when both happy and angry stimuli were concurrently present. In addition, nicotine and positive primes significantly increased attending towards neutral stimuli, while reduced attending towards negative stimuli competing for attention. This view of nicotine is consistent with the view that nicotine alters the salience (Stewart et al. 1984) of rewards and reinforcers, making them more attractive or “attention grabbing” (Robinson & Berridge, 1993). However, as both nicotine and deprivation can alter affect and attentional bias, these data may be explained by alternative explanations.
Alternative Conceptualizations
An alternative explanation of nicotine’s effect on the PACT task is that nicotine enhanced positive (or attenuated negative) mood states either directly or indirectly by means of alleviating nicotine withdrawal symptoms. That is, processing of negative affective stimuli and negative reinforcement has been proposed as the strongest or most consistent smoking motive (Baker, et al., 2004; Koob, Caine, Parsons, Markou, & Weiss, 1997). Based on an extensive review of the literature, Baker et al. (2004, pp 35), proposed that negative affect “primes previously reinforced” behaviors and cognitive processing (attentional bias) of negative stimuli, that serves to be the strongest source of affective motivation for tobacco smoking. In this affective processing model, nicotine is proposed to attenuate negative affect and negative attentional response bias. To date, there remains relative little rigorous experimental research on the effects of nicotine and abstinence on relationships of attention and affect in this area using in humans (reviewed by Buckley et al. 2007). Nevertheless, animal models support the view that nicotine can alter affect in paradigms assessing nicotine’s effects on response to reinforcers (Caggiula et al. 2002) and punishers (Szyndler et al. 2001). The importance of negative affect as a strong smoking motive is emphasized in the opponent process theory suggesting that positive reinforcers such as addictive drugs engage positive affective process, that counteract (opposes) negative affective process (Koob et al. 1997). This theory predicts that positive affective process may be important in the early stages of motivation while negative affective processes may be more important in the transition and maintenance of motivation. Taken together, these models suggest that reduced attentional bias towards negative stimuli and nicotine deprivation on negative reinforcement are important motives of smoking.
Another theoretical consideration is that the later occurring effects of nicotine on attention may be equally important in the affect modulating properties of nicotine. While a recent study (Gilbert, Rabinovich, et al. 2008) found that nicotine exerted its maximal effects on attentional bias during the latter two thirds of a two-second dual picture task, these delayed effects may reflect the relatively greater emotional potency of the target pictures used in that study, compared to the current study. Thus, it may be important to consider the potency of the target and priming stimuli in future studies.
Clinical and Theoretical Implications
Nicotine may play a critical role in redirecting momentary attentional and affective associations away from negative and toward positive contents and associations in the complex situations, with numerous brief and benign affective stimuli, that are characteristic of many contexts in the daily environment of many smokers. Nicotine may briefly act like a positive prime and have priming effects on positive affect that are dependent on priming and “target” stimuli in the natural environment (Gilbert & Welser, 1989, p. 200). This nicotine priming effect may activate positive affect-related schema and attenuate negative affect-related cognitive schemas that can sustain more prolonged moods and attentional biases and cognitive contents.
Study Limitations and Future Directions
There are several limitations of the current study. The sample size was modest, largely university based, did not include individuals who abused alcohol or psychoactive substances, was relatively young, and did not include non-smokers. While attempts were made to equate the negative and positive priming stimuli in as many dimensions as possible, differences (e.g., personality variables) other than emotional valence of the pictures may have accounted for the differential effects of nicotine. In addition, pictures with different degrees of personal relevance or salience and presentation speed of the primes (e.g., Leventhal et al. 2008) could also result in different findings than we observed. Finally, nicotine administration by patch has different pharmacokinetics than that of tobacco smoking with largely similar, though somewhat different pharmacodynamic effects. Thus, the effects of nicotine patch on attention and affect cannot be presumed to generalize to tobacco smoking; the results of this study may not generalize to other forms of affect induction and their theoretical basis.
Contrary to experimental hypotheses, nicotine and positive primes did not promote increased attention towards happy faces paired with neutral faces. One explanation for these unexpected findings is that the effects of nicotine depend upon attentional salience (Hitsman, Spring, Pingitore, Munafò, & Hedeker, 2007) as well as the emotional valence of the stimulus such that nicotine enhances attention to stimuli with greater salience and with greater positive valence given equivalent salience (Gilbert, Rabinovich et al., 2008). Alternatively, these results may hinge on the failure to find priming effects for this dual-picture condition independently of nicotine; the positive primes failed to promote increased first-gaze bias towards happy rather than neutral stimuli. It is possible that a high degree of perceptual similarity and low salience between the happy and neutral faces failed to provide effective discriminative stimuli. Another contradictory expectation was the finding that nicotine did not alter the affective priming effects for the angry and happy dual-picture condition. This may be due to the consistent effects of nicotine on reducing the attending towards angry faces, while increasing attending towards happy faces across all three prime conditions, thereby requiring a large effect size and statistical power to detect any differential effects of nicotine between the emotional and neutral prime conditions. In sum, future research may utilize increased statistical power and vary the discriminative properties or salience of emotional stimuli to better understand the effects of nicotine on primed attentional bias.
The current findings suggest that the nicotine and affective priming alters attentional bias towards emotional reinforcers, thereby suggesting that the effects of nicotine on reinforcement may be better assessed using new paradigms driven by momentary (preconscious) affective and attentional processes. To better characterize nicotine’s apparent affect-priming mechanism, it may prove useful to use the PACT in combination with other priming paradigms (e.g., memory priming using emotional stimuli) that characterize other aspects of priming. Another important area of future research is to identify additional value-altering (Laraway, Snycerski, Michael, & Poling, 2003) stimuli (e.g., food and alcohol) to better characterize tobacco smoking motivation. Future studies in this area would also benefit from larger samples that would allow the assessment of modulating effects of individual differences in genotype, personality, and psychopathology on nicotine’s effects. Finally, the PACT can be transported to a variety of assessment situations where the dependent variable can be button press responses.
Summary and Conclusions
In summary, the overall pattern of findings provides modest support for the primary hypotheses, while applying a new paradigm with promise to guide future research. Nicotine modulated attentional bias during the PACT task; however, nicotine failed to modulate performance during the classical CAP task. Results are consistent with the Affect-Priming hypothesis; that is, nicotine functioned as a positive emotional prime, promoting attention away from negative stimuli and towards positive or neutral stimuli. Nicotine primed the reinforcing value of positive stimuli, while attenuating the value of negative stimuli. Tobacco smoking may be motivated by nicotine operating on affective priming and salience of stimuli competing to grab attention. This line of research is particularly important in light of recent research findings suggesting that attention may be retrained thereby resulting in reduced levels of negative affect (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Thus, adding an attentional retraining component to the current pharmacotherapy and psychotherapy approaches may reduce the relapse rates of smoking cessation treatments. Additional clinical utility may be in the Affect-Priming hypothesis providing an explanation of the attentional and emotional benefits of tobacco smoking and treatment options in smoking cessation programs. While the results of this study may advance our understanding of mechanisms that mediate the motivation for tobacco smoking, important questions have been raised for future research. A key question to guide future research is, “Does nicotine modulate PACT performance in non-smokers and are these effects different in clinical populations?”
Acknowledgments
This research was supported in part by funding from the National Institute on Drug Abuse R01 DA017837 and DA014104 awarded to David G. Gilbert and the transdermal nicotine and placebo patches were donated by GlaxoSmithKline. This research served as a dissertation for the first author, Gregory L. Asgaard. I thank the dissertation committee (David G. Gilbert, Chair, Eric Jacobs, Benjamin Rodriguez, Rebecca Weston, and Ruth Anne Rehfeldt) for their contributions to this research and also thank Dr. Lisa T. Eyler for her comments on a draft of the manuscript. Dr. Debra Malpass is now at the University of Birmingham and Dr. Gregory L. Asgaard is now in the United States Navy.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
References
- Asgaard Gregory L. PhD dissertation. Southern Illinois University at Carbondale; United States –Illinois: 2007. Modulation of Affective Priming by Nicotine. Retrieved May 23, 2008, from Dissertations & Theses: A&I database. (Publication No. AAT 3291673) [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore M. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS. Ad lib smoking in post-traumatic stress disorder: An electronic diary study. 2008;10(7):1149–1157. doi: 10.1080/14622200802123302. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Holohan DR, Mozley SL, Walsh K, Kassel J. The effect of nicotine and attention allocation on physiological and self-report measures of induced anxiety in PTSD: A double-blind placebo-controlled trial. Experimental and clinical psychopharmacology. 2007;15(2):154–164. doi: 10.1037/1064-1297.15.2.154. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Lang PJ. Gaze patterns when looking at emotional pictures: Motivationally biased attention. Motivation and Emotion. 2004;28(3):221–243. [Google Scholar]
- Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;16(3):491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of non-pharmacological factors in nicotine self-administration. Physiology & Behavior. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry, and Behavior. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved A. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharamacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Ekman P. Emotions revealed: Recognizing faces and feelings to improve communication and emotional life. New York: Times Books/Henry Holt and Co; 2003. [Google Scholar]
- Ekman P, Friesen WV. Manual for the Facial Action Coding System. Palo Alto: Consulting Psychologists Press; 1977. [Google Scholar]
- Garolera M, Coppola R, Muñoz KE, Elvevag B, Carver FW, Weinberger, et al. Amygdala activation in affective priming: A magnetoencephalogram study. Learning and Memory. 2007;18(4):1449–1453. doi: 10.1097/WNR.0b013e3282efa253. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Carlson JM, Riise H, Rabinovich NE, Sugai C, Froeliger B. Effects of nicotine and depressive traits on affective priming of lateralized emotional word identification. Experimental and Clinical Psychopharmacology. 2008;16:293–300. doi: 10.1037/a0012871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Welser R. Emotion, anxiety, and smoking. In: Ney T, Gale A, editors. Smoking and human behavior. Chichester: John Wiley & Sons; 1989. pp. 171–196. [Google Scholar]
- Gilbert DG. Smoking: Individual differences, psychopathology, and emotion. Washington, D.C: Taylor & Francis; 1995. [Google Scholar]
- Gilbert DG, Rabinovich NE, Malpass D, Mrnak J, Riise H, Adams L, et al. Effects of nicotine on affect are moderated by stressor proximity and frequency, positive alternatives, and smoker status. Nicotine & Tobacco Research. 2008;10(7):1171–1183. doi: 10.1080/14622200802163092. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Riise H, Dillon A, Huber J, Rabinovich NE, Sugai C. Emotional stimuli and context moderate effects of nicotine on specific but not global affects. Experimental and Clinical Psychopharmacology. 2008;16:33–42. doi: 10.1037/1064-1297.16.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Rabinovich NE, McClernon FJ, Froeliger B. Brain indices of nicotine’s effects on attentional bias to smoking and emotional pictures and to task-relevant targets. Nicotine & Tobacco Research. 2007;9:351–363. doi: 10.1080/14622200701188810. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Pingitore R, Munafò M, Hedeker D. Effect of tryptophan depletion on the attentional salience of smoking cues. Psychopharmacology. 2007;192:317–324. doi: 10.1007/s00213-007-0722-2. [DOI] [PubMed] [Google Scholar]
- Hsiao JH, Cottrell G. Two fixations suffice in face recognition. Psychological Science. 2008;19998(10):1006. doi: 10.1111/j.1467-9280.2008.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. 5. Pacific Grove, CA: Duxbury; 2002. [Google Scholar]
- Izetelny A, Gilbert DG, Hammersley J, Radtke R, Rabinovich NE, Small SL. Nicotine decreases attentional bias to negative affect-related Stroop words among smokers. Nicotine & Tobacco Research. 2008;10:1029–1036. doi: 10.1080/14622200802097514. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: Support for the role of nicotine in attentionally mediated anxiolysis. Journal of Abnormal Psychology. 2000;109(1):161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch J, Klauer KC, editors. The psychology of evaluation. Lawrence Erlbaum Associates, Publishers; Mahwah, New Jersey: 2003. pp. 7–49. [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacoloogy Biochemistry and Behavior. 1997;57513(3):521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention; 1995. [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36(3):407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Breitmeyer BG, Miller EK, Tapia E, Li Y. Subliminal processing of smoking-related and affective stimuli in tobacco addiction. Experimental and Clinical Psychopharmacology. 2008;16(4):301–312. doi: 10.1037/a0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Attentional bias and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107–123. [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: Affective priming and optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–739. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Musch J, Klauer KC, editors. The psychology of evaluation. Lawrence Erlbaum Associates, Publishers; Mahwah, New Jersey: 2003. [Google Scholar]
- Olausson P, Jentsch DJ, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2003;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ, Fernandez-Duque D. Brain mechanisms of cognitive skills. Consciousness and Cognition. 1997;6:267–290. [PubMed] [Google Scholar]
- Posner MI, Snyder C. In: Rabbitt P, Dornig S, editors. Facilitation and inhibition in the processing of signals; Attention and performance 5. Attention and performance symposium; London: Academic Press; 1975. pp. 669–682. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of craving: an incentive sensitization theory of addiction. Brain Research Review. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schuller AM, Rossion B. Spatial attention triggered by eye gaze increases and speeds up early visual activity. Cognitive Neuroscience. 2001;12(11):2381–2386. doi: 10.1097/00001756-200108080-00019. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding, and reinstatement in adolescent and male adult rats: Evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2007;33(4):739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Psychological determinants of smoking behavior. In: Tollison RD, editor. Smoking and society: Toward a more balanced assessment. Lexington, MA: D. C. Heath; 1986. pp. 89–134. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91(2):251–268. [PubMed] [Google Scholar]
- Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, Siemiatkowski M, Rokicki D, Cz onkowska AI, et al. The anxiolytic-like effect of nicotine undergoes rapid tolerance in a model of contextual fear conditioning in rats. Pharmacology, Biochemistry and Behavior. 2001;69:511–518. doi: 10.1016/s0091-3057(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. HarperCollins Publishers; New York, NY: 1996. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wells A. Emotional Disorders & Metacognition: Innovative Cognitive Therapy. Chichester: John Wiley & Sons, LTD; 2001. [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill, Inc; 1991. [Google Scholar]