Abstract
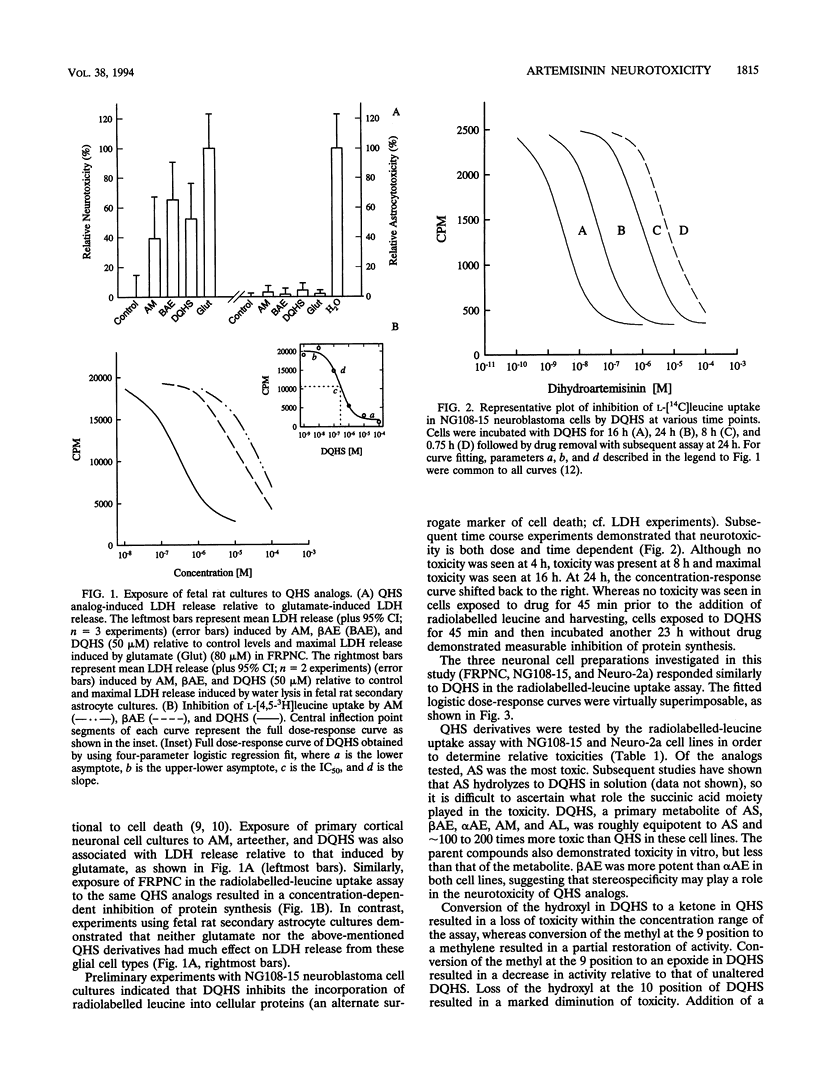
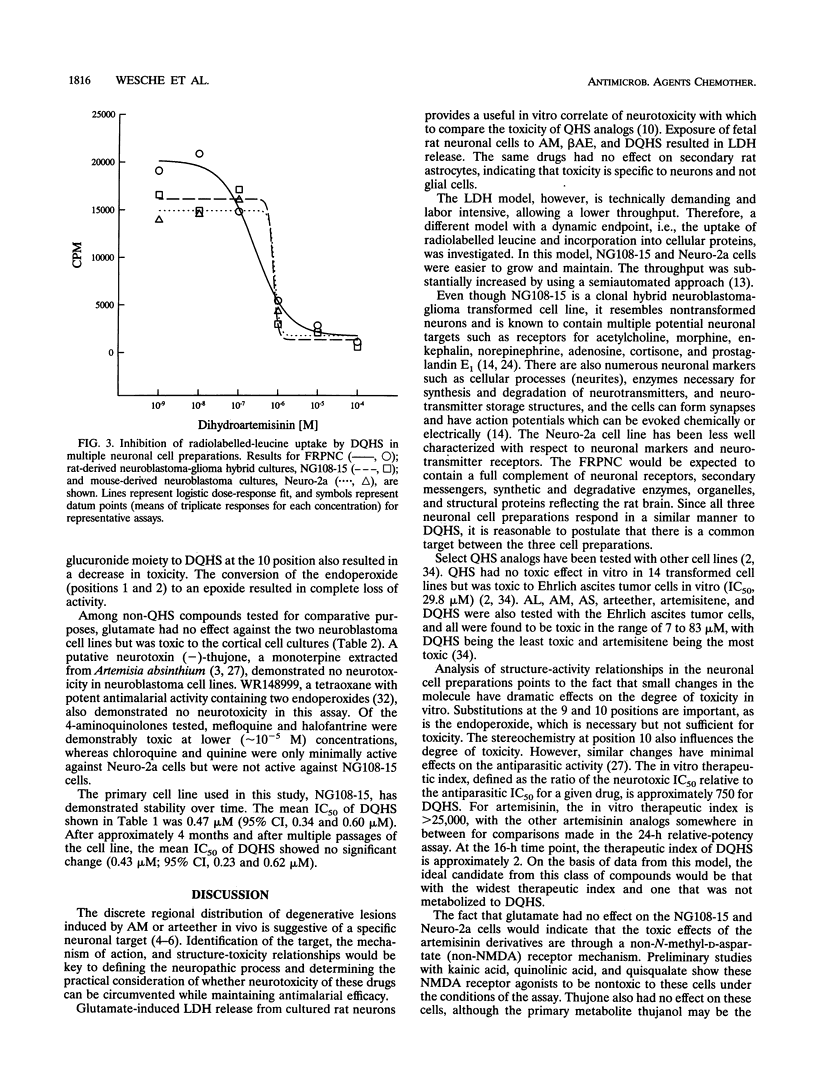
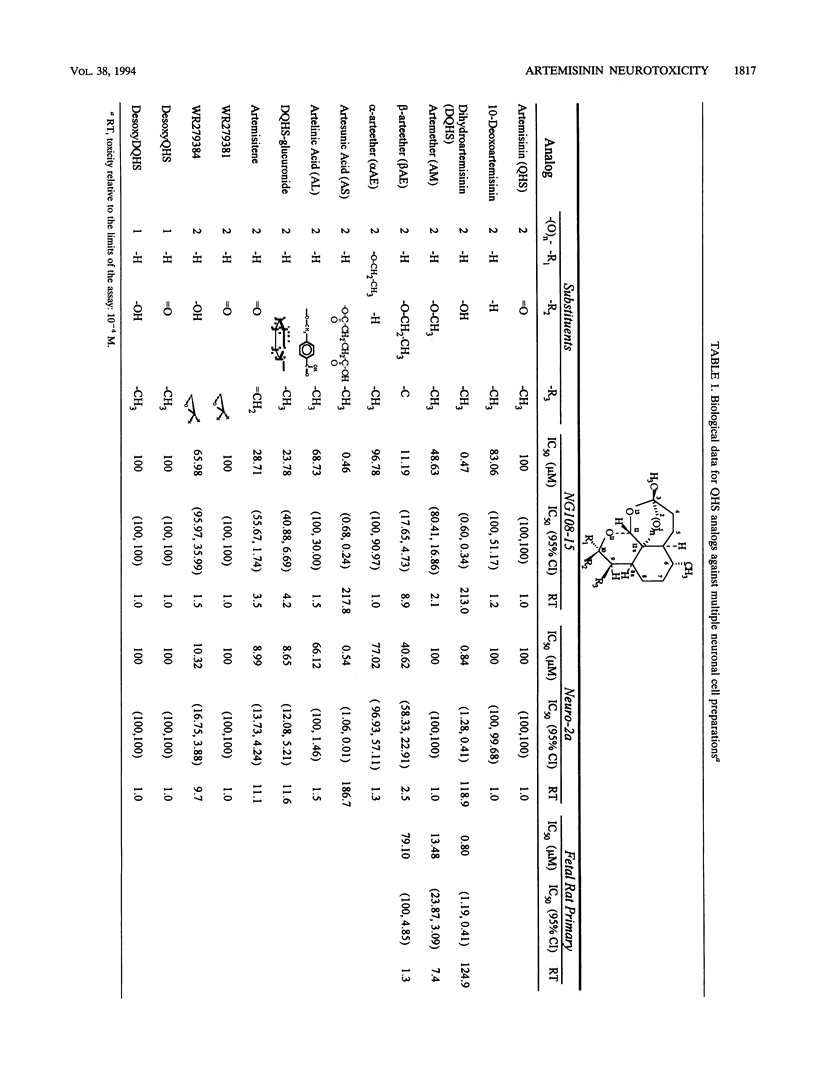
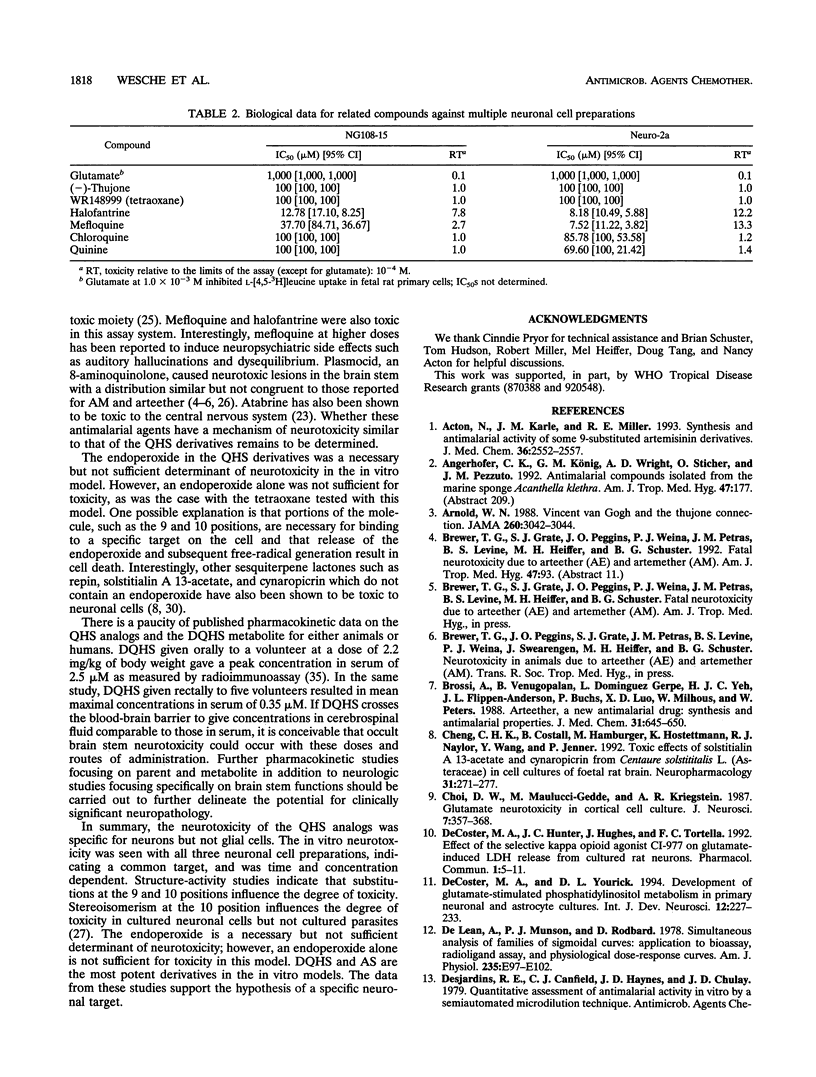
The sesquiterpene endoperoxide antimalarial agents arteether and artemether have been reported to cause neurotoxicity with a discrete distribution in the brain stems of rats and dogs after multiple doses. The nature and distribution of the brain lesions suggest a specific neuronal target, the identity of which is unknown. In order to further investigate artemisinin analog-induced neurotoxicity, we evaluated several in vitro models: fetal rat primary neuronal cultures, fetal rat secondary astrocyte cultures, and transformed neuronal cultures (rat-derived neuroblastoma NG108-15 and mouse-derived neuroblastoma Neuro-2a). Results indicate that toxicity was specific for neuronal cell types but not glial cells. Neurotoxicity, as indexed by liberation of lactate dehydrogenase and/or inhibition of radiolabelled-leucine uptake, was seen in all three neuronal culture types, implicating a common target. In vitro neurotoxicity was dose and time dependent. Acute exposure to drug results in delayed, but not immediate, manifestations of cell toxicity. Structure-activity comparisons indicate that substitutions at positions 9 and 10 and stereoisomerism at position 10 of the artemisinin backbone influence the degree of toxicity. The endoperoxide is necessary but not sufficient for toxicity. Sodium artesunate and dihydroartemisinin, a metabolite common to all artemisinin analogs currently being developed for clinical use, are the most potent of all analogs tested. These results are consistent with a specific neuronal target, but the identity of the target(s) remains unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton N., Karle J. M., Miller R. E. Synthesis and antimalarial activity of some 9-substituted artemisinin derivatives. J Med Chem. 1993 Aug 20;36(17):2552–2557. doi: 10.1021/jm00069a014. [DOI] [PubMed] [Google Scholar]
- Arnold W. N. Vincent van Gogh and the thujone connection. JAMA. 1988 Nov 25;260(20):3042–3044. [PubMed] [Google Scholar]
- Brossi A., Venugopalan B., Dominguez Gerpe L., Yeh H. J., Flippen-Anderson J. L., Buchs P., Luo X. D., Milhous W., Peters W. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J Med Chem. 1988 Mar;31(3):645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- Cheng C. H., Costall B., Hamburger M., Hostettmann K., Naylor R. J., Wang Y., Jenner P. Toxic effects of solstitialin A 13-acetate and cynaropicrin from Centaurea solstitialis L. (Asteraceae) in cell cultures of foetal rat brain. Neuropharmacology. 1992 Mar;31(3):271–277. doi: 10.1016/0028-3908(92)90177-q. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoster M. A., Yourick D. L. Development of glutamate-stimulated phosphatidylinositol metabolism in primary neuronal and astrocyte cultures. Int J Dev Neurosci. 1994 Jun;12(3):227–233. doi: 10.1016/0736-5748(94)90044-2. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Hien T. T., White N. J. Qinghaosu. Lancet. 1993 Mar 6;341(8845):603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- Hudson T. H., Grillo F. G. Brefeldin-A enhancement of ricin A-chain immunotoxins and blockade of intact ricin, modeccin, and abrin. J Biol Chem. 1991 Oct 5;266(28):18586–18592. [PubMed] [Google Scholar]
- Hudson T. H., Scharff J., Kimak M. A., Neville D. M., Jr Energy requirements for diphtheria toxin translocation are coupled to the maintenance of a plasma membrane potential and a proton gradient. J Biol Chem. 1988 Apr 5;263(10):4773–4781. [PubMed] [Google Scholar]
- Klayman D. L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985 May 31;228(4703):1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Lee I. S., Hufford C. D. Metabolism of antimalarial sesquiterpene lactones. Pharmacol Ther. 1990;48(3):345–355. doi: 10.1016/0163-7258(90)90053-5. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Viravan C., Vanijanonta S., Wilairatana P., Suntharasamai P., Charoenlarp P., Arnold K., Kyle D., Canfield C., Webster K. Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet. 1992 Apr 4;339(8797):821–824. doi: 10.1016/0140-6736(92)90276-9. [DOI] [PubMed] [Google Scholar]
- Ray P., Middleton W., Berman J. D. Mechanism of agonist-induced down-regulation and subsequent recovery of muscarinic acetylcholine receptors in a clonal neuroblastoma x glioma hybrid cell line. J Neurochem. 1989 Feb;52(2):402–409. doi: 10.1111/j.1471-4159.1989.tb09135.x. [DOI] [PubMed] [Google Scholar]
- Rice K. C., Wilson R. S. (-)-3-Isothujone, a small nonnitrogenous molecule with antinociceptive activity in mice. J Med Chem. 1976 Aug;19(8):1054–1057. doi: 10.1021/jm00230a015. [DOI] [PubMed] [Google Scholar]
- Shmuklarsky M. J., Klayman D. L., Milhous W. K., Kyle D. E., Rossan R. N., Ager A. L., Jr, Tang D. B., Heiffer M. H., Canfield C. J., Schuster B. G. Comparison of beta-artemether and beta-arteether against malaria parasites in vitro and in vivo. Am J Trop Med Hyg. 1993 Mar;48(3):377–384. doi: 10.4269/ajtmh.1993.48.377. [DOI] [PubMed] [Google Scholar]
- Shwe T., Myint P. T., Myint W., Htut Y., Soe L., Thwe M. Clinical studies on treatment of cerebral malaria with artemether and mefloquine. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):489–489. doi: 10.1016/0035-9203(89)90261-7. [DOI] [PubMed] [Google Scholar]
- Stevens K. L., Riopelle R. J., Wong R. Y. Repin, a sesquiterpene lactone from Acroptilon repens possessing exceptional biological activity. J Nat Prod. 1990 Jan-Feb;53(1):218–221. doi: 10.1021/np50067a038. [DOI] [PubMed] [Google Scholar]
- Taylor T. E., Wills B. A., Kazembe P., Chisale M., Wirima J. J., Ratsma E. Y., Molyneux M. E. Rapid coma resolution with artemether in Malawian children with cerebral malaria. Lancet. 1993 Mar 13;341(8846):661–662. doi: 10.1016/0140-6736(93)90423-e. [DOI] [PubMed] [Google Scholar]
- Tin Shwe, Pe Than Myint, Ye Htut, Win Myint, Lin Soe The effect of mefloquine-artemether compared with quinine on patients with complicated falciparum malaria. Trans R Soc Trop Med Hyg. 1988;82(5):665–666. doi: 10.1016/0035-9203(88)90187-3. [DOI] [PubMed] [Google Scholar]
- Vennerstrom J. L., Fu H. N., Ellis W. Y., Ager A. L., Jr, Wood J. K., Andersen S. L., Gerena L., Milhous W. K. Dispiro-1,2,4,5-tetraoxanes: a new class of antimalarial peroxides. J Med Chem. 1992 Aug 7;35(16):3023–3027. doi: 10.1021/jm00094a015. [DOI] [PubMed] [Google Scholar]
- White N. J., Waller D., Crawley J., Nosten F., Chapman D., Brewster D., Greenwood B. M. Comparison of artemether and chloroquine for severe malaria in Gambian children. Lancet. 1992 Feb 8;339(8789):317–321. doi: 10.1016/0140-6736(92)91644-n. [DOI] [PubMed] [Google Scholar]
- Woerdenbag H. J., Moskal T. A., Pras N., Malingré T. M., el-Feraly F. S., Kampinga H. H., Konings A. W. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod. 1993 Jun;56(6):849–856. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]
- Zhao K. C., Song Z. Y. [Pharmacokinetics of dihydroqinghaosu in human volunteers and comparison with qinghaosu]. Yao Xue Xue Bao. 1993;28(5):342–346. [PubMed] [Google Scholar]



