Abstract
Childhood obesity is associated with increased risk of behavioral/psychological disorders including depression, anxiety, poor learning, and attention deficient disorder. As the majority of women of child-bearing age are overweight or obese and consume a diet high in dietary fat, it is critical to examine the consequences of maternal overnutrition on the development of brain circuitry that regulates offspring behavior. Using a nonhuman primate model of diet-induced obesity, we found that maternal high-fat diet (HFD) consumption caused perturbations in the central serotonergic system of fetal offspring. In addition, female infants from HFD-fed mothers exhibited increased anxiety in response to threatening novel objects. These findings have important clinical implications as they demonstrate that exposure to maternal HFD consumption during gestation, independent of obesity, increases the risk of developing behavioral disorders such as anxiety.
Introduction
Childhood obesity is not only associated with increased risk of metabolic diseases, but also with a range of behavioral/psychological disorders, including depression (Rofey et al., 2009), anxiety (Rofey et al., 2009), poor learning (Cserjesi et al., 2007), and attention deficient disorder (Waring and Lapane, 2008). However, it is difficult to separate cause and effect with psychological diagnosis as there is evidence that the stigma associated with obesity increases the risk for behavioral disorders (Griffiths and Page, 2008). Furthermore, the relative contribution of early programming events caused by maternal obesity, diabetes and diet on behavioral and metabolic disorders in offspring remains unclear. Abnormalities in the serotonergic system are clearly linked to behavioral disorders, including anxiety and depression (Kiyohara and Yoshimasu, 2009), and this system is a regulator of energy homeostasis (Tecott, 2007) and is affected by energy status and diet (Hassanain and Levin, 2002). Moreover, serotonergic drugs are broadly used to treat numerous behavioral disorders.
Recently, our group used a nonhuman primate (NHP) model of diet-induced obesity to demonstrate that consumption of a high-fat diet (HFD) during pregnancy causes lipotoxicity within the developing fetus resulting in high levels of circulating inflammatory cytokines and evidence of fatty liver disease (McCurdy et al., 2009). HFD offspring displayed accelerated growth during nursing and had twice the body fat by 6 months of age. Considering that over 50% of women of child-bearing age are overweight or obese (King, 2006), and that the typical American diet is high in dietary fat, it is critical to examine the consequences on development of critical brain circuitry that regulates offspring behavior. The NHP is a uniquely qualified model as they have similar development to humans and exhibit complex social behavior. These studies used the NHP model of maternal overnutrition to examine the consequences of maternal obesity and HFD consumption on development of the serotonergic system in fetal offspring as well anxiety-like and aggressive behavior in infant offspring.
Materials and Methods
Animals.
All animal procedures were approved by the Oregon National Primate Center (ONPRC) Institutional Animal Care and Use Committee and conformed to National Institutes of Health guidelines on the ethical use of animals. A complete characterization of the maternal and fetal phenotype has been reported previously (McCurdy et al., 2009).
Adult females.
Briefly, weight- (7–9 kg) and age-matched (5–7 years of age) adult female Japanese macaques (Macaca fuscata) were fed a control (CTR) or HFD for up to 4 years. The CTR diet (Purina Mills) provided 13% of calories from fat, and the HFD (Test Diet; 5A1F; Purina Mills) provided 32% of calories from fat and was supplemented with calorically dense treats. The HFD represents a typical Western diet in regard to the saturated fat content. Monkeys were housed in indoor/outdoor pens in groups of ∼10–12 individuals (male/female ratio, 2/9–10). Monkeys had ad libitum access to food and water. During monthly health checks, animals were examined for pregnancy by palpation, and pregnancies were confirmed by ultrasound (allowing estimate of gestational age).
Fetal offspring.
Fetuses were collected by caesarean section on gestational day 130 (early third trimester; full term is 175 d) from CTR and HFD groups after 2–4 years on their respective diets. Fetal brains (CTR, n = 5; HFD, n = 6) were collected, perfused with 4% paraformaldehyde fixative, and blocked as described previously (Grayson et al., 2006).
Juvenile offspring.
Full-term offspring were maintained on their mothers' diets. Infants and mothers were left undisturbed for the first 30 d after birth. On postnatal day 30, the offspring were weighed and dual energy X-ray absorptiometry scanned to examine body composition. The mother and offspring were then left undisturbed until behavior testing on postnatal day 130. For the CTR group, 4 male and 4 female offspring were examined, and for the HFD group, 12 male and 11 female offspring were examined.
In situ hybridization. Fetal hypothalamic and midbrain blocks were sectioned at 35 μm using a freezing microtome and collected in 1:24 series. Sections were stored in ethylene glycol cryoprotectant at −20°C until use. For in situ hybridization (ISH), 1:8 series of sections were slide mounted in potassium PBS (KPBS), pH 7.4, and vacuum-desiccated overnight. cRNA probes were transcribed from cDNA clones (kindly provided by C. L. Bethea) (Pecins-Thompson et al., 1998) Tryptophan hydroxylase 2 (TPH2; 300 bp), serotonin transporter (SERT; 253 bp), and serotonin 1A receptor subtype (5-HT1AR; 431 bp), and transcribed in the presence of 100% P33-labeled UTP (PerkinElmer). Standard ISH methods were used (Grayson et al., 2006). For visualization, probe-labeled sections were exposed to film (Biomax MR; Kodak) for 2 d (SERT) or 5 d (TPH2, 5-HT1AR). Autoradiographic images were captured using a CoolSnap HQ camera (Photometrics) and MetaMorph software (Universal Imaging). Integrated morphometry analysis was used to measure total density by multiplying total area by optical density of the three levels of the midbrain: rostral (approximately bregma −17.78 ± 1 mm), medial (bregma −19.75 ± 1 mm), and caudal (bregma −23.40 ± 1 mm; reported in relative units). Three or four matched sections were analyzed for each level in each animal.
Fluorescent immunohistochemistry.
Two experiments were performed using standard fluorescent immunohistochemistry methods (Grayson et al., 2010): (1) serotonin (5-HT)-ir projections in sections containing the arcuate nucleus of the hypothalamus (ARH) to determine the terminal field density and (2) TPH2-ir in raphe-containing sections to determine the relative numbers of TPH2-containing neurons. Sections were washed in KPBS and blocked in 2% donkey serum in 0.4% Triton X-100/KPBS. Rabbit anti-5-HT (lot #057K4753, #S5545; 1:5000; Sigma-Aldrich) and rabbit anti-TPH2 (lot number #1068-102, #NB100-74555; 1:2500; Novus Biologicals) primary antibodies were used. The specificity of these antibodies has been validated by preabsorption studies (Sakowski et al., 2006; Dai et al., 2008). Primary antibodies were diluted with 2% donkey serum in 0.4% Triton X-100/KPBS and incubated with the tissue overnight at 4°C. After incubation, the tissue was rinsed in KPBS and incubated in FITC or rhodamine-labeled donkey anti-rabbit or mouse IgG (1:200; Jackson Immunoresearch) in 0.4% Triton X-100/KPBS for 1 h. Sections were wet mounted and coverslipped with glycerol-based mountant and stored at 4°C before analysis.
Image analysis.
Confocal laser microscopy, as described previously (Grove et al., 2000), was used to capture immunofluorescent images. Images were captured with a 25× oil objective (numerical aperture, 0.75). For fluorescence intensity measurements of 5-HT in the ARH, a series of optical planes at 0.5 μm intervals along the z-axis of the section were scanned for each fluorescent signal and stored as a stack of 1024 × 1024 pixel images, processed with MetaMorph and presented as maximum projections totaling 5 μm. Two fields of view per section (right and left) in three equally spaced, anatomically matched sections per animal were imaged and analyzed (CTR and HFD, n = 3). Total immunoreactive fluorescent intensity was measured using the same conditions and threshold parameters for all images. The total gray value was measured for each field. Analysis was performed by individuals blind with respect to group. The MetaMorph Imaging System was used to process the images, and brightness and contrast levels of the digital images were adjusted with Adobe Photoshop (Adobe Systems).
Cell counting.
Immunohistochemical images were captured under fluorescent illumination using a Photometrics CoolSnap HQ camera (Roper Scientific) connected to a Nikon microscope (E800) with a Plan Apo 4× objective. Total numbers of immunoreactive cells were counted in all raphe-containing sections.
Behavioral testing.
Behavior tests commonly used to assess anxious, fearful, and inhibited behavior in young children and NHPs were used (i.e., the human intruder and novel objects tests; file 2001) (Goldsmith and Rieser-Danner 1990; Kalin et al. 1991). On post-natal day 130, each infant and mother were removed from their home pen and placed in a cage located in an adjacent room at 900 h. Ten minutes before the test, the mother was sedated with ketamine HCl (5 mg/kg, i.m.; Fort Dodge Animal Health), and the mother and infant were transported to the behavioral testing suite in a covered transfer box. All behavior tests were initiated and completed between 900 and 1200 h. Upon arrival at the behavioral suite, the infant was hand caught and placed in a standard primate cage in a separate room from its mother. The infant's behavior throughout the tests was videotaped from an adjacent room through a one-way mirror.
Human intruder test.
This test reliably assesses individual differences in primate stress response and anxiety (Williamson et al., 2003) by assessing behavior in three stress-inducing conditions. This test began with a 10 min acclimation period, after which a human intruder (unfamiliar to the monkey) entered the testing room and stood 0.3 m from the cage with their facial profile (a nonthreatening social stimulus) to the monkey for 2 min (profile period). The intruder exited the room leaving the monkey alone for a 2 min control period. The human intruder then reentered the room and made continuous direct eye contact (a threatening social stimulus) with the monkey for 2 min (stare period) before exiting. Behaviors scored included vocalizations, exploration, movement, and response to the human intruder.
Novel objects test.
This test was designed to assess response to a variety of potentially threatening and nonthreatening novel objects. Novel object tests have been used to assess anxiety-like behavior in a variety of species (Belzung and Le Pape, 1994). Latency to examine novelty has been shown to be heritable and stable over time (Williamson et al., 2003), and has been pharmacologically validated (Belzung and Le Pape, 1994). Moreover, evidence in children indicates that decreased latency to explore novel objects or situations is associated with early-onset anxiety (Schwartz et al., 1999).
This test began 2 min after the end of the stare period. The infant was presented with a series of four novel objects. For each object, the human intruder entered the room taking care to avoid direct eye contact, quickly introduced the object, removed the previous object (except for the novel fruit, which was left in the cage), and immediately left the room (within 5 s). Each object was left with the infant for 5 min. The first object to be presented to the infants was a piece of novel fruit (kiwi), which was placed in the cage. After the kiwi, the human intruder then placed a potentially threatening novel object (a commercially available toy, Mr. Potato Head, selected because it has large eyes). Only the eyes and feet were attached to Mr. Potato Head's body to emphasize the eyes. The next object was a colorful hanging bird toy (a nonthreatening novel object), which was hung on the cage. Last, a realistic rubber snake (similar to natural Japanese macaque predators) and a piece of apple (a familiar food) were placed on the tray in such a way that the monkey had to reach across the snake to access the apple. This was done to examine the monkey's willingness to overcome a threatening stimulus (the snake) to get to a desirable object (apple). When the test was complete, the infant was hand caught and placed in the transfer cage with its mother, and both monkeys were returned to their home pen.
The videotapes were scored by an observer blind to maternal diet using the Observer XT software (Noldus Information Technology). The latency (seconds) to intentionally touch each item was scored, as well as behaviors such as movement, exploration, vocalizations, and other responses to the novel objects. We defined latencies to touch the novel object greater than two standard deviations (SDs) above the mean as an anxious response.
CSF assays.
CSF was collected from juvenile offspring at 13 months of age. Samples were snap frozen in liquid nitrogen and stored at −80°C until time of assay. 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) ELISAs were performed by the ONPRC Endocrine Services Core using commercially available kits (5-HT, #RE59121; 5-HIAA, #RE59131; IBL Transatlantic) in 50 μl of monkey CSF according to the manufacturer's specifications (CTR, n = 6; HFD, n = 11).
Statistical analysis.
For all analyses, normality and homogeneity of variance were initially tested. An independent t test was used to compare TPH2, 5-HT1AR, and SERT mRNA and SERT immunoreactivity in the rostral (rDR) and caudal dorsal raphe (cDR) and CSF 5-HT of CTR and HFD offspring. The latency to touch the novel objects and the number of vocalizations during the acclimation were nonparametric; thus, a Mann–Whitney U test was used to compare CTR and HFD offspring. A univariate ANOVA was used to examine the amount of time spent being active or sedentary. Data are presented as mean ± SEM. Alpha values are considered significant with p ≤ 0.05. Statistical analyses were conducted using the SPSS software package, version 16.0.
Results
The 5-HT system of fetal offspring
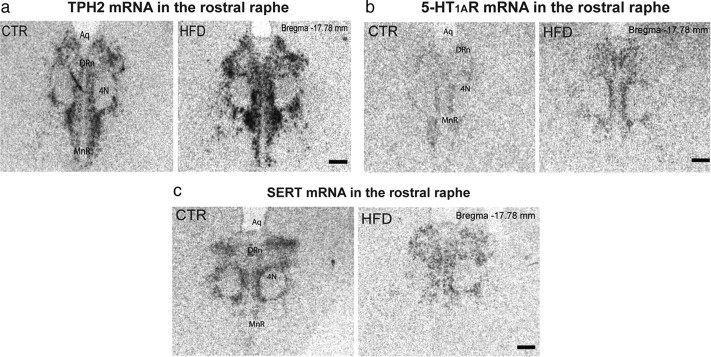
TPH2 (the rate-limiting enzyme for central 5-HT synthesis) mRNA expression was increased by more than twofold in the rDR region of fetal HFD offspring (CTR, 3.23 ± 1.06; HFD, 12.88 ± 3.69 relative units; t = −2.51; df = 14; p = 0.03) (Fig. 1a). However, TPH2 expression in the cDR was similar in HFD and CTR fetuses (CTR, 1.81 ± 0.39; HFD, 2.12 ± 0.62 relative units; t = −0.44; df = 14; p = 0.66). The increase in TPH2 mRNA was caused by increased expression per cell, as the number of TPH2-ir cell bodies was similar between groups (CTR, 287 ± 28.99; HFD, 270.67 ± 67.71 relative units; t = 0.22; df = 4; p = 0.84). Activity of the inhibitory 5-HT1A autoreceptor (5-HT1AR) is critical for controlling midbrain 5-HT production. Expression of the 5-HT1AR was upregulated in the rDR (CTR, 0.44 ± 0.29; HFD, 11.66 ± 4.36 relative units; t = −2.57; df = 10; p = 0.028) (Fig. 1b), but not in the cDR (CTR, 2.23 ± 1.18; HFD, 2.96 ± 1.35 relative units; t = −0.40; df = 8; p = 0.70) of HFD fetuses. Expression of SERT was unchanged in either the rDR (CTR, 3.73 ± 1.03; HFD, 3.03 ± 0.77 relative units; t = 0.55; df = 8; p = 0.56) (Fig. 1c) or cDR (CTR, 3.80 ± 0.90; HFD, 3.16 ± 0.84 relative units; t = 0.52; df = 9; p = 0.61) in HFD fetal offspring. Hypothalamic 5-HT immunoreactivity and SERT-ir were similar in fetuses from CTR and HFD-fed mothers (data not shown), indicating that serotonergic projections are unaffected by treatment. The disturbances in the expression of TPH2 and 5-HT1AR were independent of maternal obesity or metabolic abnormalities, as the differences were consistent among all HFD offspring, whether the mother was obese and insulin resistant, or lean and insulin sensitive (see McCurdy et al., 2009). Furthermore, changes were not sex dependent (data not shown).
Figure 1.
Maternal high-fat diet consumption causes perturbations in the serotonergic system of fetal offspring. a–c, HFD offspring displayed increased TPH2 mRNA in the rostral raphe (a; p = 0.03, n = 8), increased 5-HT1AR mRNA (b; p = 0.03, n = 6); and no difference in SERT mRNA (c; p = 0.60, n = 5) compared to CTR offspring. Aq, Aqueduct; 4N, trochlear nerve; MnR, median raphe nucleus. Scale bars, 1 mm.
Behavior of juvenile offspring
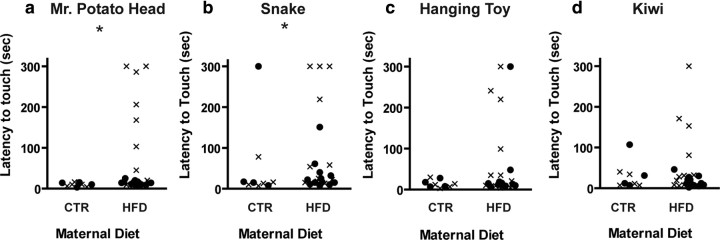
Female (p = 0.01) but not male HFD offspring (p = 0.36) exhibited increased latency to touch a potentially threatening novel object (Mr. Potato Head) compared to CTR offspring (Fig. 2a). Overall, 50% of female HFD offspring had a latency greater than 2 SD above the group mean (i.e., were anxious in response to this stimulus), whereas no CTR offspring or male HFD offspring exhibited increased latency. HFD female offspring also took longer to touch a rubber snake (a threatening object; p = 0.04) (Fig. 2b) compared to CTR female offspring. Although there was no significant difference in the latency of female HFD offspring to touch a nonthreatening novel object (the hanging bird toy; p = 0.16) (Fig. 2c), four female offspring from HFD mothers exhibited anxious behavior toward the toy, as opposed to none of the CTR offspring. Female HFD offspring exhibited no differences in latency to touch either a novel (kiwi; p = 0.28) (Fig. 2d) or familiar food item located near a potential threat (apple; p = 0.85; data not shown). Male HFD and CTR offspring did not differ in their latencies to inspect the rubber snake (p = 0.74), hanging toy (p = 0.65), or kiwi (p = 0.95) (Fig. 2b–d). Interestingly, the same animals exhibited anxious behavior across the novel object tests. Monkeys that showed increased latency to touch one novel object also showed increased latency to touch the other novel objects. For example, latency to touch Mr. Potato Head correlated with latency to touch the snake (rs = 0.69; p < 0.0001), hanging toy (rs = 0.51; p = 0.003), and kiwi (rs = 0.56; p = 0.001).
Figure 2.
Maternal high-fat diet consumption increased anxiety-like behavior in female offspring. a, b, Female offspring from HFD-fed mothers display increased latency to touch Mr. Potato Head (a; p = 0.01) and the rubber snake (b; p = 0.04) compared to female CTR offspring. c, d, There was no difference in the latency to touch the hanging bird toy (c; p = 0.16) or the kiwi fruit (d; p = 0.32) between female HFD and CTR offspring. Male HFD and CTR offspring had a similar response to Mr. Potato Head (a; p = 0.41), the rubber snake (b; p = 0.75), the hanging bird toy (c; p = 0.66), and the kiwi fruit (d; p = 0.95). Data are presented such that each point represents an animal (●, male offspring; ×, female offspring. *p < 0.05 when comparing female CTR and HFD offspring. For the CTR group, n = 4 male and 4 female offspring; for the HFD group, n = 12 male and 11 female offspring.
Male HFD offspring exhibited a nonsignificant increase in aggressive behavior toward the snake and unfamiliar human intruder compared to CTR offspring and female HFD offspring. During the stare period none, of the CTR offspring threatened the human intruder, whereas 42% of HFD male offspring threatened the intruder. Overall, 78% (18 of 23) of HFD offspring displayed some sort of anxious and/or aggressive behavior, whereas 11% (1 of 9) of CTR offspring displayed such behavior during the tests. In contrast, there was no difference in the amount of time spent being active or sedentary, or in the number of vocalizations (p = 0.11; data not shown) during the acclimation period between CTR and HFD offspring. Although we were unable to examine the brain 5-HT system at the time of the behavior tests, at 13 months of age, CSF 5-HT was decreased in HFD offspring (t = 2.67; df = 15; p = 0.02).
Discussion
As exposure to inflammatory cytokines and early stress are known to cause disturbances in development of the serotonergic system (Ishikawa et al., 2007), the 5-HT systems of fetuses were examined. Maternal HFD consumption resulted in an increase in gene expression of the rate-limiting enzyme for serotonin synthesis, TPH2, and an increase in the 5-HT1AR inhibitory autoreceptor expression in the rostral raphe, which is the primary source of hypothalamic 5-HT (Willoughby and Blessing, 1987). In contrast, the serotonergic system in the caudal raphe, which primarily projects to higher brain regions (Willoughby and Blessing, 1987), was similar in HFD and CTR fetuses. Maternal HFD consumption did not change SERT expression or 5-HT immunoreactivity in the hypothalamus, indicating that serotonergic projections were unaffected by treatment. Together, these data clearly indicate that maternal HFD consumption results in perturbations in the fetal serotonergic system.
The increased 5-HT1AR expression in the rDR in fetal offspring and decreased CSF 5-HT in the juvenile HFD offspring suggest that the serotonergic system is suppressed in offspring from HFD-consuming mothers. The increased expression of TPH2 is likely a compensatory response to the increased expression of 5-HT1ARs within the DR. An increased expression of TPH2 mRNA and protein in the DR has also been reported in depressed patients (Bach-Mizrachi et al., 2008; Lowry et al., 2008) who also have decreased CSF 5-HT (Mann and Malone, 1997). These studies also speculate that the increased TPH2 mRNA in the DR is a homeostatic response to deficient 5-HT neurotransmission. Alternatively, it is possible that the increase in TPH2 expression in the DR of fetal HFD offspring is a response to exposure to a stressful prenatal environment, as acute stress upregulates TPH2 expression in the DR (Shishkina et al., 2007), and that the prolonged exposure to stress during the postnatal period leads to death of 5-HT neurons and decreased serotonergic tone (Mizoguchi et al., 2008; Bambico et al., 2009).
Maternal HFD consumption resulted in behavioral changes in female offspring, with 55% displaying increased anxiety in the novel object tests. Male offspring from HFD mothers did not exhibit anxiety, but did exhibit increased aggression. Overall, the majority of HFD offspring (78%) displayed some sort of aberrant behavior (anxious and/or aggressive) during the test, whereas only 11% of CTR offspring displayed such behavior. As there were no difference in the amount of time spent being active or sedentary during the acclimation period, we hypothesize that the maternal HFD consumption had a specific effect on anxiety-like behavior. The observed increase in anxious and aggressive behavior is consistent with decreased serotonergic tone (Lesch et al., 1996). It is interesting that although both male and female offspring exhibit perturbations in the 5-HT system, only female offspring exhibit increased anxiety. This is consistent with evidence in humans that suggests that females are more prone to anxiety than males, and that the association between obesity and anxiety is stronger in women than men (Desai et al., 2009). As animal models have demonstrated that reduced 5-HT tone during early development also leads to impaired neuronal development (Khozhai and Otellin, 2006), learning deficits (Mazer et al., 1997), and enhanced stress response (McCormack et al., 2009), future studies need to examine the impact of maternal HFD consumption on neuronal development, stress response, and cognition.
Although additional work is needed to determine whether abnormalities in the central serotonergic system and behavior persist beyond weaning and into adulthood, these data have important clinical implications as they suggest that, in primates, exposure to maternal HFD consumption can increase the risk of early development of behavioral disorders such as anxiety. Moreover, as perturbation in the serotonergic system underlies a number of behavioral disorders, developmental changes in the central serotonergic circuitry could explain the comorbidity of obesity with anxiety (Rofey et al., 2009), depression (Rofey et al., 2009), and attention deficit hyperactivity disorder (Waring and Lapane, 2008).
Footnotes
This work was supported by U.S. National Institutes of Health Grants RO1DK079194, DK60685-S2, DK7919481, DK079194-S1, and R00163. We thank Greg Johnson for help with the behavioral tests and the Department of Animal Resources at the Oregon National Primate Center for their excellent care of the animals.
References
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49:675–678. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Dai JX, Hu ZL, Shi M, Guo C, Ding YQ. Postnatal ontogeny of the transcription factor Lmx1b in the mouse central nervous system. J Comp Neurol. 2008;509:341–355. doi: 10.1002/cne.21759. [DOI] [PubMed] [Google Scholar]
- Desai RA, Manley M, Desai MM, Potenza MN. Gender differences in the association between body mass index and psychopathology. CNS Spectr. 2009;14:372–383. doi: 10.1017/s1092852900023026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rieser-Danner L. Assessing early temperament. In: Reynolds CR, Kamphaus R, editors. Handbook of psychological and educational assessment of children. New York: Guilford; 1990. pp. 345–378. [Google Scholar]
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Grayson BE, LeVasseur P, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010 doi: 10.1210/en.2009-1019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths LJ, Page AS. The impact of weight-related victimization on peer relationships: the female adolescent perspective. Obesity (Silver Spring) 2008;16(Suppl 2):S39–S45. doi: 10.1038/oby.2008.449. [DOI] [PubMed] [Google Scholar]
- Grove KL, Campbell RE, Ffrench-Mullen JM, Cowley MA, Smith MS. Neuropeptide Y Y5 receptor protein in the cortical/limbic system and brainstem of the rat: expression on gamma-aminobutyric acid and corticotropin-releasing hormone neurons. Neuroscience. 2000;100:731–740. doi: 10.1016/s0306-4522(00)00308-0. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Levin BE. Dysregulation of hypothalamic serotonin turnover in diet-induced obese rats. Brain Res. 2002;929:175–180. doi: 10.1016/s0006-8993(01)03387-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Ishikawa A, Nakamura S. Interferon-alpha reduces the density of monoaminergic axons in the rat brain. Neuroreport. 2007;18:137–140. doi: 10.1097/WNR.0b013e328010231a. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Khozhai LI, Otellin VA. Formation of the neocortex in mice developing in conditions of prenatal serotonin deficiency. Neurosci Behav Physiol. 2006;36:513–517. doi: 10.1007/s11055-006-0048-2. [DOI] [PubMed] [Google Scholar]
- King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- Kiyohara C, Yoshimasu K. Molecular epidemiology of major depressive disorder. Environ Health Prev Med. 2009;14:71–87. doi: 10.1007/s12199-008-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol Biochem Behav. 2008;91:170–175. doi: 10.1016/j.pbb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res. 1998;53:120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Rofey DL, Kolko RP, Iosif AM, Silk JS, Bost JE, Feng W, Szigethy EM, Noll RB, Ryan ND, Dahl RE. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40:517–526. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Tecott LH. Serotonin and the orchestration of energy balance. Cell Metab. 2007;6:352–361. doi: 10.1016/j.cmet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122:e1–e6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Coleman K, Bacanu SA, Devlin BJ, Rogers J, Ryan ND, Cameron JL. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: a preliminary report. Biol Psychiatry. 2003;53:284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Blessing WW. Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Res. 1987;418:170–173. doi: 10.1016/0006-8993(87)90975-9. [DOI] [PubMed] [Google Scholar]