Abstract
Background & Aims
Although anti–tumor necrosis factor (TNF) therapy can effectively treat Crohn's disease (CD), there is concern that it might increase the risk of non-Hodgkin's lymphoma (NHL). A meta-analysis was performed to determine the rate of NHL in adult CD patients who have received anti-TNF therapy and to compare this rate with that of a population-based registry and a population of CD patients treated with immunomodulators.
Methods
MEDLINE, EMBASE, Cochrane Collaboration, and Web of Science were searched. Inclusion criteria included randomized controlled trials, cohort studies, or case series reporting on anti-TNF therapy in adult CD patients. Standardized incidence ratios (SIR) were calculated by comparing the pooled rate of NHL with the expected rate of NHL derived from the Surveillance Epidemiology & End Results (SEER) database and a meta-analysis of CD patients treated with immunomodulators.
Results
Twenty-six studies involving 8905 patients and 21,178 patient-years of follow-up were included. Among anti-TNF treated subjects, 13 cases of NHL were reported (6.1 per 10,000 patient-years). The majority of these patients had previous immuno-modulator exposure. Compared with the expected rate of NHL in the SEER database (1.9 per 10,000 patient-years), anti-TNF treated subjects had a significantly elevated risk (SIR, 3.23; 95% confidence interval, 1.5–6.9). When compared with the NHL rate in CD patients treated with immunomodulators alone (4 per 10,000 patient-years), the SIR was 1.7 (95% confidence interval, 0.5–7.1).
Conclusions
The use of anti-TNF agents with immunomodulators is associated with an increased risk of NHL in adult CD patients, but the absolute rate of these events remains low and should be weighed against the substantial benefits associated with treatment.
Crohn's disease (CD) is a chronic inflammatory bowel disease that affects approximately half a million people in the United States.1 Patients are most commonly diagnosed in young adulthood, but others might not develop symptoms until they are older. Symptoms range from mildly active disease with occasional diarrhea and rectal bleeding to severely active disease that might result in 10–20 bloody bowel movements per day, associated abdominal pain, and the possible need for surgery. Many patients are refractory to standard treatments and require the addition of anti–tumor necrosis factor (TNF) agents. Anti-TNF agents can be very effective for improving symptoms and inducing remission of CD2,3 and have shown promise in improving quality of life and decreasing the rates of hospitalizations and surgery.4,5 The currently available anti-TNF drugs for the treatment of CD include infliximab (IFX), adalimumab (ADA), and certolizumab pegol (CTZ).
Shortly after anti-TNF agents became widely available, concern was raised of a possible association with an increased risk of lymphoma, specifically non-Hodgkin's lymphoma (NHL).6 Studies aimed at quantifying potential risk among CD patients have arrived at estimates ranging from no increased risk (TREAT registry)7 to a 1.5% absolute annual risk of lymphoma.8 CD, in and of itself, does not appear to have an increased risk of lymphoma,9,10 but patients with CD treated with immunomodulators such as azathioprine and 6-mercaptopurine (6MP) might have a 4-fold increased risk.11 The magnitude of lymphoma risk added by the anti-TNF agents has been a matter of much debate.
For patients and physicians to make better informed treatment decisions regarding anti-TNF drugs, it is critical to understand the balance of risks and benefits. The current spectrum of risk estimates makes it very difficult to use the data in a meaningful way. This meta-analysis systematically evaluates the NHL rate among adult CD patients exposed to anti-TNF agents in a study setting. This rate is compared with the rate in externally derived controls including a population-based cancer registry and a pooled cohort of CD patients treated with immunomodulators without anti-TNF exposure.
Methods
Data Sources and Searches
A literature search was conducted by using the databases MEDLINE via Ovid (1950–October 2007), EMBASE (1974–2007), and Cochrane Reviews/CENTRAL (1990–2007), and meeting abstracts were searched via Web of Science (1996–2007). The search terms included “Crohn's” and related terms “Infliximab,” “Adalimumab,” “Certolizumab pegol,” and related pharmaceutical names. There were no limits used in our search strategy.
Additional search methods
Additional search methods included a manual review of reference lists of relevant articles and an electronic search of ClinicalTrials.gov. Inflammatory bowel disease clinical trialists and relevant pharmaceutical companies were contacted to determine whether additional unpublished safety data or updated results were available that would meet inclusion criteria.
Study Selection
Studies were included for analysis if they met the following inclusion criteria: study design of randomized controlled trials (RCTs), prospective or retrospective cohort studies, or case series of consecutive patients (to avoid selection bias); published articles or meeting abstracts; treatment included IFX, ADA, or CTZ; population of adult patients with CD; clearly reported adverse outcomes; and a minimum of a median follow-up of 48 weeks. There was no minimum study size, and both induction and maintenance studies could be included. Two reviewers (C.S., S.M., or S.P.) independently evaluated each of the articles for eligibility. Disagreement regarding eligibility was resolved by joint review and discussion between the authors.
Data Extraction
Data from all eligible studies were extracted by 2 independent reviewers (C.S., S.M., or S.P.) by using a standardized data abstraction form. This electronic data collection form (Excel; Microsoft, Redmond, WA) included study design, population size and median age, median time of follow-up, duration of disease, gender, specific anti-TNF agent, method of delivery and dosage, percent taking immunomodulators, dropout rate, and number of NHL cases. A second section of the data abstraction form included details of the patients who developed NHL. Discrepancies between the 2 reviewers were resolved by joint review and discussion between the authors. Corresponding authors were contacted to obtain any necessary missing data from the original publications.
Data Synthesis and Analysis
Pooled summary estimates
To calculate the total rate of NHL, we summed the number of lymphomas in all of the included studies and divided by the total number of patient-years. Patient-years were calculated by converting the follow-up time from weeks to years and multiplying by the total number of subjects.
The expected rate of NHL among subjects not exposed to anti-TNF agents was derived from 2 sources, the Surveillance Epidemiology & End Results (SEER) cancer registry12 and a meta-analysis of patients treated with 6MP or azathioprine (Kandiel et al11). The analysis by Kandiel et al included both CD and ulcerative colitis patients and reported both Hodgkin's lymphoma and NHL. Therefore, the numerator used to calculate the Kandiel rate was only NHL in CD patients, and denominator was patient-years of follow-up in CD patients treated with 6MP or azathioprine. Relative rates were calculated as standardized incidence ratios (SIR), first comparing the pooled NHL rate from anti-TNF studies with population-based NHL rates from SEER and then with the study by Kandiel et al by using the STATA “IR” command (STATA 10.0, College Station, TX).
To adjust for age and gender in the SEER comparisons, we had to develop age- and gender specific lymphoma rates from our data. Age categories were chosen to match those reported in SEER. To determine the exact age distribution of patients within included studies, investigators were contacted for patient level data. When these data were not available,13–25 we calculated an estimated distribution by deducing age structure on the basis of the available mean or median age and the given standard deviation (SD). If the SD was not provided, it was estimated by dividing the range by 4 or the interquartile range by 1.35.26 When neither the range nor interquartile range was provided for a study, it was imputed as the average SD from all studies.26 Assuming a normal distribution, medians were handled as mean age. Once mean age and SDs were ascertained for each study, with STATA 10.0, a random normal age and gender distribution was calculated. If the generated distribution did not include the entire range of study participants, the random distribution program was run until a representative group resulted. By using the actual or estimated age and gender distributions for each study, we calculated age-/gender-specific patient-year denominators and finally categorical NHL rates to make direct comparisons with SEER. SIRs were then calculated for each age/gender category. Because age and gender distribution was not available uniformly for the CD patients included in the meta-analysis by Kandiel et al,11 we performed a pooled analysis only and did not calculate age- and gender-specific comparisons to this patient population like we did with SEER.
Sensitivity and subgroup analyses
To address the concern that NHL rates might be underestimated if patients who drop out of studies are more likely to have or develop lymphoma, a sensitivity analysis was performed by removing studies that had a dropout rate >15%. Because different study designs might attract or enroll different types of subjects and likely have different intensity of treatment or follow-up, subgroup analyses were performed on the basis of the design of included studies.
Results
Description of Studies
Results of search
Our initial electronic search of MEDLINE identified 644 potentially relevant publications. After eligibility screening by abstract and title, 55 articles were obtained for more detailed review, of which 35 were excluded for reasons shown in Figure 1. A search of Web of Science identified 6 additional abstracts. If meeting abstracts were identified that included more recent and updated information than a previously published article, data from the meeting abstract replaced those of the full article.7,13,27 Ultimately, 26 studies met our inclusion criteria. A review of EMBASE, Cochrane Reviews/ CENTRAL, ClinicalTrials.gov, and contact with relevant pharmaceutical companies and experts in the field yielded no additional studies. Data from one study identified through our MEDLINE search were updated after experts indicated that new data were available.7
Figure 1.
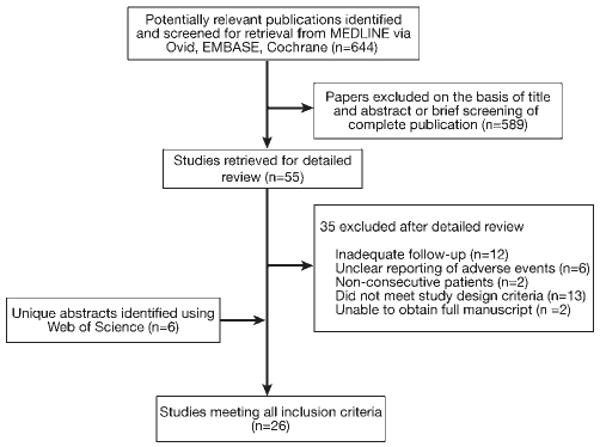
Flow diagram of the studies identified in search, and reasons for study exclusion.
Characteristics of included studies
Twenty-six studies involving 8905 patients were included in this review. As shown in Table 1, nine were RCTs,2,13,14,17,27–31 three were cohort studies,3,7,32 and 14 were case series of consecutive patients.8,20–25,34–40 Patients had a mean age of 36.9 years and a mean duration of CD of 9.3 years. Twenty-two of the included studies involved IFX, 3 involved ADA, and 1 involved CTZ. An average of 66% of participants across the studies were concomitantly taking immunomodulators. The mean duration of follow-up was 74 weeks, with a dropout rate ranging from 0%–33%.
Table 1.
Characteristics of Included Studies
| Study | Year | Publication type | N | CD duration (y)a | Anti-TNF agent | % on IMb | Median follow-up (wk) | Patient-Yearsc | % Drop out | Number of NHL |
|---|---|---|---|---|---|---|---|---|---|---|
| RCT | ||||||||||
| Colombel et al27 (P3/4) | 2007 | Abstract | 905 | 6 | CTZ | NR | 53 | 922.4 | NR | 0 |
| Colombel et al13 (OLE) | 2007 | Abstract | 1169 | NR | ADA | 46.6 | 58 | 1299 | 1 | 0 |
| Sandborn et al18 | 2007 | Full | 276 | 8.7 | ADA | 30.5 | 56 | 552 | 1.8 | 1 |
| Lemann et al15 | 2006 | Full | 57 | 4.8 | IFX | 100 | 52 | 57.0 | 3.5 | 0 |
| Schroder et al31 | 2006 | Full | 19 | 8.9 | IFX | 100 | 48 | 17.5 | 0 | 0 |
| Mantzaris et al14 | 2004 | Abstract | 45 | NR | IFX | 53 | 60 | 51.9 | 0.04 | 0 |
| Sands et al3 | 2004 | Full | 282 | 11.5 | IFX | 34.4 | 54 | 292.8 | 0 | 0 |
| Hanauer et al2 | 2002 | Full | 573 | 8.2 | IFX | 29 | 54 | 595.0 | 0 | 0d |
| Rutgeerts et al17 | 1999 | Full | 73 | 10.4 | IFX | 35 | 48 | 67.4 | 0 | 1 |
| Cohort | ||||||||||
| Lichtenstein et al7 | 2007 | Abstract | 3396 | 10.7 | IFX | 49 | 201 | 13,126 | 21 | 6 |
| Biancone et al32 | 2006 | Full | 404 | 9.5 | IFX | 53 | 109 | 846.8 | 0 | 0 |
| Doumit et al33 | 2004 | Abstract | 322 | 13.7 | IFX | 88.2 | 104 | 644 | 0 | 1 |
| Case series | ||||||||||
| Carbone et al21 | 2007 | Full | 34 | NR | IFX | 48 | 52 | 159.5 | 0 | 0 |
| Peyrin-Biroulet et al36 | 2007 | Full | 52 | 11 | ADA | 71 | 52 | 52.0 | 0 | 0 |
| Hyder et al23 | 2006 | Full | 22 | 6 | IFX | 95 | 91 | 38.5 | 0 | 0 |
| Pacault et al40 | 2006 | Abstract | 137 | NR | IFX | NR | 150 | 395.2 | NR | 0 |
| Talbot et al24 | 2005 | Full | 21 | 6 | IFX | 57 | 87 | 35.1 | 0 | 0 |
| Choi et al22 | 2005 | Full | 13 | 8.3 | IFX | 84.6 | 57 | 14.3 | 0 | 0 |
| Ardizzone et al20 | 2004 | Full | 20 | 9 | IFX | 23.2 | 67 | 25.8 | 33 | 0 |
| Colombel et al35 | 2004 | Full | 500 | 8 | IFX | 85 | 74 | 711.5 | 0 | 1 |
| Rodrigo et al37 | 2004 | Full | 44 | 7.6 | IFX | 89 | 72 | 60.9 | 0 | 0 |
| Schroder et al38 | 2004 | Full | 12 | 11.5 | IFX | 100 | 57.6 | 13.3 | 0 | 0 |
| Seiderer et al19 | 2004 | Full | 92 | 8.4 | IFX | 82 | 113 | 199.9 | 0 | 0 |
| Ljung et al16 | 2003 | Full | 191 | NR | IFX | 51 | 55 | 202.0 | 0 | 3 |
| Kinney et al25 | 2003 | Full | 117 | 13.3 | IFX | 48 | 52 | 117.0 | 9.6 | 0 |
| Cohen34 | 2000 | Full | 129 | 13.5 | IFX | 54 | 52 | 129.0 | 0 | 0 |
Abbreviations: IM, immunomodulator; N, total number exposed to anti-TNF; NR, not reported; P3/4, PRECISE 3/4; OLE, open label extension of GAIN and CHARM trials.
Disease duration based on best estimate from available data; mixed reporting of mean and median.
Patients taking concomitant IMs (azathioprine, 6MP, methotrexate) during study.
Calculated by converting follow-up time from weeks to years and multiplying by total N.
There was one NHL from Hanauer et al, but also reported in Ljung et al.
Rate of Lymphoma
There were a total of 13 lymphomas in 21,178 patient-years of observation, yielding a rate of 6.1 NHLs per 10,000 patient-years. When compared with the expected rate of NHL in all age groups combined in SEER (1.9 per 10,000 patient-years), as shown in Table 2, the SIR was 3.23 (95% confidence interval [CI], 1.5–6.9). Table 3 shows the age- and gender-specific NHL rate and SIR as compared with the expected NHL rate from SEER. In both this meta-analysis and SEER, male patients consistently have a higher rate of NHL. Although the crude NHL rate among anti-TNF exposed subjects increased with age, the age- and gender-specific SIR was only significant for male patients between the ages of 20 and 54, with an SIR of 5.4 (95% CI, 1.3–18.1). When compared with the observed rate of NHL in CD patients treated with immunomodulators alone from the study by Kandiel et al11 (3.6 per 10,000 patient-years), the SIR was 1.7 (95% CI, 0.5–7.1).
Table 2.
Rate of NHL for SEER, Immunomodulator, and Anti-TNF Treated Patients
| NHL rate per 10,000 pt-yrs | SIR | 95% CI | |
|---|---|---|---|
| SEER all ages | 1.9 | — | — |
| IM alonea | 3.6 | — | — |
| Anti-TNF vs SEER | 6.1 | 3.23 | 1.5–6.9 |
| Anti-TNF vs IM alone | 6.1 | 1.7 | 0.5–7.1 |
Abbreviation: IM, immunomodulator; pt-yrs, patient years.
IM alone is the rate of NHL in CD patients from the Kandiel meta-analysis.11
Table 3.
Age- and Gender-Specific NHL Rate and SIR
| Age | Pooled NHL rate per 10,000 pt-yrs | SEER NHL rate per 10,000 pt-yrs | SIR | 95% CI |
|---|---|---|---|---|
| 20–54 | ||||
| Male | 5.9 | 1.1 | 5.4 | 1.3–18.1 |
| Female | 3.1 | 0.8 | 3.8 | 0.7–15.9 |
| 55–64 | ||||
| Male | 23 | 4.3 | 5.4 | 0.6–20.5 |
| Female | 8.5 | 3.2 | 2.7 | 0.1–15.9 |
| 65–74 | ||||
| Male | 27 | 8.4 | 3.2 | 0.1–18.4 |
| Female | 20.9 | 6.3 | 3.3 | 0.1–19.1 |
| 75+ | ||||
| Male | 91.5 | 13.2 | 6.9 | 0.2–39.3 |
| Female | 0 | 9.26 | — | — |
There was significant heterogeneity of the rate of NHL across studies. Twenty of the included studies did not have any patients with NHL, whereas 6 reported at least 1 case. The highest rate was seen in the study by Ljung et al.16 Figure 2 displays the variation of the relative risk of NHL in individual studies compared with SEER.
Figure 2.
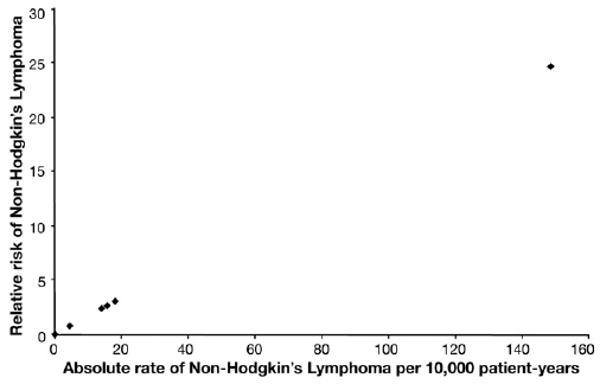
Heterogeneity among the studies. The relative risk of NHL (compared with SEER) is plotted against the absolute rate per 10,000 patient-years for each study. Twenty of the 26 studies did not have any reported cases of lymphoma. The outlier study with the highest rate is Ljung et al.16
Characteristics of Patients With Lymphoma
Table 4 shows the characteristics of the 13 patients with NHL. The mean patient age was 52 years (range, 24–79 years). Twelve of the patients with NHL were treated with IFX, and 1 was treated with ADA. Because of the heterogeneity of trial design (mixed group of anti-TNF naïve and maintenance patients), we are unable to confidently give a range of the dose and number of doses each of these patients received during their lifetime. The subtype of NHL is shown in Table 4. Patient outcomes were available for 12 of the patients with NHL, and at the time of most recent follow-up, 6 had died as a result of lymphoma or its related treatment.
Table 4.
Characteristics of Patients With NHL
| Age (y) | Gender | Agent | IM use | Type of NHL | Died & related to NHLa | |
|---|---|---|---|---|---|---|
| 1 | 70 | M | IFX | AZA | B cell | NR |
| 2 | 25 | M | IFX | AZA | NK cell | Yes |
| 3 | 79 | M | IFX | No | B cell | Yes |
| 4 | 24 | F | IFX | NR | B cell | No |
| 5 | 47 | M | IFX | MTX | NR | Yes |
| 6 | 61 | M | IFX | H/o IM use | B cell | Yes |
| 7 | 54 | M | IFX | AZA | Parotidb | No |
| 8 | 71 | F | IFX | NR | B cell | No |
| 9 | 55 | F | IFX | AZA | NR | No |
| 10 | 51 | F | IFX | AZA | NR | No |
| 11 | 42 | F | IFX | AZA | T-cell | Yes |
| 12 | 32 | M | IFX | 6MP | T-cellc | Yes |
| 13 | 61 | M | ADA | AZA | NR | No |
Abbreviations: AZA, azathioprine; H/o, history of; IM, immunomodulator; MTX, methotrexate; NK, natural killer; NR, not reported.
At last reported follow-up.
No other information available.
This T-cell lymphoma was a hepatosplenic T-cell lymphoma.
Sensitivity and Subgroup Analyses
The results of our sensitivity analysis excluding anti-TNF studies with a dropout rate greater than 15% are shown in Table 5. The sensitivity analysis is shown only for male patients, because the SIR for female patients did not meet significance in any age category. Excluding the 2 studies with a high dropout rate (Lichtenstein et al7 and Ardizzone et al20) increased the overall absolute risk of NHL to 9.4 per 10,000 patient-years, with an SIR of 9.4 (95% CI, 1.8–12.3). The NHL rate and corresponding SIRs again increased as patients got older, but the only age category in which the SIR reached statistical significance was in men ages 55–64 years.
Table 5.
Sensitivity Analysis Excluding Studies With Drop-Out >15%a
| Age (men) | Sensitivity analysis | NHL rate per 10,000b | SEER rate per 10,000b | SIR | 95% CI |
|---|---|---|---|---|---|
| All | All studies | 6.1 | 1.9 | 3.23 | 1.5–6.9 |
| >15% loss excluded | 9.4 | 9.4 | 1.8–12.3 | ||
| 20–54 | All studies | 5.9 | 1.1 | 5.4 | 1.3–18.1 |
| >15% loss excluded | 7.3 | 6.7 | 0.7–30.6 | ||
| 55–64 | All studies | 23 | 4.3 | 5.4 | 0.6–20.5 |
| >15% loss excluded | 72 | 16.8 | 2.0–64.4 | ||
| 65–74 | All studies | 27 | 8.4 | 3.2 | 0.1–18.4 |
| >15% loss excluded | 106 | 12.6 | 0.3–72.3 | ||
| 75+ | All studies | 91.5 | 13.2 | 6.9 | 0.2–39.3 |
| >15% loss excluded | 370 | 28 | 0.7–159.1 |
Excluding Lichtenstein 20077 and Ardizzone 2004
per 10,000 patient-years.
Subgroup analyses to evaluate the NHL rates in anti-TNF treated patients across different study designs are shown in Table 6. In 9 randomized control trials, there were 2 lymphomas observed in 3860 patient-years, yielding an incidence rate of 5.2 per 10,000 patient-years. In the 3 cohort studies including 15,192 patient-years, there were 7 lymphomas, resulting in an incidence rate of 4.6 per 10,000 patient-years. Finally, in the 14 case series, 4 lymphomas were observed in 2215 patient-years, yielding an incidence rate of 18.8 per 10,000 patient-years. Only the case series remained statistically significant, with an SIR of 9.4 (95% CI, 1.35–104.0).
Table 6.
Subgroup Analysis by Study Design
| Design | Lymphomas | Patient-Years | NHL rate per 10,000 pt-years | SIR* | 95% CI |
|---|---|---|---|---|---|
| RCT | 2 | 3860 | 5.2 | 2.6 | 0.19–35.7 |
| Cohort | 7 | 15,192 | 4.6 | 2.3 | 0.44–22.7 |
| Case series | 4 | 2125 | 18.8 | 9.4 | 1.35–104.0 |
SIR compared with SEER.
Discussion
Anti-TNF drugs for the treatment of CD appear to be associated with an increased risk of NHL. Although the increased risk is statistically significant when compared with the general population, the absolute risk remains small (6.1 per 10,000 patient-years). When compared with CD patients taking immunomodulators alone, there is a nonstatistically significant increased rate of NHL for those exposed to anti-TNF agents.
The baseline risk of NHL increases with age and is male-predominant.41 Therefore, when communicating the NHL risk to patients, conversations should be tailored for individuals. Although the SEER age categories are still fairly broad, it is possible to discuss more specific observed and expected rates for patients, depending on their age and gender, as opposed to our overall summary estimate.
We excluded the 2 studies with a greater than 15% dropout rate for the sensitivity analysis because we were concerned that these studies might not accurately represent the risk of lymphoma in their patient populations. Despite the fact that 6 of the observed lymphomas were then excluded, the disproportionate contribution of patient-years from the TREAT registry7 dramatically decreased the denominator, thereby increasing the NHL rate across all groups. Although statistical significance was only reached for one age category (men ages 55–64 had an SIR of 16.8), there is a dramatic increase in the absolute rate and SIR as patients get older. Because of infrequent anti-TNF use in older patients in the included studies, our analysis might be underpowered to detect a statistically significant difference in those older than 65 years.
Subgroup analyses were performed to determine whether the risk of lymphoma changed across different study designs. Patients in RCTs probably do not characterize the average patient receiving anti-TNF agents (ie, patients in early clinical trials might be sicker than those who received the medication after Food and Drug Administration approval), but they might correspond to a group at a higher risk of disease and treatment-related complications. On the other hand, clinical trials oftentimes exclude those with significant comorbidities, so it is unclear which direction the bias might favor. The case series might be more representative of patients treated with anti-TNF agents in clinical practice and embody a broad range of patients worldwide who have been treated in more diverse treatment settings. This subgroup had the highest rate of lymphoma, 18.8 per 10000 patient-years, which is nearly 2 per 1000 or 1 per 500 patient-years. This high rate was mostly driven by the study by Ljung et al,16 which had 3 cases in only 202 patient-years. This study is the outlier in Figure 2 contributing significantly to the heterogeneity among the included studies. Although the SIR for the case series is statistically significant, it is with wide CIs based on the relatively small sample.
These results contribute to the growing body of knowledge about the risk of NHL in patients with CD, but some confounding might be present. The disease itself does not appear to carry an increased risk of lymphoma,9,10 although this is controversial.42 It is important to note that almost all of the NHL patients had current or prior exposure to 6MP or azathioprine. Therefore, our rates of NHL are really reported rates of exposure to a combination of anti-TNF and immunomodulator therapy. Although numerically higher, this combination rate is not statistically higher than the Kandiel immunomodulator rate. This begs the question whether the major contributors to the increased risk are the immunomodulators or anti-TNF drugs. Patients in the study by Kandiel et al11 overall had a longer period of follow-up than those included in the 26 studies for this analysis. Although we do not know whether NHL risk accumulates over time, it is possible that our estimate for immunomodulator alone is an overestimate of what the 1-year incidence would be. However, a recent large French analysis of the lymphoma rate with immunomodulator exposure (but very little anti-TNF exposure) corroborates the possibility that immunomodulators are significantly contributing to the risk.43 On the basis of our analysis we cannot comment on the NHL risk associated with anti-TNF monotherapy. There simply have not been enough patients treated with anti-TNFs without immunomodulators to make any meaningful conclusions. Another possibility for confounding is radiation exposure. CD patients are potentially exposed to harmful levels of diagnostic radiation from repeated radiographic imaging,44 which might be associated with an increased risk of lymphoma.45 We were not able to ascertain the amount of radiation exposure in this analysis.
The most important limitation to this study is the lack of an ideal comparison group. Because the most common study design for anti-TNF agents includes an initial induction phase in which all patients receive active treatment, the control arms in the RCTs are not true placebo groups. A recent analysis compared adverse events between active and control arms in these anti-TNF RCTs and did not find a difference in lymphoma rates,46 but because of the above reason we do not believe that this was an adequate comparison to anti-TNF unexposed patients. The case series did not have controls by design, and although the cohort studies did have matched controls, the selective nature of patient enrollment is concerning. Although suboptimal, we chose SEER as a comparator on the basis of the knowledge that the largest study of lymphoma risk in CD patients did not show an increased baseline risk when compared with the general population.10 Because SEER is a US database and the included studies are international, we explored the variation of NHL rates worldwide. Although there is significant variation in the incidence of Hodgkin's lymphoma, the rate of NHL across continents is similar.47 Therefore, we believe that SEER is a reasonable representation for comparison.
The time horizon of 1 year was used to give enough time for adverse outcomes to develop, but we do not know whether the lymphoma risk will continue (or accumulate) as time progresses. Dosing data are incomplete, and the question of how much exposure is needed to increase the risk of lymphoma remains unanswered, but it is concerning that at least 4 of the patients had received only 1 infusion of an anti-TNF agent. This might mean that even 1 dose is enough to increase the lymphoma risk, or alternatively, it might raise questions about the biologic plausibility of these cases being attributed to a single episode of exposure.
We did not calculate quality scores for the included articles. Because our primary outcome was an objective event (eg, lymphoma), we did not believe that classic measures of quality such as blinded outcome assessment would have an impact on reporting. In addition, because we did not use the comparison groups from the studies, details of the randomization process seemed less relevant. Concerns regarding attrition were investigated by the sensitivity analysis, and although sampling bias related concerns regarding generalizability remain, the inclusion of multiple study designs might attenuate these problems. A formal analysis of publication bias was not performed because we believed that plotting a “treatment effect” against sample size was not practically relevant to our analysis of rare adverse events. However, we did have a range of included studies, with the smallest study having 13.3 patient-years and the largest having 10,796 patient-years. The smallest study that identified a case of lymphoma included 67.4 patient-years.
Patients, parents of patients, and physicians are concerned about the risk of lymphoma and have different thresholds for how much risk they are willing to accept.48–50 These results will help us understand the risk of NHL associated with anti-TNF and immunomodulator therapy and facilitate decisions about when it is most appropriate to use these agents. Although these estimates are a helpful step in determining treatment risks, further prospective data are necessary for a more accurate assessment. Large inception cohorts of patients with CD are being developed, and it will be critical to build monitoring of drug side effects into these programs.
Acknowledgments
The authors thank Dr H. Gilbert Welch and Thomas Mead for statistical and search support (Dartmouth Institute for Health Policy and Clinical Practice) and the pharmaceutical companies (Abbott, Centocor, and UCB) and individual authors (L. Biancone, R. Cohen, J. Doumit, S. Hanauer, M. Lemann, V. Pacault, C. Petruzziello, L. Peyrin-Biroulet, L. Rodrigo, B. Sandborn, B. Sands, and O. Schroeder) who supplemented the existing published data to provide further details of their patients.
Funding: Dr Siegel is supported by a CCFA career development award and by grant number K23DK078678 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and dose not necessarily represent the official views of the National Institute of Diabetes And Digestive and Kidney Diseases or the National Institute of Health.
Abbreviations used in this paper
- ADA
adalimumab
- CD
Crohn's disease
- CI
confidence interval
- CTZ
certolizumab pegol
- IFX
infliximab
- 6MP
6-mercaptopurine
- NHL
non-Hodgkin's lymphoma
- RCT
randomized controlled trial
- SD
standard deviation
- SEER
Surveillance, Epidemiology & End Results
- SIR
standardized incidence ratio
- TNF
tumor necrosis factor
Footnotes
Podcast interview: www.gastro.org/cghpodcast; see related article, Herrington LS et al, on page 502 in Gastroenterology.
Conflicts of interest: The authors disclose the following: Dr Siegel has served as a consultant or on a scientific advisory board for Abbott, Elan, and UCB; has received honoraria for speaking from Abbott, P&G, and UCB; and has received grant support from P&G. Dr Sands has served as a consultant or on a scientific advisory board for Abbott, Biogen/IDEC, Bristol-Myers Squibb, Centocor, Elan, Millenium Pharmaceuticals, Novartis Pharmaceuticals, Otsuka America Pharmaceuticals Inc, and UCB; has received honoraria for speaking from Abbott, Schering-Plough, and UCB; and has received grant support from Abbott, Bristol-Myers Squibb, Centocor, Elan, Millenium Pharmaceutical, Novartis Pharmaceuticals and Otsuka America Pharmaceutical Inc. The remaining authors disclose no conflicts.
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128:862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein GR, Cohen RD, Feagan BG, et al. Safety of infliximab and other Crohn's disease therapies—Treat (TM) registry data with nearly 20,000 patient-years of follow-up. Gastroenterology. 2007;132:A178. [Google Scholar]
- 8.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853. doi: 10.1136/gut.2003.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus EV, Jr, Tremaine WJ, Habermann TM, et al. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308–2312. doi: 10.1111/j.1572-0241.2000.02316.x. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JD, Bilker WB, Brensinger C, et al. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080–1087. doi: 10.1053/gast.2001.28703. [DOI] [PubMed] [Google Scholar]
- 11.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SEER. Surveillance, Epidemiology, and End Results Database. [November 6, 2007]; Available at: http://seer.cancer.gov/
- 13.Colombel JF, Rutgeerts P, Sandborn WJ, et al. Adalimumab safety in Crohn's disease patients: open-label maintenance following the GAIN and CHARM trials. Am J Gastroenterol. 2007;102:S496–S497. [Google Scholar]
- 14.Mantzaris GJ, Ployzou P, Karagiannidis A, et al. A prospective, randomized trial of infliximab (IFX) and azathioprine (AZA) for the induction and maintenance of remission of steroid-dependent Crohn's disease (CD) Gastroenterology. 2004;126:A54. [Google Scholar]
- 15.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–1061. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853. doi: 10.1136/gut.2003.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 18.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiderer J, Goke B, Ochsenkuhn T. Safety aspects of infliximab in inflammatory bowel disease patients: a retrospective cohort study in 100 patients of a German University Hospital. Digestion. 2004;70:3–9. doi: 10.1159/000080075. [DOI] [PubMed] [Google Scholar]
- 20.Ardizzone S, Maconi G, Colombo E, et al. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10:91–96. doi: 10.1097/00054725-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Carbone J, Gonzalez-Lara V, Sarmiento E, et al. Humoral and cellular monitoring to predict the development of infection in Crohn's disease patients beginning treatment with infliximab. Ann N Y Acad Sci. 2007;1107:346–355. doi: 10.1196/annals.1381.036. [DOI] [PubMed] [Google Scholar]
- 22.Choi KD, Song HJ, Kim JS, et al. Efficacy and safety of treatment with infliximab in Crohn's disease—the experience of single center in Korea. Korean Journal of Gastroenterology/Taehan Sohwagi Hakhoe Chi. 2005;46:48–55. [PubMed] [Google Scholar]
- 23.Hyder SA, Travis SP, Jewell DP, et al. Fistulating anal Crohn's disease: results of combined surgical and infliximab treatment. Dis Colon Rectum. 2006;49:1837–1841. doi: 10.1007/s10350-006-0656-5. [DOI] [PubMed] [Google Scholar]
- 24.Talbot C, Sagar PM, Johnston MJ, et al. Infliximab in the surgical management of complex fistulating anal Crohn's disease. Colorectal Disease. 2005;7:164–168. doi: 10.1111/j.1463-1318.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 25.Kinney T, Rawlins M, Kozarek R, et al. Immunomodulators and “on demand” therapy with infliximab in Crohn's disease: clinical experience with 400 infusions. Am J Gastroenterol. 2003;98:608–612. doi: 10.1111/j.1572-0241.2003.07286.x. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane handbook for systematic reviews of interventions. [June 25, 2008]; Available at: http://www.cochrane-handbook.org/
- 27.Colombel JF, Schreiber S, Hanauer SB, et al. Long-term tolerability of subcutaneous certolizumab pegol in active Crohn's disease: results from precise 3 and 4. Gastroenterology. 2007;132:A503. [Google Scholar]
- 28.Sands BE, Blank MA, Patel K, et al. Long-term treatment of rectovaginal fistulas in Crohn's disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004;2:912–920. doi: 10.1016/s1542-3565(04)00414-8. [DOI] [PubMed] [Google Scholar]
- 29.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–1061. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder O, Blumenstein I, Stein J. Combining infliximab with methotrexate for the induction and maintenance of remission in refractory Crohn's disease: a controlled pilot study. Eur J Gastroenterol Hepatol. 2006;18:11–16. doi: 10.1097/00042737-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Biancone L, Orlando A, Kohn A, et al. Infliximab and newly diagnosed neoplasia in Crohn's disease: a multicentre matched pair study. Gut. 2006;55:228–233. doi: 10.1136/gut.2005.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doumit J, Brzezinski A, Lashner B, et al. Comparison of safety and mortality of infliximab therapy to immunomodulator therapy in Crohn's disease: a cohort study. Am J Gastroenterol. 2005;100:S306. [Google Scholar]
- 34.Cohen RD. Efficacy and safety of repeated infliximab infusions for Crohn's disease: 1-year clinical experience. Inflamm Bowel Dis. 2001;7(Suppl 1):S17–S22. doi: 10.1002/ibd.3780070505. [DOI] [PubMed] [Google Scholar]
- 35.Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Peyrin-Biroulet L, Laclotte C, Bigard MA. Adalimumab maintenance therapy for Crohn's disease with intolerance or lost response to infliximab: an open-label study. Aliment Pharmacol Ther. 2007;25:675–680. doi: 10.1111/j.1365-2036.2007.03254.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigo L, Perez-Pariente JM, Fuentes D, et al. Retreatment and maintenance therapy with infliximab in fistulizing Crohn's disease [erratum appears in Rev Esp Enferm Dig 2004;96: 737] Revista Espanola de Enfermedades Digestivas. 2004;96:548–558. doi: 10.4321/s1130-01082004000800004. [DOI] [PubMed] [Google Scholar]
- 38.Schroder O, Blumenstein I, Schulte-Bockholt A, et al. Combining infliximab and methotrexate in fistulizing Crohn's disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther. 2004;19:295–301. doi: 10.1111/j.1365-2036.2004.01850.x. [DOI] [PubMed] [Google Scholar]
- 39.Seiderer J, Goke B, Ochsenkuhn T. Safety aspects of infliximab in inflammatory bowel disease patients: a retrospective cohort study in 100 patients of a German University Hospital. Digestion. 2004;70:3–9. doi: 10.1159/000080075. [DOI] [PubMed] [Google Scholar]
- 40.Pacault V, Hriz FB, Gornet JM, et al. Long term follow up of Crohn's disease patients treated with infliximab using an episodic strategy. Gastroenterology. 2006;130:A655. [Google Scholar]
- 41.Peloquin JM, Pardi DS, Sandborn WJ, et al. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:2015–2022. doi: 10.1111/j.1572-0241.2008.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Beaugerie L, Carrat F, Bouvier AM, et al. Excess risk of lymphoproliferative disorders (LPD) in inflammatory bowel diseases (IBD): interim results of the CESAME cohort. Gastroenterology. 2008;134:A-116. [Google Scholar]
- 44.Desmond AN, O'Regan KN, Curran C, et al. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gastroenterology. 2008;134:A-20. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 45.Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 46.Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Katanoda K, Yako-Suketomo H. Comparison of time trends in Hodgkin and non-Hodgkin lymphoma incidence (1973-97) in East Asia, Europe and USA, from cancer incidence in five continents. Vol IV-VIII. Jpn J Clin Oncol. 2008;38:391–393. doi: 10.1093/jjco/hyn037. [DOI] [PubMed] [Google Scholar]
- 48.Johnson FR, Ozdemir S, Mansfield C, et al. Crohn's disease patients' risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133:769–779. doi: 10.1053/j.gastro.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 49.Sands BE, Siegel CA, Johnson FR, et al. Gastroenterologists' tolerance for Crohn's disease treatment risks. Presented at the United European Gastroenterology Week; Paris, France. October 29, 2007. [Google Scholar]
- 50.Johnson FR, Ozdemir S, Mansfield C, et al. Are adult patients more tolerant of treatment risks than parents of juvenile patients? Risk Anal. 2009;29:121–136. doi: 10.1111/j.1539-6924.2008.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


