Abstract
Consumption of green tea is associated with a decrease in cardiovascular mortality. The beneficial health effects of green tea are attributed in part to polyphenols, organic compounds found in tea that lower blood pressure, reduce body fat, decrease LDL cholesterol, and inhibit inflammation. We hypothesized that epigallocatechin gallate (EGCG), the most abundant polyphenol in tea, inhibits endothelial exocytosis, the initial step in leukocyte trafficking and vascular inflammation. To test this hypothesis, we treated human umbilical-vein endothelial cells with EGCG and other polyphenols, and then measured endothelial exocytosis. We found that EGCG decreases endothelial exocytosis in a concentration-dependent manner, with the effects most prominent after 4 h of treatment. Other catechin polyphenols had no effect on endothelial cells. By inhibiting endothelial exocytosis, EGCG decreases leukocyte adherence to endothelial cells. In searching for the mechanism by which EGCG affects endothelial cells, we found that EGCG increases Akt phosphorylation, eNOS phosphorylation, and nitric oxide (NO) production. NOS inhibition revealed that NO mediates the anti-inflammatory effects of EGCG. Our data suggest that polyphenols can decrease vascular inflammation by increasing the synthesis of NO, which blocks endothelial exocytosis.
Keywords: flavonoid, granule, green tea, nitric oxide, vesicle trafficking, von Willebrand Factor (VWF)
Introduction
Consumption of green tea is associated with a decrease in cardiovascular mortality (Kuriyama et al., 2006). Green tea has a variety of anti-atherogenic effects: it is reported to lower blood pressure, reduce body fat, decrease LDL cholesterol, improve glucose tolerance, and decrease oxidant stress (Freese et al., 1999; Sung et al., 2000; Klotz and Sies, 2003; Nagao et al., 2005; Yang et al., 2004). In addition, green tea decreases inflammation, which plays an important role in atherogenesis.
One anti-inflammatory component of green tea is the polyphenol epigallocatechin gallate (EGCG). Polyphenols are a group of compounds including tannins, lignins, and flavonoids that are synthesized by plants; polyphenols are found in foods and beverages such as green tea, wine, olive oil, and chocolate. The major flavonoids in green tea are the catechins: catechin, epicatechin, epicatechin gallate, and EGCG.
EGCG contributes to the anti-inflammatory effects of green tea. It reduces inflammation in a variety of animal models, including endotoxemia (Yang et al., 1998), asthma (Bani et al., 2006; Kim et al., 2006), autoimmune encephalitis (Aktas et al., 2004), cystitis (Ozcan et al., 2005), and myocardial reperfusion (Aneja et al., 2004). EGCG even limits infiltration of leukocytes into the skin of humans exposed to UV light (Katiyar et al., 1999; Afaq et al., 2003). EGCG decreases inflammation in part by inhibiting nuclear factor-κB (NF-κB) activation (Lin and Lin, 1997; Singh et al., 2002). EGCG decreases NF-κB regulated expression of TNF-α, iNOS, and COX-2 following lipopolysaccharide treatment of mice or isolated macrophages (Lin and Lin, 1997). Another pro-inflammatory pathway inhibited by EGCG is the mitogen-activated protein kinase (MAPK) cascade. EGCG limits MAPK activation following interleukin-1β treatment (Barthelman et al., 1998; Chung et al., 2001). EGCG also inhibits STAT-1 activation (Townsend et al., 2004). However, EGCG may limit inflammation through additional pathways not yet identified.
The first step in vascular inflammation is endothelial activation. Agonists released by injured tissue activate endothelial cells to release granules called Weibel-Palade bodies (Lowenstein et al., 2005; Lowenstein and Tsuda, 2006). Exocytosis of these granules externalizes P-selectin into the vessel lumen, where it interacts with leukocyte P-selectin glycoprotein ligand-1 (PSGL-1), mediating leukocyte rolling along the vessel wall. Further inflammation activates endothelial cells and leukocytes to express intercellular adhesion molecules and their integrin receptors, leading to leukocyte adhesion to the vessel wall. We hypothesized that EGCG inhibits leukocyte adherence to endothelial cells in part by blocking endothelial exocytosis.
Results
EGCG inhibits endothelial exocytosis of Weibel-Palade bodies
We hypothesized that EGCG, a major polyphenol component of green tea, has anti-inflammatory effects by inhibiting Weibel-Palade body exocytosis from endothelial cells. To examine the effect of EGCG upon endothelial cell exocytosis, we pretreated human umbilical-vein epithelial cells (HUVECs) with increasing concentrations of EGCG for 2 h, then stimulated the cells with 1 U/ml thrombin for 45 min and measured the amount of von Willebrand factor (VWF) released in the media. Thrombin triggered VWF release from endothelial cells, and EGCG inhibited this release in a concentration-dependent manner, with an EC50 value well within physiological levels (Figure 1A). The inhibitory effect of EGCG was evident within 30 min of pretreatment, and increased slightly in a time-dependent manner (Figure 1B). To confirm that EGCG inhibits exocytosis, we also measured the effect of EGCG on P-selectin externalization. Thrombin increased P-selectin translocation on endothelial cells, and EGCG blocked P-selectin translocation (Figure 1C). EGCG treatment for 24 h had no effect on endothelial viability (Figure 1D). Serum minimally influenced the effects of EGCG (Figure 1E).
Figure 1.
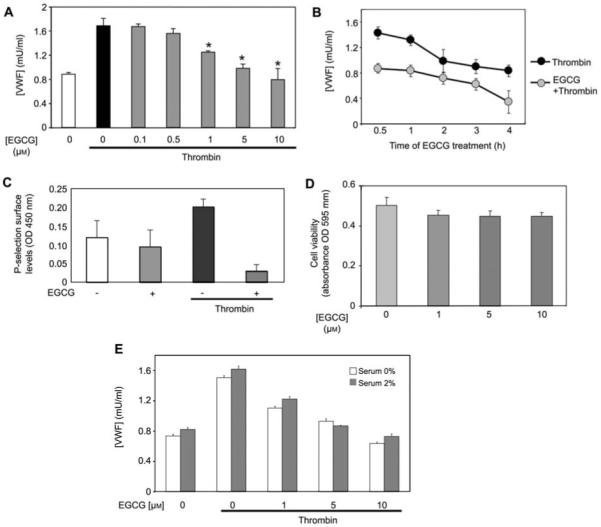
EGCG decreases endothelial exocytosis.
(A) Concentration-response plot. HUVECs were pretreated with 0–10 μm EGCG for 1 h, and then stimulated with 1 U/ml thrombin for 45 min. Exocytosis was measured by analyzing the media for VWF with an ELISA (n=3, mean±SD). (B) Time course plot. HUVECs were pretreated with EGCG for 0–4 h, and then stimulated with 1 U/ml thrombin for 45 min. Exocytosis was measured as above (n=3, mean±SD). (C) EGCG decreases P-selectin translocation. HUVECs were pretreated with EGCG for 2 h, and then stimulated with thrombin for 15 min. The cells were fixed in PFA and surface P-selectin was measured by incubation with an antibody to P-selectin and then a HRP-conjugated secondary antibody (n=4, mean±SD; *p<0.05). (D) Cell viability assay. HUVECs were pretreated with EGCG for 4 h, and then change to complete media and incubated for 24 h. The absorbance at 570 nm was measured using an MTT assay (n=3, mean±SD). (E) Serum does not affect EGCG regulation of exocytosis. HUVECs were pretreated with EGCG for 4 h in the presence or absence of serum and then treated with thrombin for 1 h; VWF was measured as above (n=3, mean±SD).
Other polyphenols do not inhibit endothelial exocytosis
Green tea polyphenols include four types of catechins, EC, ECG, EGC and EGCG. We tested which green tea polyphenols inhibit endothelial exocytosis. We pretreated HUVECs with each catechin at varying concentrations and measured VWF release. Among these polyphenols, only EGCG inhibited endothelial cells exocytosis at concentrations <10 μm (Figure 2).
Figure 2.
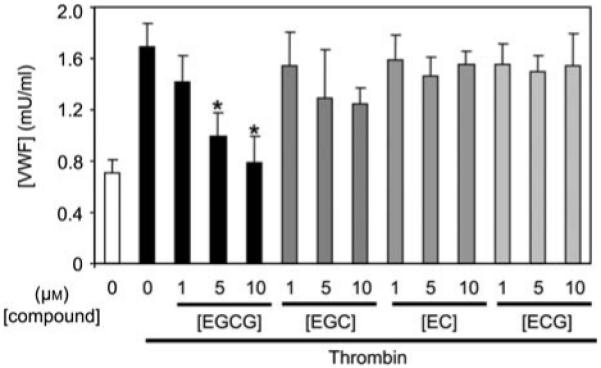
EGCG inhibition of exocytosis is greater than that of other catechins.
HUVECs were pretreated with catechins for 1 h and then stimulated with 1 U/ml thrombin for 45 min. Exocytosis was measured as described for Figure 1 (n=3, mean±SD; *p<0.01 vs. control).
EGCG decreases thrombin-induced leukocyte adherence to endothelial cells
To obtain functional evidence that EGCG inhibits the activation of endothelial exocytosis, we performed an ex vivo assay for leukocyte adherence. We treated HUVECs with EGCG, stimulated the cells with thrombin, added fluorescently labeled THP-1 leukocytes, washed the cells, and measured the number of adherent leukocytes. Thrombin increased leukocyte adherence to endothelial cells within 30 min. However, pretreatment with EGCG decreased thrombin-induced leukocyte adherence (Figure 3). Pretreatment with an antibody to P-selectin also decreased leukocyte adherence, suggesting that P-selectin mediates the interaction between leukocytes and endothelial cells (Figure 3). Translocation of P-selectin to the cell surface is a hallmark of endothelial granule exocytosis. Taken together, these data suggest that EGCG inhibits leukocyte adhesion to endothelial cells, a process mediated by endothelial exocytosis and P-selectin expression.
Figure 3.
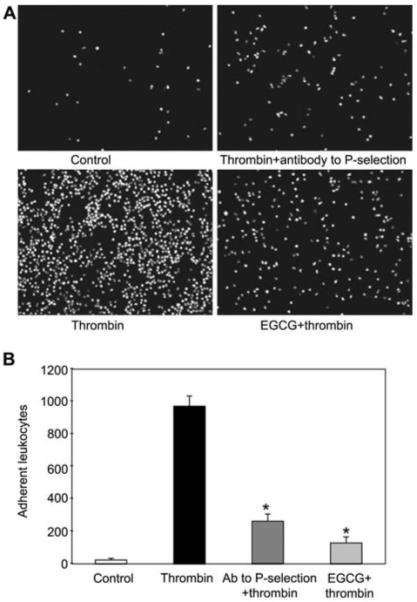
EGCG inhibits leukocyte adhesion to endothelial cells.
(A) Photomicrographs of adherent leukocytes. HUVECs were pretreated with EGCG for 1 h and with thrombin for 30 min, and then incubated with BCECF-AM labeled THP-1 leukocytes for 1 h. The cells were washed and adherent leukocytes were photographed. (B) Quantification of the effect of EGCG on adherent leukocytes (n=3, mean±SD; *p<0.005 vs. thrombin alone).
NO mediates EGCG inhibition of endothelial cell exocytosis
To examine the mechanisms responsible for the inhibitory effect of endothelial exocytosis by EGCG, we analyzed the effect of EGCG on eNOS activation. HUVECs were treated with or without EGCG for 0.5, 1, and 2 h, and cell lysates were immunoblotted for phospho-eNOS (Ser-1177) and phospho-Akt (Ser-473). EGCG increased phosphorylation level of eNOS and Akt in a time-dependent manner, but the amount of total eNOS and Akt did not change (Figure 4A). In contrast, other polyphenols had a minimal effect on eNOS and Akt phosphorylation (Figure 4B). These data demonstrate that EGCG stimulates eNOS phosphorylation.
Figure 4.
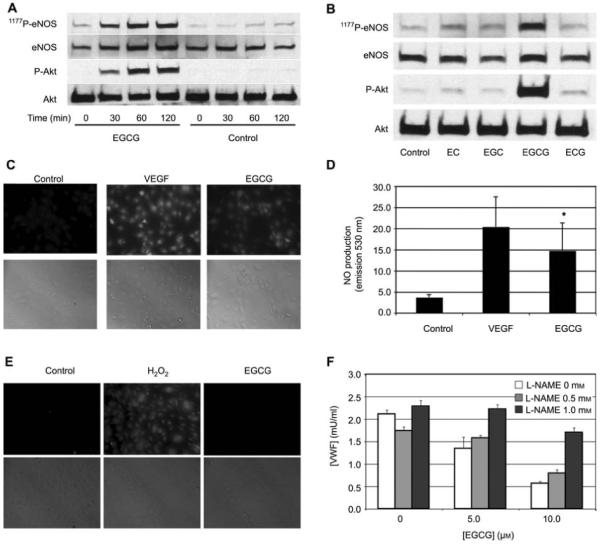
NO mediates EGCG inhibition of exocytosis.
(A) EGCG increases phosphorylation of Akt and eNOS over time. HUVECs were pretreated with 10 μm EGCG or control for 0–120 min. Cell lysates were immunoblotted for total eNOS, eNOS phosphorylated on serine 1177, total Akt, and phosphorylated Akt. (B) The increase in phosphorylation of eNOS induced by EGCG is greater than that induced by other flavonoids. HUVECs were pretreated with 10 μm flavonoids for 2 h, and then stimulated or not with thrombin for 5 min. Cell lysates were immunoblotted as for (A). (C) EGCG activates NO production. HUVECs were loaded with DAF-AM and then stimulated with VEGF or EGCG. NO production was measured by immunofluorescence (top) and cells were imaged by bright-field microscopy (bottom). (D) Quantification of NO production stimulated by EGCG (n=2–3, mean±SD; *p<0.01 vs. control). (E) EGCG does not trigger hydrogen peroxide production. HUVECs were loaded with DCF-DA, treated with EGCG or H2O2 as a control, and imaged using fluorescent microscopy. (F) EGCG inhibits exocytosis through an NO pathway. HUVECs were pretreated with l-NAME for 1 h and then EGCG was added for 1 h. HUVECs were stimulated with thrombin and VWF exocytosis was measured as described for Figure 1 (n=3, mean±SD; *p<0.05 compared to 0 mm l-NAME).
Phosphorylation of eNOS on S1177 activates eNOS to make NO (Dimmeler et al., 1999; Fulton et al., 1999). Since EGCG activates eNOS phosphorylation, we next tested the ability of EGCG to stimulate NO production. A fluorescent assay demonstrated that EGCG activates HUVEC synthesis of NO (Figure 4C,D). We confirmed this result using VEGF as a positive control. Although H2O2 can also inhibit exocytosis (Matsushita et al., 2005), EGCG did not stimulate H2O2 production (Figure 4E).
Since NO can inhibit exocytosis, we next determined whether or not NO mediates EGCG inhibition of endothelial cell exocytosis. We pretreated HUVECs with l-NAME, an inhibitor of NO synthase, added EGCG, stimulated the cells with thrombin, and then measured VWF release. EGCG inhibited thrombin-induced VWF release in the absence of l-NAME. However, l-NAME blocked much of the inhibitory effects of EGCG on exocytosis, especially at low EGCG concentrations (Figure 4F). These data suggest that EGCG-induced NO production plays a pivotal role in the inhibition of endothelial exocytosis.
We then used a pharmacological approach to explore the role of PI3K in EGCG activation of eNOS. The PI3K inhibitor LY294002 diminished the ability of EGCG to activate phosphorylation of Akt and eNOS (Figure 5A). LY294002 also blocked the effect of EGCG on endothelial exocytosis (Figure 5B). Taken together, these data suggest that EGCG activates a pathway that includes PI3K, Akt, and eNOS, and that this pathway mediates EGCG inhibition of exocytosis (Figure 5C).
Figure 5.
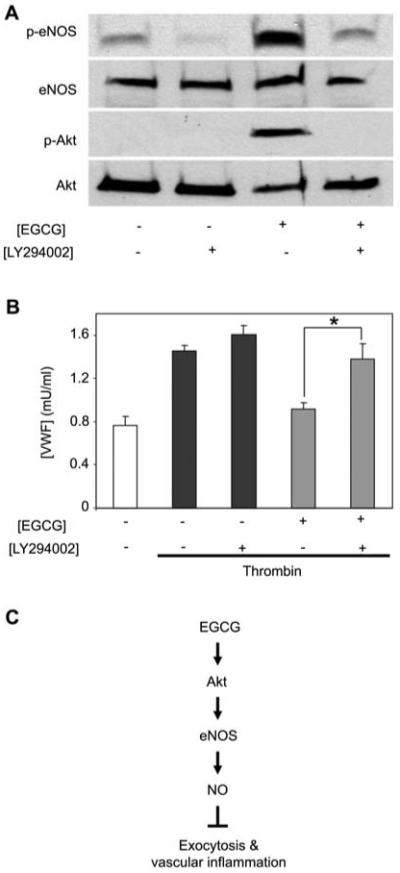
ECGC inhibits exocytosis through a PI3K-Akt pathway.
(A) HUVECs were pretreated with LY294002 for 15 min and then EGCG was added for 1 h. Cell lysates were immunoblotted as described for Figure 4. (B) HUVECs were pretreated with LY294002 for 15 min and then EGCG was added for 1 h. HUVECs were stimulated with thrombin and VWF exocytosis was measured as described for Figure 1 (n=3, mean±SD; *p<0.05 vs. EGCG alone). (C) Proposed scheme for the effects of EGCG on exocytosis.
Discussion
Our study demonstrates that EGCG inhibits endothelial exocytosis through an NO-dependent pathway. By limiting exocytosis, EGCG decreases leukocyte adherence to endothelial cells. Our observations may explain part of the anti-inflammatory effects of EGCG.
EGCG and leukocyte trafficking
Our data suggest that EGCG decreases leukocyte adherence to the vessel wall by limiting endothelial secretion of pro-inflammatory granules. By restraining Weibel-Palade body exocytosis, EGCG blocks endothelial externalization of P-selectin, which would otherwise promote leukocyte rolling. Other studies have shown that EGCG interferes with a subsequent step in leukocyte trafficking, leukocyte adhesion. EGCG blocks endothelial synthesis of VCAM-1, which mediates monocyte adhesion (Ludwig et al., 2004). Moreover, EGCG can interrupt leukocyte CD11b interactions with ICAM-1 (Kawai et al., 2004). Finally, EGCG also blocks chemotaxis, the final step in leukocyte trafficking (Takano et al., 2004). Thus, EGCG may inhibit multiple stages of leukocyte rolling by targeting leukocytes and endothelial cells.
EGCG specificity
We observed that of the four catechins found in green tea, only EGCG inhibited endothelial exocytosis (Figure 2). The effects of EGC, EC, and ECG on exocytosis at low concentrations were not significant. Furthermore, only EGCG increased Akt activation (Figure 4B). The molecular basis for this specificity is unknown. Several studies have compared the potency of these green tea polyphenols. EGC has a greater ability to scavenge radicals than EGCG, EC, and ECG (Guo et al., 1996). EGC is more effective at inhibiting PDGF-stimulated cell growth (Sachinidis et al., 2002). Furthermore, other flavonoids besides EGCG can increase NO production. For example, EC increases NO bioavailability by inhibiting NADPH oxidase (Steffen et al., 2007). Thus, different catechins appear to inhibit cell proliferation and radical production through different mechanisms.
EGCG and NO
EGCG has a variety of effects on NOS isoforms. EGCG inhibits the expression of iNOS and NO, in part by suppressing NF-κB activation (Chan et al., 1997; Lin and Lin, 1997). The effect of EGCG on nNOS is less clear, with some studies demonstrating an increase and others a decrease in nNOS expression (Wei et al., 2004; Sutherland et al., 2005). Our study extends previous reports that EGCG activates eNOS phosphorylation in bovine endothelial cells (Lorenz et al., 2004). Our results also confirm that EGCG activates eNOS through an Akt-dependent pathway (Lorenz et al., 2004; Kim et al., 2007). The present study builds on these previous works by showing that other catechins have no effect on eNOS and by demonstrating that EGCG inhibits exocytosis. Finally, our results support the concept that NO modulates endothelial exocytosis (Matsushita et al., 2003; Yamakuchi et al., 2005).
Materials and methods
Materials
(−)-Epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin (EC), (−)-epigallocatechin (EGC) and N-nitro-l-arginine methyl ester (l-NAME) were purchased from Sigma (St. Louis, MO, USA). Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN, USA). 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) was purchased from Molecular Probes (Eugene, OR, USA).
Cell culture
THP-1 cells (ATCC, Manassas, VA, USA) were grown in RPMI medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS). HUVECs (Cambrex, Walkersville, MD, USA) were cultured in endothelial basal medium (EBM-2) containing endothelial growth factor supplements and 2% FBS (EGM-2 bullet kit, Cambrex).
VWF release analysis
Confluent HUVECs were cultured in supplement-free EGM-2 medium for 18 h. Cells were pretreated with catechins and l-NAME, then placed in serum-free EGM-2 media and stimulated with 1 U/ml thrombin for 1 h. The media were collected and the amount of VWF released was measured using an ELISA (American Diagnostica, Greenwich, CT, USA).
P-selectin expression on the cell surface
P-selectin expression on HUVEC surfaces was measured using a cell surface ELISA as previously described (Burns et al., 1999). Briefly, HUVECs were seeded in 96-well plates, treated for 60 min with EGCG, then stimulated for 30 min with thrombin. The cells were fixed for 20 min at room temperature in 0.25% paraformaldehyde and blocked overnight at 4°C with 1% bovine serum albumin (BSA). After washing, the cells were incubated with antibody to P-selectin (BD Bioscience, San Diego, CA, USA) for 1 h, then with goat anti-mouse HRP-conjugated secondary antibody for 1 h. The cells were washed with PBS three times, and TMB Substrate Solution (Pierce, Rockford, IL, USA) was added. The plates were read at 450 nm after stopping the reaction by adding sulfuric acid.
Western blot analysis
After supplement-free starvation for 18 h, cells were preincubated with catechins for 2 h. The cells were dissolved in lysis buffer, fractionated on 7.5% SDS-PAGE, and blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were immunoblotted with antibodies to phospho-eNOS (Ser1177) or total eNOS (BD Biosciences) and antibodies to phospho-Akt (Ser473) or total Akt (Cell Signaling Technology, Boston, MA, USA). For detection, ECL-plus reagent (GE Healthcare, Piscataway, NJ, USA) was used according to the manufacturer’s instructions.
Measurement of intracellular ROS and NO production
To detect intracellular ROS, HUVECs were treated with 10 mm EGCG for 60 min or with 100 nm H2O2 for 10 min. Then the cells were incubated for 10 min at room temperature with PBS containing CM-H2DCFDA [5- (and 6-) chloromethyl-2′,7EGCG-dichlorodihydrofluorescein diacetate, acetyl ester; Molecular Probes). For NO measurement, HUVECs were treated with 10 mm EGCG for 60 min or with 10 mm VEGF for 60 min. The cells were then incubated for 30 min with 2 μm DAF-FM diacetate (Molecular Probes). The cells were washed with HBSS and photographed using a fluorescence microscope over an area of 520 mm×700 mm. Digital images were optimized in Adobe Photoshop (Adobe Systems, San Jose, CA, USA) only by adjusting levels according to a linear function over the entire image.
Leukocyte adhesion assay
THP-1 cells were loaded with 5 μm BCECF-AM and washed with Hanks’ balanced salt solution (HBSS). HUVECs were treated with thrombin for 30 min after EGCG pretreatment, and then BCECF-AM labeled THP-1 cells were added to each well. The cells were co-cultured at 4°C for 15 min, washed with HBSS three times, and photographed using a fluorescence microscope at magnification of 10x over an area of 520 μm×700 μm. Digital images were optimized using Adobe Photoshop (Adobe Systems) only by adjusting the levels according to a linear function over the entire image.
Statistical analysis
Results are expressed as mean±SD. Significance between mean values was determined by Student’s t-test, with a value of p<0.05 considered significant.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P01 HL56091, R01 HL074061, R01 HL78635, P01 HL65608), the Ciccarone Center, the John and Cora H. Davis Foundation, and the Clarence P. Doodeman Professorship in Cardiology to C.J.L.
References
- Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor-κB in normal human epidermal keratinocytes by green tea constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S, Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-κB inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol. Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani D, Giannini L, Ciampa A, Masini E, Suzuki Y, Menegazzi M, Nistri S, Suzuki H. Epigallocatechin-3-gallate reduces allergen-induced asthma-like reaction in sensitized guinea pigs. J. Pharmacol. Exp. Ther. 2006;317:1002–1011. doi: 10.1124/jpet.106.102178. [DOI] [PubMed] [Google Scholar]
- Barthelman M, Bair WB, III, Stickland KK, Chen W, Timmermann BN, Valcic S, Dong Z, Bowden GT. (−)-Epigallocatechin-3-gallate inhibition of ultraviolet B-induced AP-1 activity. Carcinogenesis. 1998;19:2201–2204. doi: 10.1093/carcin/19.12.2201. [DOI] [PubMed] [Google Scholar]
- Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J. Leukoc. Biol. 1999;65:299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- Chan MM, Fong D, Ho CT, Huang HI. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 1997;54:1281–1286. doi: 10.1016/s0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 2001;15:2022–2024. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Freese R, Basu S, Hietanen E, Nair J, Nakachi K, Bartsch H, Mutanen M. Green tea extract decreases plasma malondialdehyde concentration but does not affect other indicators of oxidative stress, nitric oxide production, or hemostatic factors during a high-linoleic acid diet in healthy females. Eur. J. Nutr. 1999;38:149–157. doi: 10.1007/s003940050056. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta. 1996;1304:210–222. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J. Allergy Clin. Immunol. 2004;113:1211–1217. doi: 10.1016/j.jaci.2004.02.044. [DOI] [PubMed] [Google Scholar]
- Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J. Biol. Chem. 2007;282:13736–13745. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park HJ, Lee CM, Choi IW, Moon DO, Roh HJ, Lee HK, Park YM. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 2006;580:1883–1890. doi: 10.1016/j.febslet.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Klotz LO, Sies H. Defenses against peroxynitrite: selenocompounds and flavonoids. Toxicol. Lett. 2003;140-141:125–132. doi: 10.1016/s0378-4274(02)00511-8. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. J. Am. Med. Assoc. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- Lin YL, Lin JK. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Mol. Pharmacol. 1997;52:465–472. [PubMed] [Google Scholar]
- Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Tsuda H. N-Ethylmaleimide-sensitive factor: a redox sensor in exocytosis. Biol. Chem. 2006;387:1377–1383. doi: 10.1515/BC.2006.173. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc. Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O’Rourke B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Mason RJ, Yamakuchi M, Khanday FA, Irani K, Lowenstein CJ. Hydrogen peroxide regulation of endothelial exocytosis by inhibition of N-ethylmaleimide sensitive factor. J. Cell. Biol. 2005;170:73–79. doi: 10.1083/jcb.200502031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- Ozcan A, Korkmaz A, Oter S, Coskun O. Contribution of flavonoid antioxidants to the preventive effect of mesna in cyclophosphamide-induced cystitis in rats. Arch. Toxicol. 2005;79:461–465. doi: 10.1007/s00204-005-0647-7. [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Skach RA, Seul C, Ko Y, Hescheler J, Ahn HY, Fingerle J. Inhibition of the PDGF β-receptor tyrosine phosphorylation and its downstream intracellular signal transduction pathway in rat and human vascular smooth muscle cells by different catechins. FASEB J. 2002;16:893–895. doi: 10.1096/fj.01-0799fje. [DOI] [PubMed] [Google Scholar]
- Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate inhibits interleukin-1β-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor κB activation by degradation of the inhibitor of nuclear factor κB. Arthritis Rheum. 2002;46:2079–2086. doi: 10.1002/art.10443. [DOI] [PubMed] [Google Scholar]
- Steffen Y, Schewe T, Sies H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- Sung H, Nah J, Chun S, Park H, Yang SE, Min WK. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000;54:527–529. doi: 10.1038/sj.ejcn.1600994. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (−)-epigallocatechin gallate following hypoxiaischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- Takano K, Nakaima K, Nitta M, Shibata F, Nakagawa H. Inhibitory effect of (−)-epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. J. Agric. Food. Chem. 2004;52:4571–4576. doi: 10.1021/jf0355194. [DOI] [PubMed] [Google Scholar]
- Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- Wei IH, Wu YC, Wen CY, Shieh JY. Green tea polyphenol (−)-epigallocatechin gallate attenuates the neuronal NADPH-d/nNOS expression in the nodose ganglion of acute hypoxic rats. Brain Res. 2004;999:73–80. doi: 10.1016/j.brainres.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Greer JJ, Cameron SJ, Matsushita K, Morrell CN, Talbot-Fox K, Baldwin WM, III, Lefer DJ, Lowenstein CJ. HMG-CoA reductase inhibitors inhibit endothelial exocytosis and decrease myocardial infarct size. Circ. Res. 2005;96:1185–1192. doi: 10.1161/01.RES.0000170229.49776.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med. 2004;164:1534–1540. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]


