Abstract
Background
Despite its critical role in performance-monitoring, the development of posterior medial prefrontal cortex (pMFC) in goal-directed behaviors remains poorly understood. Performance monitoring depends on distinct, but related functions that may differentially activate the pMFC, such as monitoring response conflict and detecting errors. Developmental differences in conflict- and error-related activations, coupled with age-related changes in behavioral performance, may confound attempts to map the maturation of pMFC functions. To characterize the development of pMFC-based performance monitoring functions, we segregated interference and error-processing, while statistically controlling for performance.
Methods
Twenty-one adults and 23 youth performed an event-related version of the Multi-Source Interference Task during functional magnetic resonance imaging (fMRI). Linear modeling of interference and error contrast estimates derived from the pMFC were regressed on age, while covarying for performance.
Results
Interference- and error-processing were associated with robust activation of the pMFC in both youth and adults. Among youth, interference- and error-related activation of the pMFC increased with age, independent of performance. Greater accuracy associated with greater pMFC activity during error commission in both groups.
Discussion
Increasing pMFC response to interference and errors occurs with age, likely contributing to the improvement of performance monitoring capacity during development.
Introduction
The maturation of performance monitoring is a hallmark of psychological development (Casey, 2005; Posner et al., 2007). Performance monitoring involves the detection and resolution of interference between competing response options, as well as the detection and evaluation of errors to enable the flexible adjustment of goal-directed behaviors. In healthy adults, performance monitoring functions activate several brain regions, but the most intensive work has focused on the dorsal anterior cingulate cortex, or, more inclusively, the posterior medial frontal cortex (pMFC; Ridderinkhof et al., 2004). Among youth, age-related improvements on behavioral measures of performance monitoring (i.e., lower error rates, faster response times) have been well-documented (see Casey et al., 2005, for review), but the relationship of these improvements to pMFC maturation remains poorly understood. Ultimately, characterizing the maturation of performance monitoring in healthy youth may provide leverage for understanding early onset psychiatric disorders with impaired monitoring and cognitive control, such as obsessive compulsive (Rosenberg et al., 1997; Ursu et al., 2003; Maltby et al., 2005; Fitzgerald et al., 2005) and attention deficit hyperactivity disorders (Liotti et al., 2005; Plizska et al., 2006).
Despite the critical role of the pMFC for performance monitoring in adults (Roberts and Hall, 2009; Taylor et al., 2007; Ridderinkhof et al., 2004), the contribution of pMFC development to age-related improvements of performance monitoring function remain unclear. In general, developmental imaging studies of cognitive tasks that require performance-monitoring show gradual age-related increases and decreases in activations throughout the brain (Rubia et al., 2006 and 2007; Casey et al., 2005 for review), but with inconsistent findings in the pMFC. For instance, youths show age-related increases of pMFC activation to interference on the Stroop color-word (Adelman et al., 2002) and the Simon spatial (Rubia et al., 2006) interference tasks, while other studies of interference-processing show no developmental changes in the pMFC (Bunge et al., 2002; Konrad et al., 2005; Marsh et al., 2006). Response inhibition paradigms induce interference and require subjects to withhold responses to non-target stimuli, leading to robust and reliable activation of the pMFC in adults (Wager et al., 2005; Buschbaum et al., 2005; Ridderinkhof et al., 2004); however, developmental fMRI studies of response inhibition have produced varied results, showing age-related decreases of pMFC activation on the left (Tamm et al., 2002; Durston et al., 2002), increased and decreased activation in different areas of the right pMFC (Broadmann areas 32 and 6/8, respectively; Bunge et al., 2002), and no difference from adults (Casey et al., 1997; Rubia et al., 2000; Booth et al., 2003; Rubia et al., 2006; Durston et al., 2006; Rubia et al., 2007). Evaluating reasons for these inconsistencies is an important task for studies that seek to understand the role of the pMFC in the maturation of performance monitoring functions.
The impact of behavioral performance on brain activation represents a potential confound that may contribute to inconsistent findings across developmental studies of interference-processing. Adults typically perform better than children during interference and behavioral measures of performance (e.g., error rates, response times) modulate interference-related activation in the mature brain, including in the pMFC (Paulus et al., 2002; Durston et al., 2002; Kerns et al., 2004; Weissman et al., 2006). As a result, observed changes in brain activation from youth to adulthood could relate to differences in task performance, rather than brain maturation, unless performance differences are taken into account. To address this issue, younger and older subjects can be matched on performance (e.g., Brown et al., 2005); however, this approach may yield results that are specific to unique subsets of high-performing youth and low-performing adults, thus limiting generalizability. Performance-matching in developmental work has also been pursued through the use of low potency interference tasks to enable all subjects to perform at high levels, or individually-titrated task difficulty to achieve similar performance across age groups. Yet, because the pMFC is more engaged by greater interference loads (Durston et al., 2002; Hazeltine et al., 2000; Botvinick et al., 1999), low potency tasks and reduced difficulty may decrease activation altogether (Hazeltine et al., 2003; Taylor et al., 1997), possibly contributing to the absence of pMFC-localized findings in developmental studies that have used this approach (Rubia et al., 2000; Rubia et al., 2006; Konrad et al., 2005).
A critical issue related to performance concerns differential patterns of brain activity during successful performance and during error commission. Interference- and error-processing both activate the pMFC (Ridderinkhof et al., 2004), but recent work suggests that these functions represent overlapping, but distinct neurocognitive functions (Taylor et al., 2007; Hester et al., 2004; Rushworth et al., 2004) which may exhibit unique maturational trajectories (Fitzgerald et al., 2008; Rubia et al., 2007; Velanova et al., 2008), calling for their separation in studies of pMFC development. As such, results from previous developmental work utilizing block designs that include both correct and incorrect trials (Adelman et al., 2002; Marsh et al., 2006; Casey et al., 1997; Booth et al., 2003, Luna et al., 2001; Rubia et al., 2000) may be confounded by differential development of the neural substrates for error-and interference-processing as well as by age-related differences in performance, since youths typically commit more errors. An event-related experiment that separates error trials from correct trials can distinguish different patterns of activation for error-processing from successful interference resolution.
Few functional magnetic resonance imaging studies have attempted to map the development of pMFC response to errors (but see Velanova et al., 2008 and Rubia et al., 2007); however, an emerging body of electrophysiological work has demonstrated that simple cognitive mistakes elicit a midline prefrontal event-related potential, the error-related negativity (ERN), that emerges in late childhood and increases in magnitude over the course of adolescence. Age-related increases in the ERN are remarkably consistent across studies, (Davies et al., 2004; Ladouceur et al., 2004; Wiersema et al., 2007; Kim et al., 2005; Hogan et al., 2005), but the interpretation of developmental changes in the ERN is complicated by the reduction of ERN amplitude at higher error rates (Gehring et al., 1993; Falkenstein et al., 1995). Given the inverse relationship between error rates and the ERN, poorer performance could reduce pMFC signal in younger subjects. In contrast, increasing interference load, as indexed by longer response times (Botvinick, et al., 1999; Hazeltine et al., 2003; Weissman et al., 2006) leads to greater pMFC signal during correct interference. These findings suggest that behavioral performance may differentially affect the engagement of the pMFC by error-compared to interference-processing, underscoring the importance of developmental studies that 1) separate these error and correct trials and 2) control for the effects of behavioral performance when examining the development of pMFC mechanisms for performance monitoring.
An analysis of error processing can also assess the degree to which error-related pMFC activity may associate with the flexible adjustment of behavior to achieve a correct response on subsequent trials (i.e., “cognitive control”). In the mature brain, pMFC response to errors predicts greater activation of lateral prefrontal cortex on subsequent correct trials, suggesting that the pMFC may signal other areas of the brain to increase cognitive control following a mistake (Kearns et al., 2004; Hester et al., 2008). Before age 12, children fail to recruit medial frontal cortex in response to errors (Davies et al., 2005), and reduced error-signaling could hinder the adjustment of behavior to improve performance in youth. In addition, connectivity of the pMFC with lateral prefrontal cortex increases from childhood through adolescence within the “cingulo-opercular” network known for task control (Fair et al., 2008), consistent with the notion that pMFC networking with the lateral prefrontal cortex (LPFC) may contribute to the development of cognitive control.
To characterize the development of pMFC-based performance monitoring function, we isolated correct and incorrect trials using an event-related version of the Multisource Interference Task (MSIT; Bush et al., 2003). The event-related design enabled us to examine the development of pMFC response to interference separately from its response to errors. In addition, we tested for the effects of both age and performance on pMFC activation to minimize the potential confound between these measures. Given the importance of the pMFC to mature interference processing and well-documented improvements on behavioral measures of interference capacity from childhood through adolescence, interference-related pMFC activation was expected to increase with age among youth (8 to 18 years of age), but to remain stable during adulthood. Based on electrophysiological work, error-related activation of the pMFC was also expected to increase with age among youth, and to plateau in adults. Finally, post-hoc analyses were conducted to test the prediction that greater LPFC activation would occur after errors in adults, consistent with evidence implicating lateral prefrontal regions in cognitive control, an adaptive function lacking in youth.
Materials and Methods
Participants
Twenty-one healthy adults and 29 healthy children and adolescents were recruited from the community. The adults ranged in age from 23–51 years (6F; mean age = 39.8 +/−9.4) while the youth were ages 8–18 years (16F; mean age = 12.9 +/− 2.8). Exclusion criteria were history of serious medical or neurological illness, head trauma, or mental retardation. In addition, structured clinical interviews were administered to rule out the presence of any current or past psychiatric illness in child/adolescent (Kiddie-Schedule for Affective Disorders-Present and Lifetime Version; Kaufman et al., 1997) and adult (Structured Clinical Interview for Diagnosis; First et al., 1996) subjects. The purpose and risks of the study were explained to all the participants and to the parents of minors, and informed consent/assent was obtained according to the procedures approved by the University of Michigan Medical School institutional review board. Technical difficulties led to the loss of behavioral data for 2 children (a 13 year old girl and 14 year old boy), and an additional child failed to understand the task (a 10 year old boy), reducing the group of youth to 26 participants.
Task
Participants performed a simple cognitive interference task, the MSIT (Bush et al., 2003), which required subjects to identify the unique number among three digits, “1”, “2”, or “3” (e.g., for “311,” the target is “3”), and make a key press with one of three fingers, corresponding to the ordinal value of the number: “1” → thumb, “2” → index finger, “3” middle finger. Interference was enhanced by presenting the unique number in the position incongruent with the ordinal value (e.g., “3” presented at the 1st position), and always flanked by different numbers (e.g., “11”). In the congruent condition, the target number was presented in the position compatible with its ordinal value (e.g., “1” presented in the first position) and flanked by a non-number symbol (e.g., “1xx”).
In contrast to the original blocked version of the MSIT (Bush et al. 2003), the task was adapted for an event-related design to allow for the separation of fMRI BOLD signal associated with correct incongruent, correct congruent and error trials. MSIT stimuli appeared for 500 msec, followed by a 2500 msec ISI (fixation cross), to comprise a trial. A total of 120 incongruent, 120 congruent, and 60 “fixation” trials (3000 msec of a fixation cross) were presented in pseudorandom order, over five runs (3 minutes each). Errors were scattered across the experiment, effectively introducing “jitter” to facilitate the resolution of error-related fMRI BOLD response.
Functional MRI acquisition
Scanning took place on a 3.0 T GE Signa scanner (LX [8.3] release, Neuro-optimized gradients), beginning with the acquisition of a standard, axial T1-image for anatomic normalization and alignment. Next, a reverse spiral acquisition sequence was applied to collect T2* weighted images (GRE, TR = 2000ms, TE = 30ms, FA = 90, FOV = 20cm, 40 slices, 3.0mm/slice, 64×64 matrix) in the same prescription as the T1-image, with 85 volumes per session. This protocol has been shown to minimize magnetic susceptibility to enable signal recovery in regions of the brain that lie near air/tissue boundaries (Stenger et al., 2002; Yang et al., 2002). After the functional volumes were acquired, a high resolution T1 scan (3D SPGR, 1.5 mm sl, 0 skip) was obtained for anatomic normalization. Subject head movement was minimized through instructions to the participant and packing with foam padding that we have found to be well-tolerated by children.
Functional data pre-processing
All functional data were subjected to an initial series of preprocessing steps. First, the data were sinc-interpolated, weighted by a Hanning Kernal, in time, slice-by-slice, to correct for the staggered sequence of slice acquisition (Aguirre et al., 1997). Next, a six-parameter, rigid body motion correction algorithm was applied to realign all functional data from a given participant to the first image acquired during a scanning session (“mcflirt”, Jenkinson et al., 2002). The data were then automatically thresholded to exclude extra-parenchymal voxels from subsequent analysis, and the scan-wise global signals and power spectra were derived and stored. Functional volumes were anatomically normalized to the MNI152 template of the SPM2 software package, in order to place individual data into a common anatomic reference space to facilitate analysis across subjects. Co-registration of the 3D-SPGR image, T1-overlay, and realigned functional volumes yields a high resolution image which can be warped to the common reference space (i.e., canonical image) by means of non-linear transformation contained within SPM2. The brain reaches 90% of adult size by approximately 5 years of age, enabling the normalization of pediatric brains to the adult-sized canonical images included in SPM2 (Kang et al., 2004).
Movement Analysis
Measures of head movement were obtained from the output of the rigid body motion correction algorithm. Translations in the roll, pitch and yaw dimensions, and rotations in the x, y, and z dimensions were averaged across TRs for each run. Runs were excluded from further analysis if average movement in any direction exceeded 1 mm or degree, or if movement for one or more TRs exceeded 2 mm or degrees. Excessive movement led to the exclusion of 3 youth [2 girls (ages 9 and 11) and one boy 9 (age 10)] from analyses. Functional MRI analyses included data from the remaining twenty-three youth: 18 with five runs, 2 with four runs, and 3 with three runs of data. Five runs of data were included for all of the adult subjects. Average movement parameters for included runs were compared between youth and adults using 2-sample t-tests (p < .05, 2- tailed).
Data Analysis
Behavioral
Commission error rates and response times (RT) were entered as dependent measures in separate two-way ANOVAs using group (youth vs. adults) as the between-subjects factor and condition (incongruent, congruent) as the within-subjects factor. Significant group × condition interactions were followed up with 2-sample t-tests (p < .05, 2-tailed).
Whole-brain fMRI Analyses for Interference and Errors
Functional data was analyzed using a standard random effects analysis within the framework of the modified General Linear Model (Worsley et al., 1997; Worsley et al., 1992), implemented using SPM2. Correct incongruent, correct congruent, and commission error trials were modeled, with fixation trials and inter-trial intervals modeled as an implicit baseline. Omission trials were modeled as a covariate of no interest. Activation maps were first derived for individual subjects from linear contrasts of interest, Incongruent – Congruent (correct trials only) and Error – Correct (across both incongruent and congruent conditions). For inclusion in the Error contrast, subjects were required to have at least 3 errors of commission, which we have empirically determined, in other similar data sets, as the minimum number of trials to generate a reliable error signal in the BOLD response (Stern et al., 2008). In a separate model, correct trials after error responses, and correct trials after correct responses were entered as regressors to test the prediction that errors would increase LPFC engagement on subsequent correct trials (i.e., for correct trials that followed an error compared with those that followed other correct trials). Contrasts for individual subjects were entered into parametric random effects (or “second order”) analyses to produce group activation maps (Friston et al., 1999). Contrasts for interference, errors and post-error correct trials were examined within and between the youth and adult groups using voxel-by-voxel parametric maps at a height threshold of p < 0.001 (uncorrected), with cluster-level significance of p < 0.05, corrected for multiple comparisons across the brain.
Region of Interest fMRI Analyses
To test the effects of age and performance on pMFC activation, beta contrast estimates for individual subjects were extracted from the pMFC regions of interest (ROI) for the interference and error contrasts. To obtain unbiased ROIs, clusters of voxels from the combined “super-group” of youth and adults for each contrast were functionally identified in the pMFC (interference: −3, 9, 54; k = 171; error: −9, 21, 57; k = 56). ROI extractions were regressed on age, group, age × group interaction and performance to test for a linear relationship between pMFC activations and age, accounting for performance, during interference- and error-processing. Linear regression models (simultaneous entry of independent variables) were implemented in SPSS.
The first-order linear coefficients for performance were differentially specified for the interference and error contrasts: interference contrast estimates were tested against overall performance accuracy (i.e., across both incongruent and congruent trial types) and interference RT (the difference between correct incongruent and congruent RTs), since the interference contrast was derived from the difference in fMRI BOLD signal for correct incongruent compared with correct congruent trials. Error contrast estimates were tested against commission error rates for incongruent trials, which elicited the majority of errors.
Since some work has suggested that younger subjects exhibit greater extent of regional activations in response to cognitive tasks (Durston et al., 2006; see Casey et al., 2005 for review), analyses were performed to test for differential effects of age and performance on the extent of pMFC activation during interference- and error-processing. Extent was defined as the total number of activated voxels (t-score > 1.65) within a pMFC region of interest. Two large regions covering the known extent of activation foci reported for interference and error processing (Ridderinkhof et al., 2004) were defined by manually thresholding the relevant contrasts from the unbiased super-groups (t = 2.15, t = 3.02, respectively) to yield volumes of 39177 mm3 and 21735 mm3, respectively. Measures of extent were entered into linear regressions to test for effects of age, group, age × group interaction and performance.
The pMFC was the primary region of interest for the linear regression analyses; however, contrast estimates from other peak activations that fell within regions known to be engaged by interference- and error-processing were also examined (Roberts and Hall, 2009; Wager et al., 2005; Taylor et al., 2007). Based on super-group activations, interference ROIs were defined in the bilateral inferior frontal gyrus (IFG, right: 48, 9, 30; k = 32 and left: −48, 3, 33; k = 90), bilateral anterior insula (right: 36, 21, 6; k = 63 and left: −33, 27, 3, k = 22), and bilateral parietal cortex (right: 30, −66, 42 and left: 33, −72, 36 were included within a single larger cluster spanning midline, k = 717) and error ROIs were defined for the left IFG (−51, 15, 0; k = 15) and the midline superior parietal cortex (3, −72, 51; k = 15).
Results
Movement results
No group differences in movement were observed except in the roll direction, for which youth (.15 +/− .21 deg) exhibited significantly less movement than adults (.60 +/− .59 deg; t [2, 42] = −3.39, p < .01). There was also a trend towards less movement in the pitch direction for youth (.02 +/− .15) than adults (.10 +/− .24; t [42] = 1.9, p = .06). Exclusion of 3 youth with excessive movement and exclusion of specific runs for 5 additional youth likely contributed to these findings.
Behavioral results
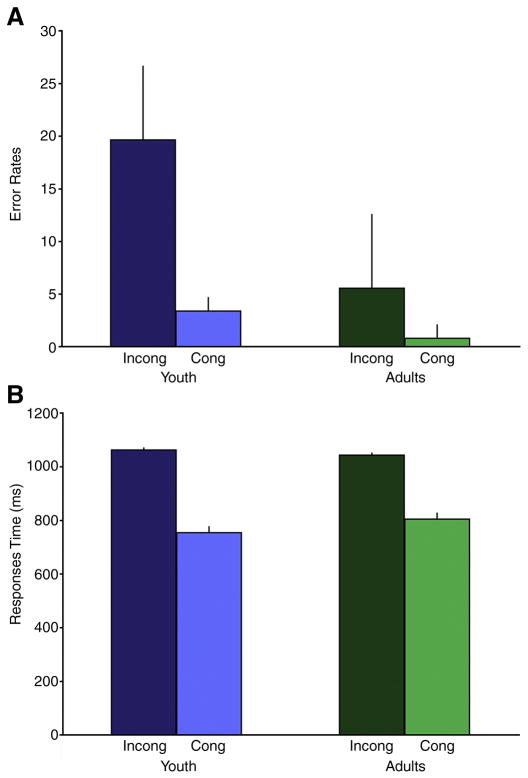
For commission error rates, there were main effects of group [F (1, 42) = 15.9; p <0.01], condition [F (1, 42) = 38.9, p < 0.01], and a group × condition interaction (F [1, 42] = 11.6, p < 0.01). Planned contrasts revealed that youth had higher error rates than adults on both incongruent (youth: 19.7 +/− 15., adult: 5.6 +/− 7.2, t [42] = 3.8, P < 0.01) and congruent trials (youth: 3.4 +/− 4.1, adult: 0.8 +/− 1.3, t [42] = 2.8, p = 0.01; Figure 1A).
Figure 1.
Youth had significantly higher error rates during incongruent and congruent trials compared with adults (Panel A), but response times were not significantly different between groups for either condition (Panel B).
Response times were not found to differ between youth and adults (no main effect of group [F (1, 42) = .048, p = .83]). However, there was a main effect of condition [F (1, 42) = 214, p< .01]), and a trend towards a group × condition interaction (F [1, 42] = 3.4, p = .07). Post-hoc contrasts revealed slower response times for incongruent than congruent trials across all subjects (t [42] = 25.4, p < .01), but no between-group differences for incongruent (youth: 1062 +/− 338, adults: 1044 +/− 193 p = .83) or congruent (youth: 754 +/− 212, adults: 803 +/− 197, p = .42) conditions (Figure 1B), suggesting that the trend-level interaction of group × condition was driven by nominally slower incongruent, but faster congruent response latencies for youth than adults.
fMRI results
Interference-processing (Incongruent - Congruent, Correct Trials) in pMFC
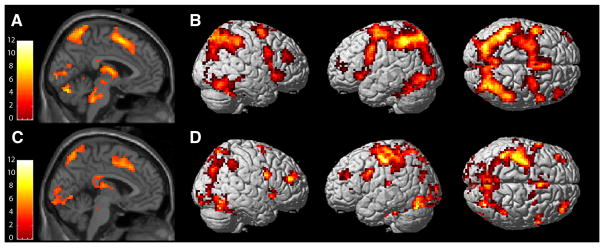
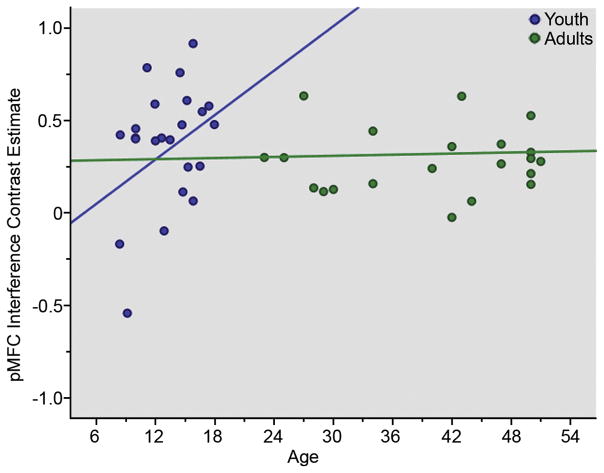
Analyses of interference-processing included 23 youth (12 F, 13.2 +/− 3.0 years) and 21 adults (6 F, 39.8 +/− 9.4 years). The whole brain, voxel-wise analyses revealed robust pMFC activations for the youth and adult groups when examined separately (Table 1, Figure 2), but no between-group differences for this region. On the other hand, when pMFC activity was regressed on age as a continuous measure, a significant developmental effect emerged. In a linear regression analysis of BOLD values extracted from an unbiased pMFC ROI (defined by combining both groups in a single analysis), significant effects of age and an age by group interaction were observed. Interference-related activation of the pMFC ROI was positively associated with age for youth, but not adults (Table 2; Figure 3), when controlling for performance (accuracy and interference RT). No independent effects of performance on pMFC activity were observed (Table 2). In a separate and secondary analysis, the extent of pMFC activation was regressed on age and performance, but no significant associations were found.
Table 1.
Interference-related activations for Adults and Youth
| Adults |
Youth |
|||||
|---|---|---|---|---|---|---|
| Region (Brodmann area) | Cluster | Coordinates | Z-score | Cluster | Coordinates | Z-score |
| Posterior Medial Frontal Cortex (32/6) | * | −3, 9, 54 | 5.25 | 325 | −3, 21, 42 | 4.65 |
| −6, 0, 57 | 5.17 | −3, 9, 48 | 4.58 | |||
| 3, 9, 57 | 5.34 | 0, −3, 48 | 4.45 | |||
| 32 | 6, −24, 30 | 4.52 | ||||
| Bilateral Anterior Insula | * | −33, 24, −6 | 5.89 | 139∂ | −30, 21, 6 | 4.53 |
| −33, 30, 3 | 4.37 | |||||
| 441 | 39, 21, 3 | 4.75 | 82 | 36, 21, 6 | 4.11 | |
| 36, 33, 3 | 4.29 | 48, 15, −3 | 4.25 | |||
| Bilateral Inferior Frontal Gyrus (9/45) | 48, 6, 30 | 4.76 | ||||
| * | −51, 3, 39 | 5.71 | 148 | −60, 9, 24 | 4.53 | |
| −51, 3, 33 | 3.92 | |||||
| 89 | 48, 9, 27 | 4.60 | ||||
| 60, 9, 27 | 5.30 | |||||
| Bilateral Middle Frontal Gyrus (6) | * | −33, −6, 60 | 5.13 | 36, 12, 30 | 3.67 | |
| −36, −3, 60 | 5.07 | 56 | −42, 39, 27 | 4.09 | ||
| 33, 3, 63 | 5.65 | −33, 45, 30 | 3.79 | |||
| Right Dorsolateral Frontal Cortex (45/46) | 93 | 51, 36, 21 | 4.48 | 133 | 33, 54, 27 | 4.63 |
| 45, 39, 27 | 3.91 | 36, 42, 21 | 4.54 | |||
| 48, 48, 18 | 3.36 | 42, 48, 21 | 4.45 | |||
| Left Precentral Gyrus (4) | 1548 | −45, −18, 51 | 5.00 | |||
| −36, −18, 66 | 4.80 | |||||
| Left Postcentral Gyrus (2, 3) | −30, −39, 72 | 4.27 | ||||
| −18, −9, 75 | 4.06 | |||||
| −51, −33, 57 | 4.05 | |||||
| Bilateral Parietal Cortex (40/7/2) | * | −39, −51, 45 | 6.68 | −45, −33, 33 | 5.01 | |
| −24, −72, 48 | 5.85 | −48, −42, 63 | 5.00 | |||
| −54, −42, 57 | 5.27 | −21, −63, 66 | 4.08 | |||
| 33, −60, 48 | 6.22 | 30, −66, 39 | 5.32 | |||
| 48, −39, 45 | 5.09 | 42, −45, 48 | 4.01 | |||
| Bilateral Occipital Cortex (18, 19) | −33, −84, 21 | 5.10 | 33, −72, 36 | 5.41 | ||
| 39, −72, 24 | 5.15 | 42 | 45, −81, 3 | 4.25 | ||
| 321 | 6, −81, 15 | 4.84 | 48, −75, 12 | 3.43 | ||
| 0, −93, 0 | 4.82 | 182 | −27, −72, 39 | 4.94 | ||
| −9, −96, 3 | 4.29 | −27, −72, 27 | 4.66 | |||
| ‡ | −39, −90, 0 | 5.06 | 783 | −45, −69, −15 | 4.75 | |
| 51, −72, −9 | 4.61 | −42, −78, −18 | 4.61 | |||
| Bilateral Cerebellum | −42, −54, −30 | 5.03 | 12, −90, 18 | 4.46 | ||
| −3, −75, −21 | 5.21 | 282 | 33, −42, −30 | 5.19 | ||
| 39, −48, −27 | 5.38 | 39, −48, −30 | 4.68 | |||
| 29 | 0, −48, −3 | 4.32 | 33, −63, −27 | 4.67 | ||
| 0, −45, −15 | 3.25 | |||||
| Left Thalamus | ‡ | −9, −6, 3 | 5.90 | |||
| Right Thalamus | 6, −3, 3 | 4.98 | 303 | 18, −21, 15 | 5.74 | |
| 3, −9, 12 | 4.70 | |||||
| 0, −18, 12 | 4.41 | |||||
| Left Temporal Cortex | ‡ | −51, −72, −6 | 5.02 | |||
| Right Temporal Cortex | 51, −63, −18 | 4.42 | ||||
| Bilateral Pons | −6, −39, −39 | 4.37 | ||||
| 3, −36, −30 | 4.94 | |||||
Height threshold: p < 0.001
Cluster threshold: size > 9 voxels, pcorr < .05
Large clusters are broken down by region for adults (4652 voxels*, 2561 voxels‡)
Cluster extends medially into left caudate (sub-peak: −12, 24, 0; 4.09)
Figure 2.
During interference, both adults (A, B) and youth (C, D) exhibited robust activations of the pMFC, bilateral insula, inferior frontal gyri and parietal cortex. Activations are displayed at a height threshold of punc < .001 for clusters comprised of more than 9 voxels.
Table 2.
Effects of Age and Accuracy on Interference-processing for Youth
| Linear Model Coefficients ± S.E. (p-value) | ||||||
|---|---|---|---|---|---|---|
| ROI |
Youth† |
Adult† |
Group€ |
AgexGroup€ |
Accuracy |
Interference RT |
| pMFC | .05 ± .02* | .001 ± .006 | .55 ± .39 | −.04 ± .02* | −.48 ± .60 | .00 +/− .00 |
| R. IFG | .03 ± .01 | .000 ± .004 | .34 ± .28 | −.03 ± .02 | −.29 ± .43 | .00 +/− .00 |
| L. IFG | .04 ± .02* | .001 ± .005 | .52 ± .34 | −.04 ± .02* | −.40 ± .53 | .00 +/− .00 |
| R. Ins | .03 ± .02* | −.003 ± .005 | .60 ± .30* | −.03 ± .02* | −.60 ± .46 | .00 +/− .00 |
| L. Ins | .02 ± .01 | .001 ± .004 | .33 ± .24 | −.02 ± .01 | −.79 ± .37* | .00 +/− .00 |
| B. Par | .02 +/− .01 | −.002 +/− .003 | .28 +/− .22 | −.02 +/− . −1 | .45 +/− .34 | .00 +/− .00 |
p < .05
Δ Estimate: Δ Age for Youth and Adults
Signs for each value are in reference to youth [same value, but invert signs for adults]
Figure 3.
Interference-related pMFC activation increased with age among youth, but plateaued in adults, when controlling for the effects of performance. Blue circles indicate youth, green circles indicate adults.
Error-processing (Error - Correct Trials) in the pMFC
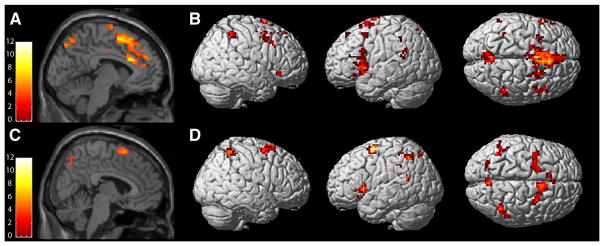
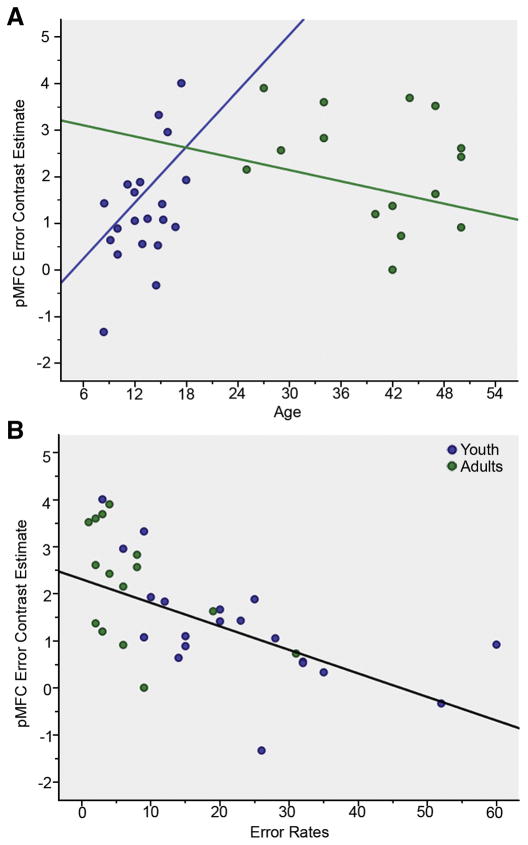
Fifteen adults (4F, 40.2 +/− 8.5 years) and 20 youth (9 F, 13.1 +/− 3.0 years) exhibited sufficient error rates for inclusion in the analyses of error-processing. The whole brain, voxel-wise analyses revealed activations for both youth and adult groups in the pMFC (Table 3; Figure 4); for the group contrasts, greater activation of the left dACC (0, 33, 6, k = 10, Z = 3.92) was observed for adults than youth, but failed to meet the threshold set for cluster level significance (p = .10). As with interference processing, the linear regression of pMFC ROI extractions revealed significant effects of age and an age by group interaction, as well as an effect of group, indicating that error-related pMFC activation was positively associated with age for youth, but not adults, when controlling for performance (error rates; Table 4, Figure 5A). Across all subjects, error-related activation in the pMFC ROI was inversely related to error rates (Table 4, Figure 5B). Secondary analyses of the extent of pMFC activation during error-processing revealed no associations with age or performance, and no age by group interaction.
Table 3.
Error-related activations for Adults and Youth.
| Adults |
Youth |
|||||
|---|---|---|---|---|---|---|
| Region (Brodmann area) | Cluster | Coordinates | Z-score | Cluster | Coordinates | Z-score |
| Posterior Medial Frontal Cortex (6) | 669 | 0, 15, 54 | 4.83 | 112❄ | 6, 12, 63 | 4.17 |
| −6, 27, 24 | 4.80 | |||||
| 9, 27, 51 | 4.78 | |||||
| Left Insula | 34 | −30, 30, −9 | 3.94 | 70 | −39, 21, 0 | 4.07 |
| −27, 24, −15 | 3.40 | −39, 12, 9 | 3.96 | |||
| Left Inferior Frontal Gyrus (49/47) | 143 | −60, 15, 3 | 4.74 | −51, 15, 0 | 3.88 | |
| −54, 9, 0 | 4.68 | |||||
| −51, 9, 9 | 4.01 | |||||
| Left Middle Frontal Gyrus (6/10) | 74 | −18, 3, 63 | 4.37 | |||
| −27, 0, 66 | 4.14 | |||||
| −39, 0, 60 | 4.05 | |||||
| Right Middle Frontal Gyrus (6/10) | 38 | 45, 18, 39 | 4.27 | |||
| 51, 12, 45 | 3.67 | |||||
| 51, 21, 33 | 3.49 | |||||
| Right Superior Frontal Gyrus (6) | 44 | 21, 9, 54 | 4.44 | |||
| 24, −3, 51 | 3.82 | |||||
| Bilateral Parietal Lobule (7/40) | 19 | −57, −48, 27 | 3.85 | 36 | −51, −54, 48 | 4.17 |
| −57, −48, 18 | 3.54 | −51, −39, 57 | 4.07 | |||
| −39, −57, 51 | 3.63 | |||||
| 29 | 48, −42, 54 | 4.15 | 53 | 42, −51, 60 | 4.16 | |
| 88 | 0, −63, 57 | 4.64 | ||||
| −6, −78, 48 | 4.11 | |||||
Height threshold: p < 0.001
Cluster threshold: size > 9 voxels, pcorr < .05
Cluster extends laterally into the right middle frontal gyrus (sub-peaks: 33, −9, 60;3.63 and 27, 3, 54; 3.54)
Figure 4.
During error-processing, both adults (A, B) and youth (C, D) exhibited robust activations of the pMFC, left insula and inferior frontal gyrus, and bilateral parietal cortex. Activations are displayed at a height threshold of punc < .001 for clusters comprised of more than 9 voxels.
Table 4.
Effects of Age and Error Rates on Error-processing for Youth
| Linear Model Coefficients ± S.E. (p-value) | |||||
|---|---|---|---|---|---|
| ROI |
Youth† |
Adult† |
Group |
AgexGroup |
ErrIncCom |
| pMFC | .20 ± .07* | −.04 ±.03 | 4.3 ± 1.6* | −.24 ± .08 * | −.05 ± .01* |
| L IFG | .13 ± .10 | .06 ±.04 | −.13 ± 2.1 | −.07 ± .11 | −.03 ± .02 |
| Sup Par | .002 ± .10 | −.03 ± .04 | 1.1 ± 2.3 | −.03 ± .11 | −.02 ± .02 |
p < .05
Δ Estimate: Δ Age for Youth and Adults
Signs for each value are in reference to youth [same value, but invert signs for adults]
Figure 5.
Error-related pMFC activation increased with age among youth, but plateaued in adults, when controlling for the effects of performance (A). Greater error-related pMFC activation occurred at lower error rates across the combined group of subjects (B). Blue circles indicate youth, green circles indicate adults.
Performance Monitoring Outside of the pMFC
Interference
In addition to the pMFC, whole brain voxel-wise analyses also revealed interference-related activations of the bilateral inferior frontal gyri, bilateral anterior insula, and bilateral parietal cortex in both youth and adults (Figure 2, Table 1). For adults compared to youth, activation of the left angular gyrus (−39, −57, 36; k = 10; Z = 3.53) met the statistical threshold set for height-, but not cluster-level significance (pcorr = .75); youth did not exhibit greater activity than adults in any part of the brain. In contrast to the largely negative results for the whole brain group comparison, significant developmental effects were shown by regression analyses testing the effects of age on extracted values for supergroup-derived ROIs. Activity in the left IFG, right anterior insula and, at trend-level significance, the right IFG (p = .06) was positively associated with age for youth, but not adults (Table 2), controlling for performance. Across all subjects, performance accuracy was inversely associated with activation in the left anterior insula ROI (Table 2). No other significant effects of age or performance were observed in any other ROI.
Error
Whole brain voxel-wise analyses revealed error-related activations in the pMFC, but also in the left anterior insula, the left IFG and bilateral parietal cortex in both groups (Table 3, Figure 4); group comparisons showed greater activation of the left anterior insula (−30, 33, −12; k = 22; Z = 4.11) and left basal ganglia (−15, 9, 3; k = 104; Z = 3.96) in adults, but no increased activation in any area for youth. In the linear regression analyses, no age effects were observed for either of the ROIs that were tested outside of the pMFC (left IFG or the midline superior parietal; Table 4). For the ROI in the left IFG, activity was inversely correlated with error rates across all subjects at trend-level significance (p = .07).
Error-related Adjustment ( Correct Trials, Post-Error - Post-correct)
Recent theories of cognitive control posit that the pMFC response to an error may induce slower, more accurate performance on subsequent trials through recruitment of the lateral prefrontal cortex (PFC; Kerns et al., 2004; Garavan et al., 2002). Although mean post-error response times did not differ between groups (youth: 941 +/− 389 msec, adults: 1009 +/−243 msec; t [1, 42] = .68, p = .50), we sought to test whether development impacts error-related adjustments of the lateral prefrontal cortex (Kerns et al., 2004) in a post-hoc analysis comparing correct trials preceded by an error with those preceded by a correct response in adults and in youth. Adults exhibited activation of the right DLPFC (42, 24, 45; Z = 3.74; k = 31, pcorr < .01 in lateral prefrontal search volume) and of the right IFG (54, 18, 33; Z = 3.81; k=16, pcorr = .01), consistent with prior work (Kerns et al., 2004; Garavan et al., 2002). In contrast, youth did not exhibit activation in any lateral prefrontal region. For the group comparison, adults exhibited greater activation of the left inferior frontal gyrus (−45, 18, 6; Z = 3.63; k = 37; pcorr < .01) than youth, a difference that was driven by activation in adults compared to deactivation in youth. Across all subjects (adults and youth), error-related activation for the pMFC tended to associate with extracted values from this left IFG region (B = .26 +/− .12, p = .06), controlling for age and performance accuracy. No relations were observed between the pMFC and post-error response times, or between the left IFG and overall performance accuracy.
Discussion
To address the question of how the pMFC contributes to the development of performance-monitoring, we used fMRI to assess pMFC response to interference and errors in youth and adults performing the MSIT. Both interference- and error-processing were associated with activations of the pMFC in adults, as well as youth. These findings are consistent with a recent meta-analysis demonstrating overlapping clusters of interference- and error-related activation across the pMFC in adults (Ridderinkhof et al., 2004), and extend this work by demonstrating the engagement of the pMFC by both performance monitoring functions in the developing brain.
To disentangle the effects of age and performance on pMFC activation, contrast estimates for interference and errors were extracted from functionally defined pMFC regions of interest and regressed on age and performance measures. As predicted, increasing age was positively associated with pMFC activation to both performance monitoring functions among youth, but not adults, when covarying for behavioral performance. Behavioral performance was not significantly associated with interference-related pMFC activation while, in contrast, greater pMFC response to errors occurred with declining error rates. Developmental differences in performance monitoring function outside of the pMFC, including in the lateral prefrontal cortex following errors, were also examined and are discussed below.
Effects of Age on pMFC Activation
Given the complicated differential effects of age and performance on pMFC function for interference and error processing, it is not surprising that prior attempts to map the development of interference-processing have produced inconsistent findings in the pMFC. Age-related increases of pMFC activation to interference have been previously demonstrated (Adelman et al., 2002; Rubia et al., 2006), but negative findings from developmental studies of interference and interference-inducing response inhibition tasks (Interference: Bunge et al., 2002; Konrad et al., 2005; Marsh et al., 2006; Response Inhibition: Casey et al., 1997; Booth et al., 2003; Durston et al., 2006; Rubia et al., 2006), reports of age-related decreases (Response Inhibition: Durston et al., 2002; Tamm et al., 2002; Velanova et al., 2008) or shifts in laterality (Response Inhibition: Bunge et al., 2002) have left an inconclusive picture. Prior work may have been confounded by block designs in which error and correct trials were mixed, and/or a failure to control for performance differences between adults and youth. By separating correct interference from errors and covarying for performance effects, we demonstrated that pMFC activation to interference increases with age in the developing brain.
Evidence for maturational increases in error-related engagement of the medial prefrontal cortex comes from electrophysiological work, which has collectively demonstrated a dramatic increase of the ERN over the course of adolescence (Davies et al., 2004; Ladouceur et al., 2004). However, given the limited spatial resolution of electrophysiologic techniques, the localization of the developing ERN within the frontal midline remains to be determined. Despite the critical, and apparently distinct role of the pMFC in responding to errors in mature brain (Taylor et al., 2007), few developmental fMRI studies have examined the maturation of this pMFC function. The extant literature includes only two developmental fMRI studies of error-processing -- one with negative findings for the pMFC (Rubia et al., 2007) and the other showing decreased pMFC activation from childhood to adolescence followed by subsequent increase into adulthood (Velanova et al., 2008), with neither study controlling for the effects of performance. Covarying for performance, we have demonstrated a maturational increase of pMFC engagement by error-processing that appears to parallel the development of the ERN.
Recent work suggests that the development of pMFC structure may follow a nonlinear, quadratic trajectory, with the peaking of cortical thickness in adolescence, followed by subsequent thinning through adulthood (Shaw et al., 2006), and some evidence suggests that functional development may also follow an inverted ‘U-shaped’ relationship with age. For instance, Luna and colleagues (2001) found that during an anti-saccade task, pMFC activity increased from pre-adolescent children (8–13 years) to adolescents (14–17 years), and then decreased from adolescents to adults. In our sample, inspection of the pMFC contrast estimates plotted against age, suggests that pMFC response to performance monitoring may peak in late adolescence/early adulthood and then decline. Greater pMFC activation in older adolescents compared with some adults may have obscured developmental differences in this region in our whole brain comparison of youth and adult groups, and could have contributed to the negative findings for the pMFC in prior developmental work that has relied on simple group comparisons (Bunge et al, 2002; Konrad et al, 2005; Casey et al, 1997; Both et al, 2003). Taken together, these converging lines of evidence underscore the importance of considering age as a continuous measure to map the gradual, and possibly non-linear, shifts of pMFC function that occur during development.
Prior work has suggested that developmental improvement in performance monitoring capacity may associate with increasing magnitude, but decreasing extent of activation in task critical regions (“focalization,” Durston et al., 2006). Contrary to this possibility, the extent of pMFC activation was not found to vary across the age range represented by our youth sample, 8 – 18 years. In contrast, Durston et al. (2006) found increasing focalization of right IFG activation during response inhibition in children from 9 to 11 years of age. It is possible that this developmental pattern may be specific to particular brain regions (e.g., IFG, but not pMFC), task designs (e.g., response inhibition, but not interference- or error-processing), or even periods of development (e.g., from mid- to late childhood, rather than during adolescence). Alternatively, the inclusion of larger numbers of youth subjects might enable the detection of age-related decreases in pMFC extent that we were underpowered to detect.
Effect of performance on pMFC activity
Despite prior work demonstrating that greater interference load, reflected by reduced performance accuracy and/or longer response times, increases engagement of the pMFC on correct trials in adults (Durston et al., 2002; Botvinick et al., 1999), we did not observe a relationship between interference-related activation of the pMFC and performance measures across our combined group of subjects. However, our linear regression model tested the effect of performance on pMFC activation across the combined group of subjects and would not have been sensitive to detecting a situation in which adults, but not youth, exhibit a relationship between performance and pMFC response to interference. Indeed, partial correlations of accuracy and interference-related pMFC activation, controlling for age, revealed a trend towards inverse association in adults (r = −43, p = .06), but not youth (r = −.04, p = .89), suggesting that the interaction between performance and the pMFC during interference may be developmentally specific.
In contrast to interference, error-related pMFC was affected by performance. Subjects who performed more accurately exhibited a stronger pMFC signal when they committed an error. This result is consistent with electrophysiological work that has consistently found higher error rates to reduce the amplitude of the ERN (Gehring et al., 1993; Yeung et al., 2004; Stahl and Gibbons, 2007), as well as recent fMRI findings (Polli et al., 2008; Klein et al., 2007). Our work replicates and extends these findings across a broad developmental range, since lower error rates were inversely correlated with pMFC activation during error-processing across the combined group of youth and adult subjects, independent of age. There are several explanations for this finding. Infrequent errors may reflect greater subjective involvement in correct performance, and a stronger reaction to individual errors. Increasing a subject’s investment in a task with a monetary reward increases the magnitude of the ERN (Hajcak et al., 2005) and the fMRI BOLD signal (Taylor et al., 2006) when subjects commit errors. Alternatively, recent work suggests that pMFC response to errors may be directly connected to brain mechanisms that adjust performance (Kearns et al., 2004), such that greater error-related engagement of the pMFC may facilitate adjustments to improve performance, reducing errors overall.
Error-related activation of the pMFC has been previously found in association with post-error improvements in performance and recruitment of the lateral PFC (Kerns et al., 2004; Garavan et al., 2002). Consistent with this work, adults from our sample showed greater lateral prefrontal (right DLPFC and right IFG) activation on correct trials that followed errors compared with correct trials that followed other correct responses. In contrast, youth did not activate any region of the lateral PFC. Greater activation of the left IFG was observed in adults compared to youth, driven by differential patterns of activation and deactivation between groups. Across all subjects, post-error activity of the left IFG tended to associate with greater pMFC response to errors, consistent with recent theories that error-related activation of the pMFC may signal the lateral PFC to increase cognitive control (Kerns et al., 2004). In addition to predicting post-error engagement of the lateral PFC, cognitive control theory also predicts greater behavioral adjustment (i.e., post-error slowing) in better performing individuals; in our sample, adults were more accurate than youth but post- error response times, though nominally slower for adults, were not significantly different between groups. Nonetheless, findings for our adults are compatible with recent work suggesting that pMFC interactions with the lateral PFC may support the execution of a correct response following an error (Kerns et al., 2004; Garavan et al., 2002). In contrast, the absence of post-error engagement of the lateral PFC in youth may reflect under-developed networks for cognitive control, consistent with recent work demonstrating reduced pMFC-lateral PFC connectivity at resting state in youth (Fair et al., 2008).
Distinguishing between Error and Interference Processing in the pMFC
Functional neuroimaging work in adults suggests that interference and error processing may represent overlapping, but separable functions within the pMFC (Ridderinkhof et al., 2004). For the combined group of youth and adult participants, the peak activation for interference localized to y = 9, whereas the peak for error-processing was observed at y = 21, consistent with meta-analytic work suggesting that error-processing may occur in a more anterior region of the pMFC than interference processing (Hester al, 2005; Taylor et al., 2007). The precise functional topography of this region remains an area of debate (Botvinick et al., 2004), and additional study will be needed to determine whether development may influence the distribution of interference- and error-related activations within the pMFC.
Changes in pMFC activation with age may follow different slopes for interference-compared to error-processing. Velanova et al. (2008) recently reported greater differentiation of pMFC response to errors than correct trials in adults compared to youth, and developmental shifts in the timing of peak differences in pMFC activation to correct versus error trials for adults, adolescents, and youth. In our sample, the slope (Δ pMFC contrast estimate: Δ Age) was nominally steeper for error- (.40) than interference-processing (.04). From our sample, it was not possible to distinguish differential developmental trajectories, but future work with larger sample sizes may be better able to test for differences between the development of the two processes
Effects of Age and Performance on Performance Monitoring Regions Outside of the pMFC
Interference reliably activates the pMFC, anterior insula, IFG and parietal cortex in the mature brain (Roberts and Hall, 2009), and recent developmental neuroimaging work has provided evidence that “long range connections” between task-critical regions increase with age in youth (Fair et al., 2008; Stevens et al., 2007; Rubia et al., 2006 and 2007; Velanova et al., 2008). Among youth in our sample, interference-related activation was found to increase with age in the pMFC, but also in the left IFG and right anterior insula and, at trend levels, the right IFG, independent of performance. Age-related increases of pMFC and opercular (i.e., anterior insula and IFG) activations for interference are predicted by prior work demonstrating developmental increases of connectivity between these regions during response inhibition (Stevens et al., 2007) and at resting state (Fair et al., 2007). Outside of the pMFC, error-processing engaged the left IFG and superior parietal in both youth and adults, consistent with prior work (Taylor et al., 2007), however, no significant relation between age and activation in these regions was observed.
Performance accuracy was inversely associated with interference-related activation of the left anterior insula and, at a trend level, with error-related activation of the left IFG. The anterior insula and inferior frontal cortex comprise the more broadly defined anterior operculum, which has been repeatedly implicated in studies of performance monitoring (Roberts and Hall, 2009; Taylor et al. 2007), and plays a key role within the “frontal-opercular” network for task control (Dosenbach et al., 2008; Wager et al., 2005). Prior neuroimaging work has linked lower accuracy to insular activation (Paulus et al., 2008; Klein et al., 2007). The insula mediates conscious perception of changes in bodily states (Craig et al., 2002), leading Klein et al. (2007) to suggest that its engagement may enhance autonomic reactivity to errors which, in turn, could trigger negatively-valenced affect and degrade performance. Although future work will be needed to clarify the exact roles of these regions, they appear to be associated with the development of performance monitoring functions.
Limitations and Future Directions
Several limitations of the present study should be kept in mind. The MSIT was chosen because simple, small-value numbers are processed “automatically” by 6 years of age (Duncan and McFarland, 1980; Resnick et al., 1983), however, because incongruent trials involved 3 numbers compared to the only 1 number and 2 flankers for congruent trials, it is possible that task demands – unrelated to interference - may have been greater for incongruent trials, possibly impacting the developmental effects observed. A modified version of the MSIT has recently been developed to address this concern (Bush and Shin, 2006). Future work could also improve on the current study by including older adolescents and young adults; engagement of the pMFC by interference may continue to increase past 18 years – the age of our eldest youth participant - and further testing across late adolescence and early adulthood will be needed to determine whether the development of interference- and/or error-related pMFC function increases linearly with age and then plateaus (exponential function), or follows an inverted U-shaped curve (quadratic function). Our study was also limited in that adults performed at high levels of accuracy across a relatively narrow range, possibly leading to our failure to demonstrate increasing interference-related activation of the pMFC with declining performance (Durston et al., 2002). Future work could maximize interference demands among adults (and high performing youth) by parametrically modulating interference demands to test for a relationship between accuracy and pMFC response to interference. Future work eliciting higher error rates will also help to better elucidate the development of interactions between the pMFC response to errors, subsequent activation of the lateral PFC, and the adjustment of behavior to improve performance. In addition, more advanced analytic techniques, such as psychophyisologic interactions and dynamic causal modeling could be employed to characterize the development of pMFC integration with other task control regions during performance monitoring (Stevens, 2009).
Conclusion
Isolating the effects of age on pMFC performance monitoring function represents a challenge for developmental cognitive neuroscience since age-related changes in performance may confound changes in brain activation in youth compared to adults. In addition, the role of development in shaping pMFC contributions to the overlapping, but distinct performance monitoring functions of interference- and error-processing has only recently been addressed (Rubia et al., 2007; Velanova et al., 2008), and remains poorly understood. By examining correct and incorrect trials in separation, while covarying for performance, we have demonstrated age-related increases in pMFC activation to both interference and errors during development, followed by a plateau in adulthood. Greater pMFC response to errors was associated with better performance across both youth and adults and, on correct trials that followed an error, greater lateral prefrontal activation tended to associate with greater pMFC activation during errors. Taken together, these findings suggest that the maturation of the pMFC facilitates improvements in performance monitoring from childhood through adolescence, and may interact with lateral prefrontal cortex in the development of cognitive control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental Changes in Human Cerebral Functional Organization for Word Generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Buchsbaum, Greer, Chang, Berman Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Frontostriatal and Frontocerebellar Circuitry underlying Cognitive Control. In: Mayr U, Owh E, Keele SW, editors. Developing Individuality in the Human Brain. American Psychological Association; Washington, DC: 2005. [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowtiz SJ, Gavin WJ. Development of Error-Monitoring Event-Related Potentials in Adolescents. Annals of the New York Academy of Sciences. 2004;1021:324–28. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EM, McFarland CE., Jr Isolating the effects of symbolic distance and semantic congruity in comparative judgments: an additive-factors analysis. Memory and Cognition. 1980;8:612–22. doi: 10.3758/bf03213781. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Event-related potential correlates of errors in reaction tasks. Electroencephalography Clinical Neurophysiology Supplement. 1995;44:287–96. [PubMed] [Google Scholar]
- First MB, RLS, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician version: User’s guide. American Psychiatric Press; Washington, D.C: 1996. [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Zbrozek CD, Welsh RC, Britton JC, Liberzon I, Taylor SF. Pilot study of response inhibition and error processing in the posterior medial prefrontal cortex in healthy youth. J Child Psychol Psychiatry. 2008;49:986–994. doi: 10.1111/j.1469-7610.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable Executive Functions in the Dynamic Control of Behavior: Inhibition, Error Detection, and Correction. NeuroImage. 2002;17:1820–29. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Yeung N. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JDE. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JDE. Neural Activation During Response Competition. Journal of Cognitive Neuroscience. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Hester R, Barre N, Murphy K, Silk TJ, Mattingley JB. Human Medial Frontal Cortex Activity Predicts Learning from Errors. Cereb Cortex. 2008;18:1933–940. doi: 10.1093/cercor/bhm219. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Kirkham FJ, Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Dev Sci. 2005;8:525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kang E, Lee DS, Kang H, Lee JS, Oh SH, Lee MC, Kim CS. Age-associated changes of cerebral glucose metabolic activity in both male and female deaf children: parametric analysis using objective volume of interest and voxel-based mapping. Neuroimage. 2004;22:1543–1553. doi: 10.1016/j.neuroimage.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. NeuroImage. 2007;34:1774–781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Ann N Y Acad Sci. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal Brain Activity Related to Performance Monitoring and Error Detection in Children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF. A Brief Motivational Intervention for Treatment-Refusing OCD Patients. Cognitive Behaviour Therapy. 2005;34:176–184. doi: 10.1080/16506070510043741. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG. Error Rate and Outcome Predictability Affect Neural Activation in Prefrontal Cortex and Anterior Cingulate during Decision-Making. NeuroImage. 2002;15:836–846. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Lovero KL, Wittmann M, Leland DS. Reduced Behavioral and Neural Activation in Stimulant Users to Different Error Rates during Decision Making. Biological Psychiatry. 2008;63:1054–060. doi: 10.1016/j.biopsych.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13:160–66. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Rossi A, Japee S, Desimone R, Ungerleider LG. Attentional control during the transient updating of cue information. Brain Research. 2009;1247:149–158. doi: 10.1016/j.brainres.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, III, Xiong J, Liotti M. Neuroimaging of Inhibitory Control Areas in Children With Attention Deficit Hyperactivity Disorder Who Were Treatment Naive or in Long-Term Treatment. Am J Psychiatry. 2006;163:1052–060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Resnick LB. Mathematics and Science Learning: A New Conception. Science. 1983;4596:477–478. doi: 10.1126/science.220.4596.477. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a Supramodal Network for Conflict Processing: A Systematic Review and Novel Functional Magnetic Resonance Imaging Data for Related Visual and Auditory Stroop Tasks. Journal of Cognitive Neuroscience. 2008;20:1063–078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, Dick EL, Bagwell WW, Master FPM, Birmaher B. Corpus callosal morphology in treatment-naive pediatric obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1269–283. doi: 10.1016/s0278-5846(97)00163-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, Woolley, Nosarti, Heyman, Taylor, Brammer Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Stenger VA, Boada FE, Noll DC. Multishot 3D slice-select tailored RF pulses for MRI. Magn Reson Med. 2002;48:157–165. doi: 10.1002/mrm.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Lennert T. BOLD signal in intraparietal sulcus covaries with magnitude of implicitly driven attention shifts. NeuroImage. 2009;45:1314–328. doi: 10.1016/j.neuroimage.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Stahl J, Gibbons H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: An electrophysiological investigation using a stop-signal task. Clinical Neurophysiology. 2007;118:581–596. doi: 10.1016/j.clinph.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Stern ER, Welch RC, Fitzgerald KD, Taylor SF. Topographic analysis of individual activation patterns in medial frontal cortex in schizophrenia. Human Brain Mapping. 2009;30:2146–156. doi: 10.1002/hbm.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70(1):1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of Specific Interference Processing in the Stroop Task: PET Activation Studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial Frontal Cortex Activity and Loss-Related Responses to Errors. J Neurosci. 2006;26:4063–070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Ursu S. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE. Toward a taxonomy of attention shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:127–143. doi: 10.3758/cabn.5.2.127. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. 2006;9:971–78. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;54(8):1649–57. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. Neuroimage. 1997;6:305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig DA, Stern E. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magn Reson Med. 2002;48:278–289. doi: 10.1002/mrm.10196. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]