Abstract
Few randomized trials attempt to improve insulin sensitivity and associated metabolic risks in overweight Latino youth. The purpose of this study is to examine the effects of a modified carbohydrate nutrition program combined with strength training on insulin sensitivity, adiposity, and other type 2 diabetes risk factors in overweight Latino adolescents. In a 16-week randomized trial, 54 overweight Latino adolescents (15.5 ± 1.0 years) were randomly assigned to: (i) Control (C; n = 16), (ii) Nutrition (N; n = 21), or (iii) Nutrition + Strength training (N+ST; n = 17). The N group received modified carbohydrate nutrition classes (once per week), while the N+ST received the same nutrition classes plus strength training (twice per week). The following were measured at pre- and postintervention: strength by 1-repetition maximum, dietary intake by 3-day records, body composition by dual-energy X-ray absorptiometry, glucose/insulin indices by oral glucose tolerance test (OGTT) and intravenous glucose tolerance test with minimal modeling. Across intervention group effects were tested using analysis of covariance with post hoc pairwise comparisons. A significant overall intervention effect was found for improvement in bench press (P < 0.001) and reductions in energy (P = 0.05), carbohydrate (P = 0.04) and fat intake (P = 0.03). There were no significant intervention effects on insulin sensitivity, body composition, or most glucose/insulin indices with the exception of glucose incremental area under the curve (IAUC) (P = 0.05), which decreased in the N and N+ST group by 18 and 6.3% compared to a 32% increase in the C group. In conclusion, this intense, culturally tailored intervention resulted in no significant intervention effects on measured risk factors with the exception of a beneficial effect on glycemic response to oral glucose.
INTRODUCTION
Latino youth are more likely to be at risk of overweight than white children, with 40.5 and 37.1% of Latino boys and girls (aged 12–19 years), respectively, being overweight (≥85th CDC percentile) compared to 34.5 and 31.7% of white children being overweight (1,2). Prediabetes and type 2 diabetes have emerged as significant health issues in overweight adolescents, especially among certain ethnic groups including Latinos (3). Our group has shown that over 30% of overweight Latino children (aged 8–14 years) in the Los Angeles area have prediabetes (4,5) and the metabolic syndrome (6). While there are numerous interventions aimed at decreasing obesity (7-9), few randomized trials address the underlying metabolic abnormalities, specifically insulin resistance, in overweight Latino youth.
There is evidence that modifying the quality of carbohydrates in the diet may be effective for reducing obesity and improving glucose control (10,11). We have previously shown in overweight Latino youth (aged 19–17 years) population that high intakes of total added sugar and sugary beverages were the only dietary components associated with adiposity and poor β-cell function (10,12). We also recently showed that in a 12-week, modified, carbohydrate nutrition education pilot study called ALAS (Adolescent Latinas Adjusting Sugar) conducted with overweight Latina girls (aged 12–17 years), resulted in a 5% reductions in BMI z scores and those girls with the greatest reductions in added sugar intake had a 7% improvement in insulin secretion (13,14). Therefore, a modified carbohydrate intervention has the potential to reduce type 2 diabetes risks in overweight youth who have insulin resistance.
Recently, strength training has also been linked to improvements in insulin sensitivity in adults (15,16). Poehlman et al. (16) showed that a 6-month strength training study resulted in a 10% improvement in insulin sensitivity in nonobese young women (18–35 years). While the study by Ishii et al. (15) showed that an intensive 6-week strength training intervention improved insulin sensitivity by 48% in sedentary nonobese type 2 diabetic adults (35–60 years). To our knowledge, we were the first to demonstrate through a 16-week randomized pilot study called STEALTH (Strength Training Exercise for Adolescent Latinos To improve Health) that strength training significantly increased insulin sensitivity by 45% in overweight Latino adolescent males (aged 12–17 years), independent of changes in body composition (17). However, this pilot study did not include nutrition education and was conducted only in boys.
The purpose of this study was to therefore conduct a 16-week randomized control trial to examine the incremental effects of combining a modified carbohydrate nutrition education program like ALAS with the strength training regimen like STEALTH in high-risk overweight Latino boys and girls. There were three intervention groups: (i) control, (ii) a culturally tailored, modified carbohydrate nutrition education program once per week, and (iii) the same nutrition education program with twice per week strength training. We hypothesized that the combination group would have the greatest reductions in adiposity and the greatest increases in insulin sensitivity compared to the nutrition only and control groups.
METHODS AND PROCEDURES
Participants
Study participants satisfied the following criteria for inclusion: ageand gender-specific BMI ≥85th percentile (18), Latino ethnicity (i.e., parents and grandparents of Latino descent by parental self report), and grades 9th through 12th (14–18 years of age). Participants were excluded based on the following criteria: (i) were using medication or were diagnosed with any disease that could influence dietary intake, exercise ability, body composition, or insulin indices; (ii) were previously diagnosed with any major illness since birth; (iii) had any diagnostic criteria for diabetes; or (iv) participated in structured exercise, nutrition, or weight loss program in the past 6 months. For logistical reasons, intervention groups were gender-specific. Prior to any testing procedure, informed written consent from parents and assent from the children were obtained. This study was approved by the Institutional Review Board of the University of Southern California, Health Sciences Campus.
Outpatient visit
Participants arrived at the General Clinical Research Center (GCRC) at ~7:30 am after an overnight fast. A licensed pediatric health care provider conducted a detailed medical history exam and determined Tanner staging using established guidelines (19,20). Following the exam, a 3-h oral glucose tolerance test (OGTT) was conducted. This test included the application of a topical anesthetic to one arm and ~30 min later a flexible intravenous catheter was placed in an antecubital vein. Subjects then ingested 1.75 g oral glucose solution/kg body weight (to a maximum 75 g). Blood samples were drawn at baseline and every 10 min for 3 h (a total of 18 samples) and were assayed for glucose, insulin, and c-peptide. Fasting and 2-h glucose levels were used to determine normal or impaired glucose tolerance as defined by the American Diabetes Association (3). Three-hour insulin area under the curve (AUC) and incremental insulin area under the curve (IAUC) were calculated from the OGTT data, in nmol/l/min.
Anthropometry and body composition
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a beam medical scale and wall-mounted stadiometer. BMI and BMI percentiles were determined (CDC, 2000). Whole body fat and soft lean tissue was measured by dual-energy X-ray absorptiometry using a Hologic QDR 4500W (Hologic, Bedford, MA).
In-patient visit
Approximately 7–14 days following the outpatient visit, participants were admitted to the GCRC and served a standardized dinner and an evening snack. An insulin-modified frequently sampled intravenous glucose tolerance test (FSIVGTT) (21) was performed the following morning. At time 0, glucose (25% dextrose, 0.3 g/kg body weight) was administered intravenously. Blood samples were collected at time points −15, −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min. Insulin (0.02 units/kg body weight, Humulin R (regular insulin for human injection); Eli Lilly, Indianapolis, IN) was injected intravenously at 20 min. Plasma collected during the FSIVGTT was analyzed for glucose and insulin, and values were entered into the MINMOD Millenium 2003 computer program (version 5.16, Bergman, USC) to determine insulin sensitivity, acute insulin response, and disposition index (an index of β-cell function).
Assays
Fasting and 2-h samples taken during the OGTT for clinical diagnosis were separated for plasma and immediately transported on ice to the Los Angeles County–USC Medical Center Core Laboratory where glucose was analyzed on a Dimension clinical chemistry system using an in vitro hexokinase method (Dade Behring, Deerfield, IL). Blood samples from all time points taken during the OGTT and FSIVGTT were centrifuged immediately for 10 min at 2500 rpm and 8–10 °C to obtain plasma, and aliquots were frozen at −70 °C until assayed. Glucose was assayed in duplicate on a Yellow Springs Instrument 2700 Analyzer (Yellow Springs Instrument, Yellow Springs, OH) using the glucose oxidase method. Insulin was assayed in duplicate using a specific human insulin enzyme-linked immunosorbent assay kit from Linco (St. Charles, MO).
Dietary intake and strength assessment
At the outpatient visit, participants were given 3-day diet records to complete at home before and after the intervention and were instructed to bring them to their next GCRC visit. Participants were given a short lesson (10 min) on how to estimate portion sizes and complete the diet records and were given measuring cups and rulers to aid in accurate reporting. At the in-patient visit, research staff clarified all dietary records. Nutrition data were analyzed using the Nutrition Data System for Research (NDS-R version 5.0_35).
Upper- and lower-body strength were assessed before and after intervention by 1 repetition maximum (1-RM) in the bench press and leg press, respectively, using established procedures (22).
Randomization
Allocations were concealed from participants until after their in-patient visit. Sixty-six participants were randomized to one of three groups. Randomization was blocked by gender to achieve balance in randomization between genders. Siblings (five pairs) were randomized into the same experimental group (one in C, two in N, and two in N+ST), and data from both siblings was used.
Description of interventions
Nutrition education only (N)
Participants in the N group reported to the Veronica Atkins Lifestyle Intervention Laboratory once per week (~90 min) for 16 weeks for a culturally tailored dietary intervention. This dietary intervention was modified from a pilot study and specifics are described in detail elsewhere (13,14). In brief, the dietary intervention targeted two goals: (i) ≤10% of total daily calorie intake from added sugar; and (ii) consuming at least 14 g/1,000 kcal of dietary fiber a day. Participants received four individual motivational interviewing (MI) sessions throughout the 16-week program by trained research staff. MI is a client-centered counseling approach designed to enhance intrinsic motivation for behavior change by creating, exploring, and resolving ambivalence toward changing behaviors and habits (23). Participants were given $25 grocery gift cards each week and transportation was provided to and from the classes if needed. Parent(s) were asked to attend a minimum of four classes and were taught the same curriculum separately from the participants.
Nutrition education plus strength training (N+ST)
In addition to the nutrition education class described above, participants in the N+ST group also received strength training twice per week (~60 min/session) for 16 weeks at Veronica Atkins Lifestyle Intervention Laboratory. Training sessions took place on 2 nonconsecutive days per week and the nutrition education took place once per week. The exercise program has been explained in detail elsewhere (17). In brief, the program was personalized and progressive such that the resistance increased as the participant's technique and strength improved. One day a week focused on compound lower body exercises (i.e., leg press and dead lift) and isolated upper body exercises (i.e., biceps curl, triceps extension, and shoulder press) and the other training day included the reverse (i.e., bench press, lat pull-down, leg extension, leg curl, and calf raises). A 3:1 child/trainer ratio was employed. In addition, the N+ST received a minimum of four group MI sessions by trained research staff in order to enhance intrinsic motivation for physical activity. Participants were also given $25 grocery gift cards each week and transportation to and from the classes was provided if needed.
Control group (C)
Participants randomized to the C group received no intervention between pre and postintervention data collection. After post-testing, participants were offered an abbreviated 1-month intervention, consisting of biweekly nutrition and strength training classes. No testing was done after the abbreviated intervention.
Post-testing procedures
At the conclusion of the 16-week intervention/control period, participants returned to the GCRC for follow-up testing. The procedures were identical to those previously described in the outpatient and in-patient visits. The FSIVGTT test took place within 1 week of the last nutrition class and within 48–72 h after the final training session for the N+ST group to minimize the acute effects of the last training bout on insulin action and glucose homeostasis.
Statistical considerations
Sample size considerations
Sample size calculations were based on results from the STEALTH pilot study (17). Using a conservative estimate of the standard deviation of the insulin sensitivity change score, a sample size of 60 (20/group) had 0.80 power to detect a meaningful difference >0.57 units in mean change in insulin sensitivity between the C and N+ST groups.
Statistical analyses
Across-group comparisons of baseline characteristics were conducted for evaluable participants using ANOVA to identify possible randomization imbalance. Except for 2-h insulin during the OGTT, no differences were found. Subsequent intent to treat analyses found no differences in OGTT related outcomes with and without controlling for baseline OGTT 2-h insulin as a potential confounder.
The overall effects of the interventions were tested for evaluable participants using analysis of covariance on the post-prechange score, after controlling for pretest values and preplanned covariates. Change scores were evaluated for normality and transformations were made if needed. Covariates included age and gender, as well as outcome variable-specific covariates based on theoretical and biological decisions. When significant differences across groups were identified, post hoc pairwise comparisons with Bonferroni adjustments were conducted. Statistical analyses were carried out at the 0.05 level using the Statistical Analysis System 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
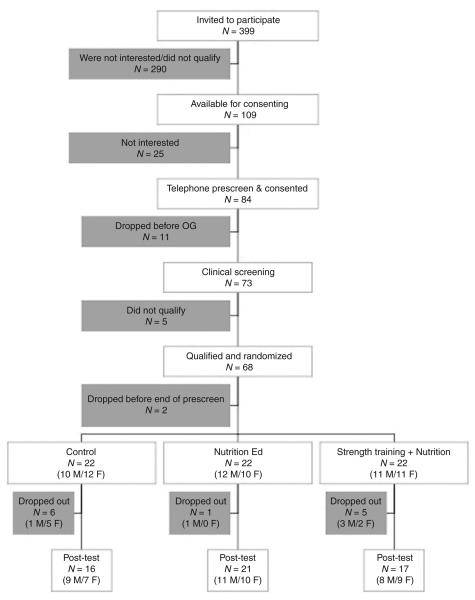
Figure 1 shows the flow of participants through the study. Of the 109 potential subjects (aged 14 to 18 years) who were available for consenting, 68 qualified subjects were randomized to one of the three groups. Of these, 54 completed the program (evaluable participants), 16 in the C group, 21 in the N group, and 17 in the N+ST group. There were no statistically significant differences in baseline demographics, anthropometrics, or body composition measures between the 12 participants who dropped out of the program and the 54 participants who completed the program.
Figure 1.
Consort diagram of participant flow (screening, randomization, completion of study).
Evaluable participants in the intervention groups attended a minimum of 12 of the 16-week nutrition classes and a minimum of 28 of the 32 exercise classes. Evaluable participants in the N and N+ST groups received a minimum of four individual MI sessions and participants in the N+ST also received a minimum of four group MI sessions.
Participants were 15.5 ± 1.0 years of age, had a Tanner stage of 4.5 ± 0.8 and 52% were male. There were no significant group differences across the intervention groups in gender, baseline age, weight, BMI, BMI z score, BMI percentile, or Tanner stage for the evaluable participants (all P > 0.32).
Strength and dietary variables
Table 1 presents the strength and dietary outcomes for the evaluable participants stratified by intervention group. There was a significant across group effect for bench press (P < 0.001). Post hoc comparisons showed that the N+ST increased more than the C group (27 ± 2% vs. 12 ± 7%; P = 0.004) and the N group (27 ± 2% vs. 2 ± 3%; P < 0.001).
Table 1.
Strength and dietary outcomes: across groups effects for evaluable participants (N = 54)
| Control (C) (n = 16) |
Nutrition education (N) (n = 21) |
Nutrition + strength training (N+ST) (n = 17) |
|||||
|---|---|---|---|---|---|---|---|
| Outcomesa | Pretest | Post-test | Pretest | Post-test | Pretest | Post-test | P valueb |
| Strength | |||||||
| Bench press (kg) | 42.5 (13.0) | 47.7 (13.9)c | 42.6 (11.9) | 43.5 (11.6)c | 44.3 (18.6) | 56.1 (19.0)d | <0.001 |
| Leg press (kg) | 233.0 (87.4) | 255.4 (116.9) | 232.4 (95.8) | 226.8 (66.0) | 216.6 (91.8) | 271.5 (107.3) | 0.06 |
| Dietary | |||||||
| Energy (kcals/d) | 1,957.7 (721.9) | 2,146.6 (987.0)c | 1,954.5 (678.8) | 1,752.1 (616.0)c | 1,788.1 (455.9) | 1,436.1(472.3)d | 0.05 |
| Carbohydrate (g/d) | 262.9 (94.2) | 282.0 (133.2)c | 256.3 (107.2) | 234.0 (92.3)c | 226.4 (61.1) | 185.1 (57.9)d | 0.04 |
| Protein (g/d) | 69.7 (29.5) | 80.0 (39.1) | 76.4 (22.2) | 71.7 (24.5) | 73.8 (23.5) | 65.0 (27.3) | 0.50 |
| Fat (g/d) | 72.6 (34.4) | 80.3 (43.7)c | 71.7 (26.3) | 61.5 (23.8)c | 67.4 (24.9) | 51.3 (22.9)d | 0.03 |
| Total sugar (g/d) | 120.9 (52.8) | 118.5 (65.7) | 111.1 (58.8) | 101.0 (55.6) | 102.2 (35.3) | 87.1 (40.6) | 0.70 |
| Added sugar (g/d) | 92.6 (53.7) | 84.2 (49.8) | 75.2 (48.9) | 57.6 (49.4) | 59.7 (30.0) | 50.4 (29.2) | 0.62 |
| Fiber (g/d) | 14.2 (4.8) | 17.1 (7.4) | 16.8 (9.9) | 17.9 (7.0) | 14.8 (4.8) | 15.2 (7.9) | 0.52 |
Data are mean (s.d.). Sample sizes for dietary variables are as follows: C (n = 14); N (n = 20); N+ST (n = 15).
P values were calculated using analysis of covariance. Covariates included: age, sex, dual-energy X-ray absorptiometry fat and lean tissue mass. Dietary variables included calories not attributed to nutrient-dependent variable as a covariate. Analyses were based on log scores for the following variables: protein, fat, total sugar, added sugar, and fiber.
Means with different letters (c and d) across intervention groups are signifcantly different from one another using Bonferroni multiple comparisons (P < 0.05).
Means with different letters (c and d) across intervention groups are signifcantly different from one another using Bonferroni multiple comparisons (P < 0.05).
There was an across group effect for total energy intake (P ≤ 0.05), the N+ST group decreased their energy intake by 20 ± 4% compared to the C group that increased their energy intake by 10 ± 3% (P = 0.04). The N group had a 10 ± 9% decrease in energy intake, although not significant from the other two groups. There was a significant across group effect for carbohydrate intake (P = 0.04), the N+ST group decreased by 18 ± 5% compared to the C group that increased by 7 ± 4% (P = 0.03). The N group decreased carbohydrate intake by 9 ± 14%, but this was not significantly different from the other two groups. There was also a significant across group effect for dietary fat (P = 0.03), the N+ST group decreased by 24 ± 8% compared to the C group that increased by 11 ± 3% (P = 0.03). The N group decreased dietary fat by 14 ± 9%, although not significantly different from the other groups. There were no significant across group effects for dietary protein, total or added sugar or dietary fiber intake.
When stratified by gender (data not shown), there were several across group effects for strength and dietary outcomes. For both genders there was an across group effect for bench and leg press. For males, the N group had only a 3 ± 4% increase in bench press compared to the 23 ± 6% increase seen in the N+ST group (P < 0.001). For females, the N group had a 5 ± 6% decrease in bench press compared to the 40 ± 23% increase seen in the N+ST group (P < 0.001). Additionally, C females did significantly better (a 12 ± 2% increase) on bench press than N females (P = 0.02).
For males only, there was a significant across group effect for carbohydrate intake (P = 0.01), the N group decreased by 13 ± 18% compared to N+ST who decreased by only 9 ± 18% (P = 0.03). For females only, there was a significant across group effect for added sugar (P = 0.05); the C females reported a 4 ± 4% decrease in added sugar intake compared to the reported 16 ± 3% decrease by the N females (P < 0.001).
Anthropometric and body composition
Anthropometric and body composition (dual-energy X-ray absorptiometry) data, across group effects are presented in Table 2. There were no significant across group difference for BMI, BMI z score, BMI percentile, or body weight (kg). There were also no significant across group effects for total fat mass, lean mass, or % body fat. However, for females (data not shown), there was a significant across group effect for weight (P = 0.03), with the C group increasing by 1.3 ± 1.4% and the N+ST group increasing by 0.6 ± 1.5% (P = 0.02). The N group increased by 1.1 ± 1.3%, although not significantly different from the other groups.
Table 2.
Anthropometric and body composition (DXA): across groups effects
| Control (C) (n = 16) |
Nutrition education (N) (n = 21) |
Nutrition + strength training (N+ST) (n = 17) |
|||||
|---|---|---|---|---|---|---|---|
| Outcomesa | Pretest | Post-test | Pretest | Post-test | Pretest | Post-test |
P valueb |
| BMI (kg/m2) | 33.7 (8.5) | 33.9 (8.3) | 32.3 (6.0) | 32.2 (6.1) | 34.9 (6.8) | 34.9 (6.3) | 0.74 |
| BMI z -score | 2.1 (0.7) | 2.1 (0.6) | 2.0 (0.5) | 2.0 (0.5) | 2.2 (0.6) | 2.2 (0.5) | 0.39 |
| BMI percentile | 95.5 (7.2) | 96.2 (5.0) | 96.2 (4.2) | 95.7 (5.5) | 97.0 (3.8) | 97.4 (3.3) | 0.16 |
| Weight (kg) | 93.0 (25.8) | 93.6 (26.0) | 87.9 (18.4) | 88.0 (18.0) | 95.3 (24.8) | 95.0 (21.3) | 0.86 |
| Total fat (kg) | 34.5 (16.9) | 34.4 (14.7) | 31.7 (10.4) | 31.6 (10.0) | 36.7 (13.1) | 35.4 (11.1) | 0.66 |
| Total lean (kg) | 54.7 (9.5) | 55.8 (11.7) | 53.8 (0.8) | 53.8 (9.2) | 55.5 (11.4) | 56.6 (11.4) | 0.27 |
Data are mean (s.d.).
P values were calculated using analysis of covariance. Covariates included: age and sex for all; dual-energy X-ray absorptiometry (DXA) fat was adjusted for lean, and lean for fat. While raw scores are reported here for all variables, analyses were based on log scores for weight and total fat. For BMI percentile, a transformation involving ln(highest value + 1)-Y was used.
Glucose and insulin indices
The group effects for glucose and insulin indices from the OGTT and FSIVGTT are presented in Table 3. There was a significant across group effect for glucose IAUC (P = 0.05), with N group decreasing by 17 ± 19% compared to the C group that increased by 29 ± 19% (P = 0.05). The N+ST group also improved (reduction of 6 ± 4%), although not significantly different from the other groups. There were no other significant across group effects for fasting and 2-h glucose and insulin, insulin IAUC, or for insulin sensitivity, acute insulin response, or disposition index measured by the FSIVGTT.
Table 3.
Glucose and insulin dynamics from OGTT and FSIVGTT: across groups effects for evaluable participants (N = 54)
| Control (C) (n = 16) |
Nutrition education (N) (n = 21) |
Nutrition + strength training (N+ST) (n = 17) |
|||||
|---|---|---|---|---|---|---|---|
| Outcomesa | Pretest | Post-test | Pretest | Post-test | Pretest | Post-test | P valueb |
| OGTT (3-h) | |||||||
| Fasting glucose (mg/dl) | 93.7 (7.1) | 88.7 (8.0) | 92.2 (6.1) | 91.4 (6.5) | 90.9 (7.3) | 88.5 (8.6) | 0.11 |
| 2-h Glucose (mg/dl) | 120.0 (25.3) | 120.9 (32.3) | 134.6 (18.5) | 117.6 (24.6) | 127.6 (30.3) | 124.3 (17.5) | 0.16 |
| Fasting insulin (μU/ml) | 27.1 (17.3) | 26.1 (19.7) | 26.1 (16.3) | 24.3 (14.5) | 31.7 (10.6) | 27.8 (12.5) | 0.82 |
| 2-h Insulin (μU/ml) | 132.7 (91.4) | 164.5 (183.5) | 249.9 (204.3) | 169.0 (259.6) | 184.6 (108.1) | 150.9 (92.5) | 0.23 |
| Glucose IAUC (nmol/min/l) | 80.7 (50.7) | 103.9 (60.4)c | 114.1 (41.6) | 94.8 (49.4)d | 98.2 (58.3) | 91.9 (56.0)c | 0.05 |
| Insulin IAUC (nmol/min/l) | 309.4 (178.0) | 338.4 (281.2) | 459.3 (319.5) | 368.3 (386.6) | 405.0 (279.1) | 302.6 (159.8) | 0.18 |
| HOMA | 6.2 (3.8) | 5.9 (4.8) | 6.1 (4.2) | 5.5 (3.3) | 7.1 (2.5) | 6.1 (3.0) | 0.71 |
| FSIVGTT | |||||||
| SI (×10−4/min/μU/ml) | 1.8 (1.2) | 1.9 (1.4) | 1.6 (0.9) | 1.8 (0.8) | 1.5 (0.9) | 1.5 (0.8) | 0.47 |
| AIR (μU/ml × 10/min) | 1,308.5 (930.9) | 1,404.8 (1,119.7) | 1,160.4 (869.9) | 1,222.8 (994.2) | 1,530.0 (870.4) | 1,607.0 (959.2) | 0.83 |
| DI (×10−4/min) | 1,944.4 (1,071.3) | 1,797.4 (1,038.1) | 1,297.1 (596.7) | 1,723.0 (898.3) | 1,832.6 (808.8) | 2,002.9 (994.3) | 0.43 |
AIR, acute insulin response; FSIVGTT, frequently sampled intravenous glucose tolerance test; HOMA, homeostasis model assessment; OGTT, oral glucose tolerance test.
Data are mean (s.d.). Sample sizes for insulin sensitivity (SI) and disposition index (DI) for the control group was n = 15.
P values were calculated using analysis of covariance. Covariates included: age, sex, dual-energy X-ray absorptiometry fat and lean tissue mass. AIR included SI as a covariate. While raw scores are reported here for all variables, analyses were based on log scores for all variables except fasting glucose.
Means with different letters (c and d) across intervention groups are signifcantly different from one another using Bonferroni multiple comparisons (P < 0.05).
Means with different letters (c and d) across intervention groups are signifcantly different from one another using Bonferroni multiple comparisons (P < 0.05).
For females (data not shown), there was a significant across group effect for OGTT fasting glucose (P = 0.05); the N group increased by 0.6 ± 2.2 mg/dl and N+ST group decreased by 1.1 ± 1.3 mg/dl compared to the C group that decreased by 3.8 ± 2.1 mg/dl (P = 0.05).
DISCUSSION
We hypothesized that strength gains in the N+ST group, independent of changes in body composition, would result in significant improvements in insulin sensitivity, similar to what we saw in our pilot study (17). Although we saw similar strength gains as in our pilot, ~30% (17), these strength gains did not translate into improvement in insulin sensitivity. Other adult studies have shown that 10–30% increases in strength resulted in 10–50% increases in insulin sensitivity (15,16), which suggests that although strength gains are associated with increases in insulin sensitivity, there is no clear dose-dependent effect of strength gains on insulin sensitivity. However, these adult studies did not include a dietary component and it is possible that the addition of the dietary component in our study diluted the effects.
We hypothesized that changes in carbohydrate quality in the N and N+ST group would result in reductions in adiposity and improvements in insulin secretion, similar to our ALAS pilot study (13,14). Numerous cross-sectional studies have shown that fiber and/or sugar intake are inversely associated with glycemic control and poor insulin action (12,24-26); however, few intervention studies have been conducted that focus on reducing added sugar and increasing dietary fiber. A study by Ebbeling et al. (27) found that a low glycemic load diet improved insulin resistance compared to a conventional low-fat diet in 16 overweight adolescents (aged 13–21 years). In this study, while neither intervention group significantly decreased added sugar or increased fiber intake, there were significant reductions in overall carbohydrate intake. The reductions in carbohydrate could, in part, explain the significant decrease in the glucose IAUC. In addition, females in the intervention groups did not gain as much weight compared to the C group, which suggests that reduction in carbohydrate intake, regardless the quality, may have contributed to less weight gain in females but not males, similar to what we saw with the ALAS pilot. These findings also highlight the need to further explore the response to intervention as a function of dietary compliance.
It is important to note that there were several improvements in insulin indices across intervention group, although not significant. The N and N+ST group decreased 2-h insulin by 32 and 18% compared to a 24% increase in the C group. Insulin IAUC also improved, with the N and N+ST decreasing by 20 and 25%, while the C group increased by 9%, although not significant. With a larger sample size, these improvements in insulin indices could have reached significance.
There are several possible explanations for the null findings in insulin sensitivity and adiposity parameters of this study. Although we hypothesized that the different interventions would be additive, intervening on multiple behaviors may not be optimal. Research suggests that the number of behavior targets is inversely related to the magnitude of the intervention effects for obesity (28,29). Specifically, an intensive nutrition and strength training program may have attempted to change too many health behaviors and therefore diluted the potential effects. Although little is known about the effects of energy compensation in response to exercise in children, research in adults has shown that when individuals exercise they often compensate with increased energy intake (30) or reduced physical activity outside of the training session (31) and this compensation is even more pronounced in females (32,33). Thus, the participants in the N+ST group may have altered their intake in response to the exercise throughout the program. Subsequently, the diet records collected after the intervention would not really reflect this acute compensatory intake.
Alternatively, the dose and the duration of the strength training intervention, two times a week for 16 weeks, may not have been frequent or long enough to see improvements in health outcomes. The American Academy of Pediatrics, Council on Sports Medicine and Fitness, recommends a frequency of 2–3 days a week of strength training for at least an 8-week duration (34). A 20-week intervention study conducted in obese, prepubertal girls showed that strength training three times per week resulted in improvements in intra-abdominal adipose tissue and insulin indices (35). Although, strength training two times per week for 16 weeks in STEALTH program resulted in improvements in insulin sensitivity in males, strength training 3 days/week for 20 weeks may be more optimal for females.
In secondary analyses and subsequent papers, we intend to assess whether there are differences in compliance across intervention group. Although participants attended at least 12 of the 16 nutrition classes and at least 28 of the 32 exercise sessions, we may stratify the participants into lower (12–14 classes) vs. more (15–16 classes) in order to assess changes in health outcomes. We also intend to evaluate compliance in regards to dietary intake strength and/or physical activity levels in subsequent analyses. We are currently examining whether dietary compliers, those who reduced sugar intake and increased dietary fiber, had more improvements in insulin indices and adiposity parameters compared to dietary noncompliers. In future analyses, we will also assess whether participants who increased their strength and physical activity levels had greater improvements in adiposity and metabolic parameters compared to those who decreased their strength and physical activity levels. In addition, the motivation of the participants must also be considered. Motivational questionnaires that were administered pre- and postintervention will be useful in identifying the potential mediating role of motivational factors on health outcomes.
One potential limitation to consider relates to the small sample size. Although, the power calculation showed that we would be able to detect significant differences in change in insulin sensitivity across intervention groups, this calculation was based on pilot data from only boys and the intervention did not include a nutrition component. In addition, this study was not powered to detect differences in gender and a larger sample size would have allowed for better exploration of gender differences across groups.
In conclusion, this intensive, randomized control trial, designed specifically for overweight Latino teenagers based on previous successful pilot studies, did not result in the expected adiposity and metabolic improvements. These findings highlight the need for either different or stricter dietary (i.e., more aggressive modification of carbohydrate intake) and exercise (i.e., increased dose, duration, and intensity and different modality) approaches to elicit improvements in adiposity and metabolic parameters in this high-risk population. These findings also emphasize the need to further investigate the intervention response as a function of dietary, strength, and physical activity compliance, the potential mediating effect of motivation, and whether intervening on multiple health behaviors is optimal.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Cancer (NCI), University of Southern California Center for Transdisciplinary Research on Energetics and Cancer (U54 CA 116848), the National Institute of Child Health and Human Development (RO1 HD/HL 33064), the Dr Robert C. and Veronica Atkins Foundation, the National Cancer Institute (Cancer Control and Epidemiology Research Training Grant, T32 CA 09492) and the M01 RR 00043 from NCRR/NIH. We thank the SANO LA (Strength and Nutrition Outcomes for Latino Adolescents) team as well as the nursing staff at the GCRC. In addition, we are grateful for our study participants and their families for their involvement.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents. JAMA. 2008;299:2442–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Krebs N, Himes J, Jacobson D, et al. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120:193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 3.ADA Type 2 diabetes in children and adolescents. Pediatrics. 2000;105:671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 4.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Bergman RN, Avilla Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 6.Cruz ML, Weigensberg MJ, Huang T, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 7.Caballero B, Clay T, Davis SM, et al. Pathways: a school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am J Clin Nutr. 2003;78:1030–1038. doi: 10.1093/ajcn/78.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gortmaker SL, Peterson K, Wiecha J, et al. Reducing obesity via a school-based interdisciplinary intervention among youth: Planet Health. Arch Pediatr Adolesc Med. 1999;153:409–418. doi: 10.1001/archpedi.153.4.409. [DOI] [PubMed] [Google Scholar]
- 9.Pangrazi RP, Beighle A, Vehige T, Vack C. Impact of Promoting Lifestyle Activity for Youth (PLAY) on children's physical activity. J Sch Health. 2003;73:317–321. doi: 10.1111/j.1746-1561.2003.tb06589.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis J, Ventura E, Weigensberg M, et al. The relation of sugar intake to beta-cell function in overweight Latino children. Am J Clin Nutr. 2005;82:1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffen LM, Jacobs DR, Stevens J, et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- 12.Davis JN, Alexander KE, Ventura EE, et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr. 2007;86:1331–1338. doi: 10.1093/ajcn/86.5.1331. [DOI] [PubMed] [Google Scholar]
- 13.Davis JN, Ventura EE, Shaibi GQ, et al. Reduction in added sugar intake and improvement in insulin secretion in overweight Latina Adolescents. Met Syn Rel Dis. 2007;5:183–193. doi: 10.1089/met.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JN, Ventura EE, Alexander KA, et al. Development and testing of a culturally tailored nutrition education program for reducing sugar and increasing fiber intake in overweight Latina adolescents. Int J Ped Obes. 2007;2:22–30. [Google Scholar]
- 15.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21:1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- 16.Poehlman ET, Dvorak R, Denino W, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85:2463–2468. doi: 10.1210/jcem.85.7.6692. [DOI] [PubMed] [Google Scholar]
- 17.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38:1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention, Department of Health and Human Services Resource Guide for Nutrition and Physical Activity Interventions to Prevent Obesity and Other Chronic Diseases. Available at: < http://www.cdc/gov/nccdphp/dnpa/obesity/state_programs/index.htm>. Accessed September 14, 2007.
- 19.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutfield W, Bergman R, Menon R, Sperling M. The modified minimal model: application to measurement of insulin sensitivity in children. JCEM. 1990;70:1644–1650. doi: 10.1210/jcem-70-6-1644. [DOI] [PubMed] [Google Scholar]
- 22.Faigenbaum AD, Milliken LA, Westcott WL. Maximal strength testing in healthy children. J Strength Cond Res. 2003;17:162–166. doi: 10.1519/1533-4287(2003)017<0162:mstihc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd edn. The Guilford Press; New York: 2001. [Google Scholar]
- 24.Liese AD, Schulz M, Fang F, et al. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2832–2838. doi: 10.2337/diacare.28.12.2832. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig D, Pereira M, Kroenke C, et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282:1539–1546. doi: 10.1001/jama.282.16.1539. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig DS, Majzoub JA, Al-Zahrani A, et al. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:E26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 27.Ebbeling C, Leidig M, Sinclair K, Hangen J, Ludwig D. A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157:773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 28.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132:667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnicow K, Robinson TN. School-based cardiovascular disease prevention studies: Review and synthesis. Ann Epidemiol. 1997;7:S14–S31. [Google Scholar]
- 30.Tremblay A, Despres JP, Bouchard C. The effects of exercise-training on energy balance and adipose tissue morphology and metabolism. Sports Med. 1985;2:223–233. doi: 10.2165/00007256-198502030-00005. [DOI] [PubMed] [Google Scholar]
- 31.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263:E950–E957. doi: 10.1152/ajpendo.1992.263.5.E950. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev. 2005;33:169–174. doi: 10.1097/00003677-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Pomerleau M, Imbeault P, Parker T, Doucet E. Effects of exercise intensity on food intake and appetite in women. Am J Clin Nutr. 2004;80:1230–1236. doi: 10.1093/ajcn/80.5.1230. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics. Council on Sports Medicine and Fitness Strength training by children and adolescents. Pediatrics. 2008;121:835–840. doi: 10.1542/peds.2007-3790. [DOI] [PubMed] [Google Scholar]
- 35.Treuth MS, Hunter GR, Figueroa-Colon R, Goran MI. Effects of strength training on intra-abdominal adipose tissue in obese prepubertal girls. Med Sci Sports Exerc. 1998;30:1738–1743. doi: 10.1097/00005768-199812000-00013. [DOI] [PubMed] [Google Scholar]