Abstract
The V2 vasopressin receptor gene contains an alternative splice site in exon-3, which leads to the generation of two splice variants (V2a and V2b) first identified in the kidney. The open reading frame of the alternatively spliced V2b transcripten codes a truncated receptor, showing the same amino acid sequence as the canonical V2a receptor up to the 6th transmembrane segment, but displaying a distinct sequence to the corresponding 7th transmembrane segment and C-terminal domain relative to the V2a receptor. Here, we demonstrate the postnatal expression of V2a and V2b variants in the rat cerebellum. Most importantly, we showed by in situ hybridization and immunocytochemistry that both V2 splice variants were preferentially expressed in Purkinje cells, from early to late postnatal development. In addition, both variants were transiently expressed in the neuroblastic external granule cells and Bergmann fibers. These results indicate that the cellular distributions of both splice variants are developmentally regulated, and suggest that the transient expression of the V2 receptor is involved in the mechanisms of cerebellar cytodifferentiation by AVP. Finally, transfected CHO-K1 .expressing similar amounts of both V2 splice variants, as that found in the cerebellum, showed a significant reduction in the surface expression of V2a receptors, suggesting that the differential expression of the V2 splice variants regulate the vasopressin signaling in the cerebellum.
Keywords: arginine vasopressin, vasopressin receptor, cerebellar ontogeny, Purkinje cells, motion perception
INTRODUCTION
Vasopressin (AVP) and oxytocin are neuropeptides secreted by magnocellular neurosecretory neurons located in the supraoptic and paravetricular nuclei of the hypothalamus, whose axons project to extrahypothalamic regions, including the neurohypophysis (Pickering et al., 1983) and the cerebellum (Hawthorn et al., 1980; Hashimoto et al., 1985; Caffe et al., 1988; Hallbeck et al., 1999). AVP regulates water and salt balance in the kidney, stimulates gluconeogenesis in the liver, and induces ACTH secretion in the anterior pituitary (Fitzsimmons et al., 1992; Szczepanska-Sadowska, 1996; González and Figueroa, 1999). AVP modulates learning and memory (de Wied et al., 1984; Engelmann et al., 1992; de Wied et al., 1993; Vawter et al., 1997), and is involved in thermoregulation (Cooper et al., 1987; Naylor et al., 1987), blood pressure regulation (Stebbins et al., 1992; Stebbins et al., 1998), anti-nociception (Berson et al., 1983; Thurston et al., 1992), sexual behavior (Lim et al., 2004), and social recognition (Bielsky and Young, 2004; Bielsky et al., 2005). Notably, AVP also regulates motor coordination and motion perception through the activation of vasopressin receptors in the cerebellum (Maiti et al., 1986a; Hirasawa et al., 1994a; Hirasawa et al., 1994b; Croiset and De Wied, 1997).
Vasopressin receptors belong to the superfamily of G protein-coupled receptors and three receptor subtypes have been identified, V1, V2 and V3 (Birnbaumer, 2000). V2 receptors are highly expressed in the kidney, while V1 and V3 receptors are ubiquitous. Previously, we demonstrated the expression of two splice variants of V2 receptors in the kidney, referred as V2a and V2b (Sarmiento et al., 2004). The V2b variant is generated by using an alternative splicing site, 76 bp downstream of the V2a receptor splice site of the third exon, resulting in a frameshift in the 3′-end coding region. These two splice variants differ in size and function, the V2b amino acid sequence is identical to the V2a up to the 6th transmembrane domain sequence; however, the downstream V2b sequence is different and shorter than the canonical sequence corresponding to the 7th transmembrane segment and C-terminus of GPCRs. Unlike the V2a, the V2b is retained inside the cell and does not bind AVP (Sarmiento et al., 2004).
Although previous studies have shown the presence of V2 receptor mRNA in cerebellum and brain, the expression and localization of the V2 splice variants in these tissue remain to be determined. Here, we have examined the cellular distribution of the V2 receptor splice variants in the cerebellum using in situ hybridization, immunocytochemistry, autoradiography and RT-PCR. We showed that both V2 splice variants are localized preferentially in Purkinje cells from early to late postnatal development, whereas these variants are transiently expressed in the external granule layer and Bergmann fibers. Furthermore, we showed that the equal expression of both splice variants in CHO-K1 cells significantly inhibited the surface expression of V2a receptors, suggesting that the differential expression of the V2 splice variants 0regulate the vasopressin signaling in the cerebellum.
MATERIAL AND METHODS
Animals
Adult female Sprague-Dawley rats (200 to 250 g) were housed in a light- and temperature-controlled room with free access to chow and water. Vaginal smears were taken daily. Rats exhibiting a regular 4-day cycle were randomly caged with fertile males on the night of proestrus. The presence of sperms in the vaginal smear defined day 1 of pregnancy. Four to six rats were sacrificed between 10 am and 12 noon on days 1, 5, 15, 30 and 60 of postnatal life (P1, P3, etc). The animals were anesthetized with ether and the cerebellum was removed and immediately frozen in liquid nitrogen or fixed by immersion in 4% (v/v) formol-saline, Bouin’s fluid or periodate-lysine-paraformaldehyde fixative, as described (McLean and Nakane, 1974).
Iodination procedure
The selective rat V2 antagonist d(CH2)5[D-Ile2,Ile4,Tyr-NH29]AVP (Sawyer and Manning, 1988; Cotte et al., 1998; Tian et al., 2000; kindly provided by Prof. M. Manning, Ohio Medical College, Toledo, OH) was radiolabeled with 125I using the chloramine T method (Gonzalez et al., 1997). The iodinated peptide was separated from free iodine on a C18 column. The iodinated peptide showed a single radioactive peak on a C18 gradient HPLC analysis.
Autoradiography
Rats were sacrificed and organs were immediately frozen in liquid nitrogen. Tissue sections (16 μm thick) were freeze-dried, hydrated with 50 mM phosphate buffer, pH 7.4, and incubated with 500 or 100 pM of [125I]-labeled d(CH2)5[D-Ile2,Ile4,Tyr-NH29]AVP for 24 h at 4°C. Binding reaction was terminated by washing the tissue sections with ice-cold phosphate buffer and ice-cold distilled water. To determine the non-specific binding, tissue sections were incubated with the 125I-labeled antagonist in the presence of an excess of non-labeled antagonist, AVP or with the V2-specific agonist, desmopressin (DDAVP). The tissue sections were dried for 2 h at 37°C and exposed to Kodax Biomax MR film.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was prepared from approximately 100 mg of cerebellum using RNAsol (Biotecx, Houston, TX, USA). The cDNAs were synthesized using oligo-dT primers and SuperScript II (Gibco BRL, Rockville, MD, USA). Sequences of the primers flanking the 3′ splicing site that discriminate between the two V2 splice variants (GenBank accession no. Z22758) were 5′-cgtgggatccggaagctcctctgg-3′ (sense, positions: 1277-1300) and 5′-tcagggccaaccctagatagtcag-3′ (antisense, positions: 1715-1738). The PCR reaction mixture contained 10 pmol of each primer, 1 mM deoxynucleotides, 1 x Taq polymerase reaction buffer, 1.5 mM MgCl2 and 2.5 U Taq polymerase in a final volume of 50 μl. The PCR analysis consisted of 30 cycles and the products were fractionated by electrophoresis in 1% agarose gels.
Immunohistochemistry
Rat cerebella were fixed by immersion in 4% (v/v) formol-saline, Bouin’s fluid or periodate-lysine-paraformaldehyde fixative for 24 h to 48 h at room temperature and then dehydrated in a graded series of ethanol, and embedded in Histosec (Merck; Darmstadt, Germany). Tissue sections (5 μm thick) from different sites were analyzed from each animal; each section was mounted on glass slides pre-coated with poly-L-lysine or gelatin (Sigma-Aldrich, St. Louis, MO, USA). Sections were dewaxed with xylene, rehydrated through a graded series of ethanol followed by absolute methanol and 1% (v/v) H2O2 to block endogenous pseudoperoxidase activity. Tissue sections were rinsed three times with 50 mM Tris-HCl pH 7.8 and incubated with rabbit antiserum AS382 directed against the second intracellular loop (NH2-TLDRHRAICRPMLAYRHGGGARWNR-COOH, peptide TLD25; Sarmiento et al. 2005), which is shared by both splice variants. AS382 was diluted 1:200 in 50 mM Tris-HCl, pH 7.8, containing 1% immunoglobulin-free bovine serum albumin (BSA; Sigma). Similar sections were also incubated with a rabbit antiserum ASC-3 directed against the unique V2b C-terminus (NH2-HTAWVLKMNPVPQP-COOH). Bound antibodies were detected using a biotin-streptavidin kit (LSAB+, Dako, Carpinteria, CA, USA) following the manufacturer’s instructions. All incubations were carried out at 22°C in a water bath incubator. In between incubations the sections were washed with buffer Tris-HCl (see above). Tissue sections were stained with 0.1% (w/v) 3-3′-diaminobenzidine and 0.03% (v/v) H2O2 for 5 min at room temperature. Control procedures included preabsorption of the working dilution of primary antisera with their respective cognate peptides (10 μg/mL), substitution of the primary antiserum for the pre-immune serum and omission of the primary antiserum. For our immunocytochemical studies, we employed the AS382 antiserum raised against a peptide (TLD25) corresponding to the second intracellular loop shared by the two V2 variants. Western blot analysis of cerebellum membranes, stained with AS382, identified a single 55 kDa protein band, whereas the same antibody stained three proteins of 50, 55 and 120 kDa in kidney membranes. We previously showed that the 50 kDa band corresponds to the high mannose V2 receptor, while the 55 kDa band corresponds to the mature fully glycosylated receptor; the 120 kDa band most likely corresponds to a receptor dimer (Sarmiento et al., 2004; Sarmiento et al., 2005).
In situ hybridization
Sagittal sections of cerebella were fixed in Bouin’s fixative and embedded in Histosec (Merck). Sections were mounted on poly-L-lysine-coated slides. Sections were dewaxed, washed twice with PBS for 5 min at 37°C and then treated with proteinase K (1 μg/μl) for 20 min at 37 °C. The sections were postfixed with 4% (w/v) paraformaldehyde for 5 min, washed with PBS for 5 min and incubated for 10 min at room temperature in saline solution containing 0.25% (v/v) acetic acid and 0.1 M triethanolamine. Sections were then preincubated in hybridization buffer containing 50% (v/v) formamide and 4x SSC, 250 μg/ml sheared salmon sperm DNA (Sigma-Aldrich), 0.5x Denhardt’s solution (1% Ficoll, 1% polyvinylpyrrolidone and 1% BSA), 10% (w/v) dextran sulfate and 10 mM DDT. An antisense biotinylated oligodeoxynucleotide (positions 1558-1581, common to V2a and V2b transcripts, GenBank accession number Z22758) against the mRNAs encoding rat V2 receptor was employed as probe (Invitrogen, Carlsbad, CA, USA). The antisense sequence was 5′-cacagcaaagcaggctacgcaact-3′ and the sense sequence was 5′-agttgcgtagcctgctttgctgtg-3′. Sections were incubated with 30 p moles of the denatured probes at 50°C for 5 min in hybridization buffer for 16 h at 42 °C. After incubation, the sections were sequentially washed for 15 min each with 4x SSC at 42 °C, 2x SSC at 37 °C, 1x SSC at 37 °C, and 0.3x SSC at 37 °C. Then, sections were washed with solution A (100 mM Tris-HCl 7.4, 150 mM NaCl) for 10 min, treated with solution B (1% BSA, 0.3% Triton X-100 in solution A), and incubated with a 1:40 dilution of anti-biotin Fab fragment, conjugated to alkaline phosphatase, in solution B for 3 h at room temperature. Sections were washed twice with solution A for 10 min and three times with solution C (100 mM Tris-HCl pH 9.5, 100 mM NaCl, 50 mM MgCl2) for 10 min at room temperature. Finally, the sections were incubated in color-developing buffer containing NBT/BCIP and 10 mM levamisole for 15 h, after which the reaction was stopped by incubation with 10 mM Tris-HCl, pH 8.0 containing 1 mM EDTA, and sections were mounted in Mowiol (Polysciences, Warrington, PA, USA).
Cell culture and transfections
CHO-K1 cells were grown to subconfluency in 35 mm dishes in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B. CHO-K1 cells were transfected with 0.30 μg of plasmid encoding V2a and increasing concentrations of plasmid encoding V2b (0.10 μg or 0.30 μg) using FuGENE 6 (Roche Diagnostic Co., Indianapolis, IN, USA). After 48 h [3H]AVP binding was carried out on transfected cells.
Binding assays
Transfected CHO-K1 cells were washed three times with ice-cold DPBS (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.0 mM NaPO4, 0.9 mM CaCl2, 0.5 mM MgCl2, pH 7.4) supplemented with 1 mg/ml glucose, 20 mg/ml BSA and 165 ng/ml L-phenylalanine. The binding assay was carried essentially as described (Sarmiento et al. 2004). Transfected cells were incubated for 2 h at 37°C with 4 nM [3H]AVP in the absence or the presence of 10 μM unlabeled AVP. The reaction was terminated by washing the cells twice with ice-cold DPBS. Cells were harvested by incubating them with 0.1 N NaOH for 30 min at 37°C. Radioactivity was determined in a Packard Scintillation β-Counter. Data were analyzed by using the Sigma plot program (SPSS Science, Chicago, IL) in the ligand binding plug-in mode. Binding is reported as average of triplicates determinations. Each experiment was performed at least three times.
Biotinylation of surface membrane proteins
Transfected CHO-K1 cells in 60 mm culture dish were washed twice with ice-cold PBS pH 8.0 137mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4,2 mM KH2PO4) .and incubated with 0.5 mg/mL NHS-SS-Biotin (Pierce, Rockford, IL) solution containing 2 mM CaCl2, 150 mM NaCl, 10 mM Trietanolamina pH 8.0 for 1 h at 4°C. The biotinylation reaction was quenched by rinsing twice with PBS pH 8.0 containing 100 mM glycine and finally with PBS pH 7.4. The cells were lysed with 1 mL of radioimmune precipitation assay buffer (RIPA) (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% sodium deoxycolate, 1% Nonidet P40, 10 mM N-ethylmaleimide, 0.1 mM PMSF, 5 mg/mL soybean trypsin inhibitor and 1 μg/mL leupeptin) for 1 h on ice followed by centrifugation at 12,000 rpm for 30 min. Biotin labeled proteins were precipitated overnight with 50 ul NeutrAvidin-agarose slurry under constant agitation, and then recovered by centrifugation. The precipitates were analyzed by Western blotting.
Membrane preparation
Rat kidney medulla or cerebella were homogenized in 12 mM HEPES, pH 7.4 containing 300 mM mannitol and centrifuged for 15 min at 3,000x g. The supernatant was centrifuged for 45 min at 150,000x gav and the pellet was resuspended in PBS containing 0.1 mM PMSF. Crude membranes (150 μg) were solubilized in 450 μL of RIPA buffer.
Western blotting
Membrane proteins (80 μg per lane) were fractionated on SDS-PAGE (12.5% w/v polyacrylamide gels containg 0.1% w/v SDS) at constant voltage for 3 h. Proteins were electrotransferred onto nitrocellulose filters in 0.05% SDS, 20 mM Tris-glycine, and 20% (v/v) methanol (transfer buffer). Filters were incubated with anti-GFP or anti-HA antibodies diluted in 20 mM Tris-HCl, pH 7.6, 500 mM NaCl, 0.05 % (v/v) Tween 20. Bound immunoglobulins were detected by the chemiluminescence method (Pierce Biotechnology Inc. Rockford, IL, USA).
RESULTS
V2 receptor isoforms are expressed in Purkinje cells during postnatal development
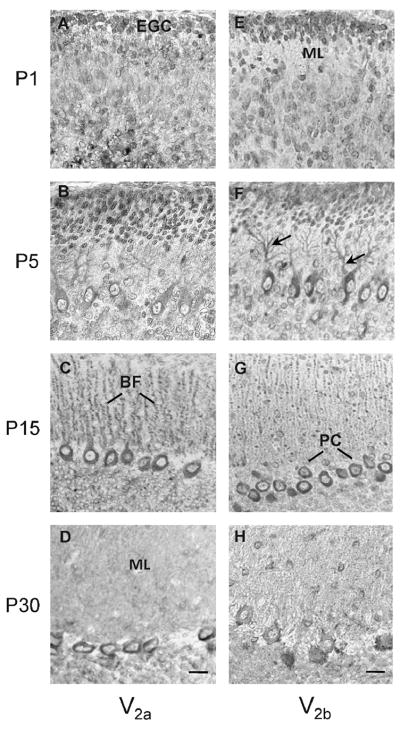
In situ hybridization with an anti-sense probe for the V2 receptor showed preferential staining in the somata of Purkinje cells and external granule layer at P1 (Fig. 1A), whereas at P5 the staining was extended to the proximal region of Purkinje cell dendrites (Fig. 1B). At P15 the staining was localized in the Purkinje cell dendrites and in the Bergmann fibers which extended into the molecular layer (Fig. 1C) At P30 the staining was confined to the emerging dendrites and somata of Purkinje cells (Fig. 1D). Staining of the rat cerebellum with the V2 receptor sense probe showed no non-specific signal (Fig 1E-H). In good agreement with the in situ hybridization studies the anti-V2 receptor antibody AS382 stained the Purkinje cells (Fig. 2A). Neither the pre-immune serum (Fig. 2B) nor AS382, previously incubated with the peptide antigen, showed specific staining (Fig. 2C). The cellular distribution of the V2b variant was determined by immunocytochemistry using an anti-V2b antiserum ASC-3 (directed against the C-terminal peptide). This antiserum specifically stained Purkinje cells (Fig 2D). Neither the preimmune (Fig 2E), nor the antiserum ASC-3, previously incubated with the antigenic peptide (Fig. 2F), stained cerebellar sections. During postnatal development of the cerebellum, at P1 the V2a receptor immunostaining was rather diffuse (Fig. 3A), whereas at P5 the somata and the dendrite proximal region of the Purkinje cells were preferentially stained (Fig. 3B). At P15 the V2a receptor immunoreactivity was detected in the somata and in the dendritic processes of Purkinje cells, and in Bergmann fibers which extended into the molecular layer (Fig 3C). At P30, the staining was clearly located in the somata and emerging dendrites of Purkinje cells (Fig. 3D). These findings indicate that the subcellular distribution of V2a receptor is developmentally regulated in the cerebellar cortex. Interestingly, the expression of the V2b variant (Fig. 3E–H) followed a similar postnatal development pattern as that of the V2a receptor. At P1 and P5 the external and the descending granule cells were stained with both anti-receptor antibodies (Fig. 3E and F); at P15 and P30 few neurons in the molecular layer were immunostained as shown at high magnification (Fig. 4A and D), but most importantly the granule cells became immune negative (Fig. 3C and D). Furthermore, at P15 the Bergmann fibers were intensively stained by both anti-receptor antibodies, showing the characteristics rod-like pattern running throughout the molecular layer (Fig. 3C and 3G, and 4B). Interestingly, at P30 the V2a receptor was detected in the somata and dendrites of the Purkinje cells, whereas, the V2b variant was found mainly in the somata of Purkinje cells (Fig. 4C and F).
Figure 1. Expression and cellular distribution of V2 receptor mRNA during postnatal development of rat cerebellum.
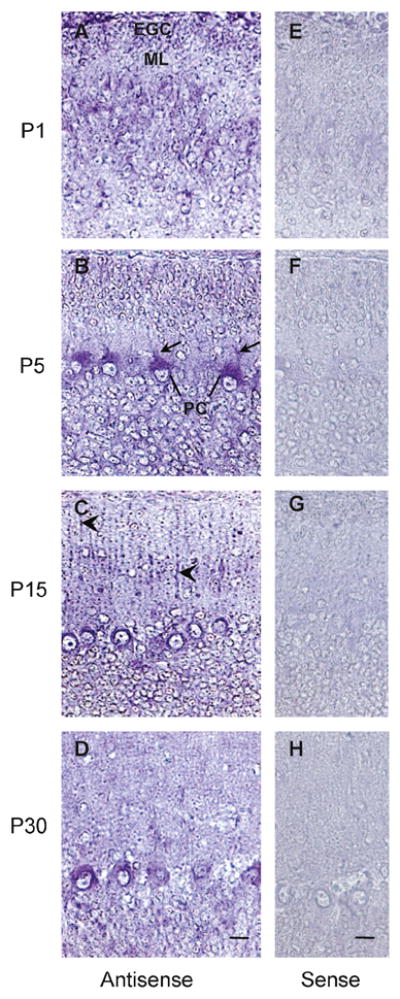
In situ hybridization of rat cerebellar sections during postnatal development, using sense (control) or antisense oligonucleotide probes. Specific staining is observed at every stage of the postnatal development. EGC, external granule cells; ML, molecular layer; PC, Purkinje cell; PC dendrites (arrows) and Bergmann fibers (arrow heads). Scale bars, 10 μm. Images are representative of two independent experiments.
Figure 2. Immunocytochemical analysis of the V2 receptor in the rat cerebellum.
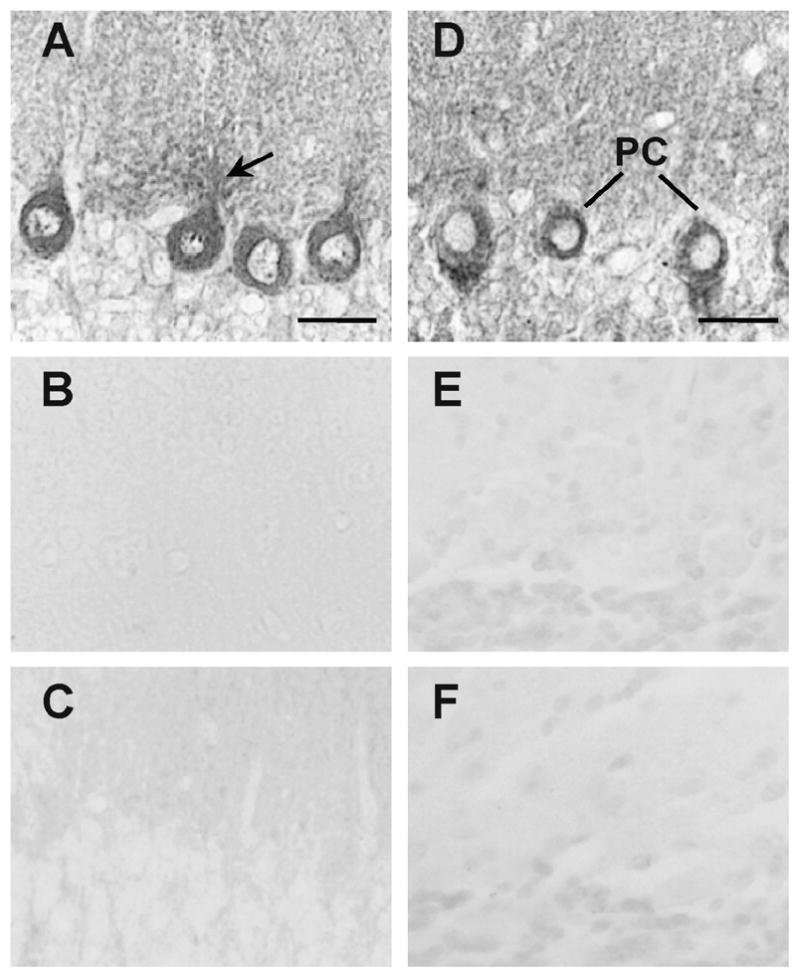
Immunostaining of rat cerebellar sections with AS382 antibody directed against the 2nd intracellular loop of the V2a receptor (A), or with the AS382 antibody pre-absorbed with the antigenic peptide TLD2 (B), or with the pre-immune serum (C), or with an antiserum (AS C-3) directed against the V2b splice variant (D), with the antiserum pre-absorbed with the V2b antigenic peptide (E) or with the pre-immune serum (F). PC, Purkinje cells; PC dendrites (arrows). Scale bars,20 μm. Images are representative of two independent experiments.
Figure 3. Expression and cellular distribution of the V2 splice variants during postnatal development of the rat cerebellum.
Immunostaining of rat cerebellar sections with AS382 directed against the 2nd intracellular loop of the V2 receptor (A-D) or with ASC-3 specific V2b antiserum (F–H). EGC, external granule cells; ML, molecular layer; PC, Purkinje cell; PC dendrites (arrows); BF, Bergmann fibers. Scale bars, 10 μm. Images are representative of four independent experiments.
Figure 4. Subcellular localization of the V2 splice variants.
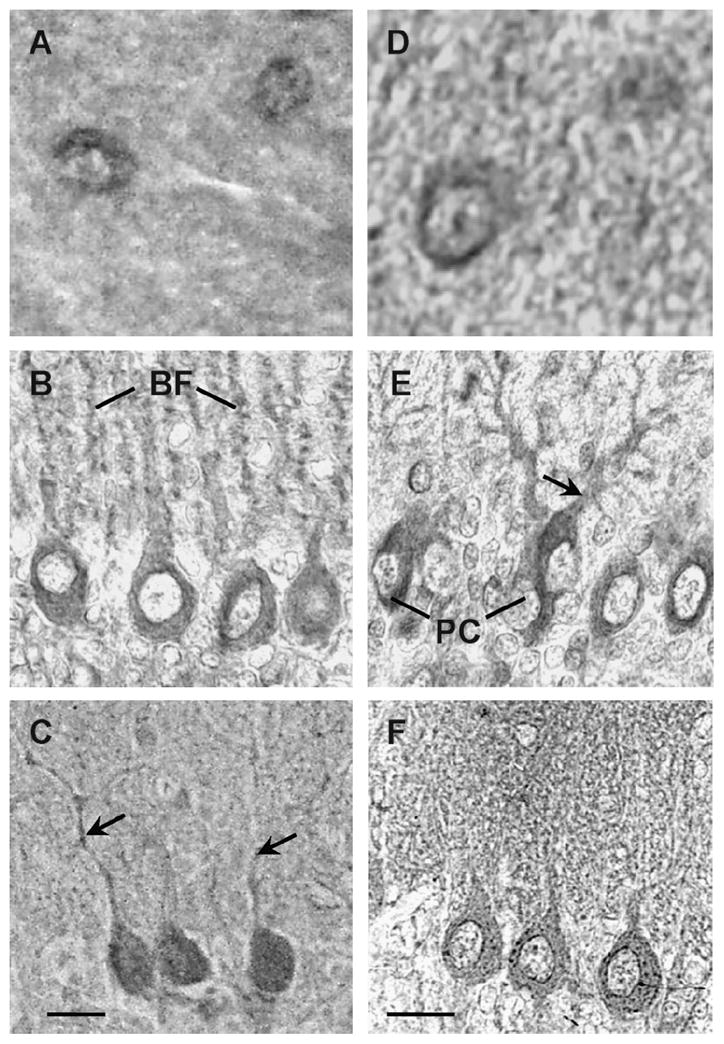
High magnification of cerebellar sections stained with the AS382 (A–C) or with ASC-3 (D–F). Immunopositive neurons in the molecular layer at P30 (A, D). At P15 thesomata of Purkinje cell (PC) and Bergmann fibers (BF) are immunostained (B); at P5 the somata of Purkinje cell and growing dendrites are immunostained (E). At P30 thesomata of Purkinje cells (C–F) and dendrites are immunopositive for the V2a (C, arrows). Scale bars, 20 μm.
Consistent with the immunocytochemistry findings, autoradiographic studies with [125I]-labeled V2 antagonist d(CH2)5[D-Ile2,Ile4,Tyr-NH29]AVP(Sawyer and Manning, 1988; Cotte et al., 1998; Tian et al., 2000) demonstrated specific labeling of Purkinje cells (Fig 5A). This specific labeling was displaced by the unlabeled V2 antagonist (Fig 5B), by AVP (Fig 5C) and by DDAVP (Fig 5D). The displacement of the V2 antagonist labeling by DDAVP, a specific V2 agonist, indicates that these binding sites correspond to V2a receptors, as the V2b does not bind AVP (Sarmiento et al., 2004).
Figure 5. Binding of [125I]-labeled V2-selective antagonist d(CH2)5[D-Ile2,Ile4,Tyr-NH29]AVP to rat cerebellum.
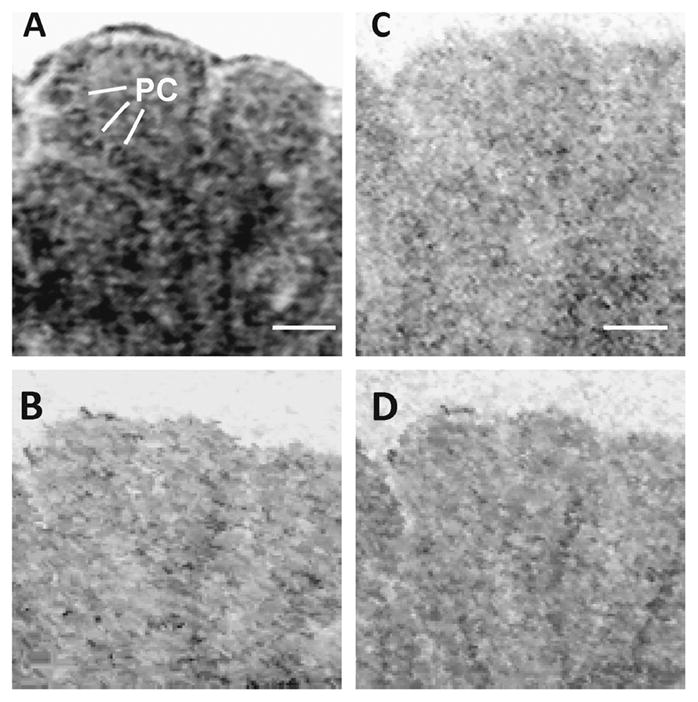
Autoradiography of sagittal cerebellar sections (P5) incubated with [125I]-labeled V2-selective antagonist d(CH2)5[D-Ile2,Ile4,Tyr-NH29]AVP in the absence (A) or presence of an excess of unlabeled V2 antagonist (B), or vasopressin (C) or DDAVP (D). Purkinje cells(PC) are shown in A. Scale bars, 50 μm. Images are representative of three independent experiments.
To further examine the expression of V2b splice variant in the cerebellum, we performed RT-PCR (Fig. 6A and B) using RNA extracted from the rat cerebellum and primers flanking the 3′ splicing site. We detected two PCR fragments of 301 bp and 225 bp corresponding to V2a and V2b splice variants, respectively (Fig. 6B). These fragments were previously identified in the kidney, in which the small fragment is about 15% of that of the large fragment (Firsov et al 1994; Sarmiento et al., 2005). In addition, we detected a large 450 bp PCR fragment in cerebellar RNA (see Fig. 6B), most likely due to the amplification of the primary non-splice transcript, which was still detected after extensive digestion of the RNA sample with DNase and identified by DNA sequencing (data not shown). Interestingly, the V2 pre-RNA was not amplified from postnatal kidney RNA extracts (Fig. 6C). Similar findings have been reported in other cell systems and might reflect differences in the gene transcription or/and RNA processing rates (Choe et al., 2003; Yue et al., 2006). On the basis of our PCR studies and previous studies on the expression of the mRNAs encoding V2a and V2b (Firsov et al 1994, and Sarmiento et al. 2004) we estimated that the relative expression of these transcripts remains constant throughout the postnatal development of the cerebellum (Fig. 6B). Regardless of the developmental stage of the cerebellum, the expression V2a was approximately similar to that of V2b, whereas in the kidney V2a is expressed at higher levels than V2b. In fact, in the kidney the V2b represents approximately 15% of the V2a mRNA (Fig. 6C; Firsov et al 1994).
Figure 6. Expression of V2 splice variant mRNA in rat cerebellum and kidney by RT-PCR.
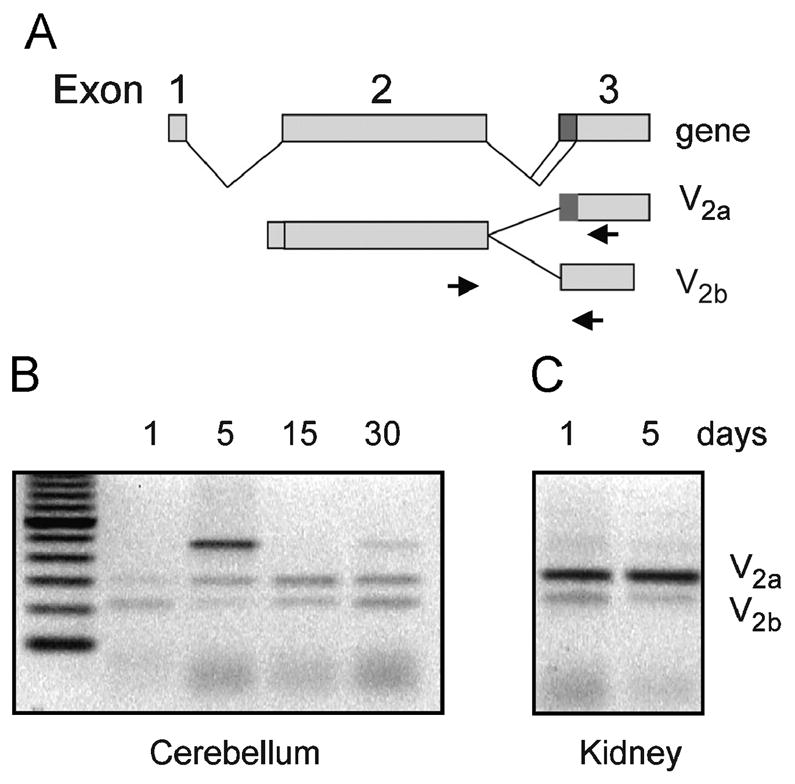
(A) Outline of the generation of the two splice variants V2a and V2b, the arrows represent the primers employed in the RT-PCR assays; (B) shows the PCR fragments corresponding to V2a and V2b splice variants using total RNA from rat cerebellum cortexes during postnatal development; (C) shows the PCR fragments using total RNA from rat kidney medulla during postnatal development. The sizes of the fragments corresponding to V2a and V2b are 301 bp and 225 bp, respectively. The fragment 450 bp corresponds to unprocessed pre-mRNA. . Note the similar amount of V2a and V2b transcripts at 30 days of postnatal development. Data are representative of three independent experiments.
V2b down-regulates the surface expression of V2a
To assess the functional significance of the co-expression of V2 splice variants in the cerebellum, we determined the binding of [3H]AVP to CHO cells expressing V2a and V2b splice variants. We found that cells co-transfected with V2a cDNA (0.30 μg) and with increased amounts of V2b cDNA (0–0.30 μg) showed a decrease in the binding to [3H]AVP. Cells transfected with equal amounts of V2a and V2b cDNAs exhibited approximately 20% of [3H]AVP binding relative to cells transfected with V2a cDNA alone (Fig. 7). This condition mimics the almost equal expression levels of the two splice variants found in the cerebellum (cf. Fig. 6). To ruled out potential changes in the protein expression of V2a during co-transfections, we evaluated the protein expression of V2a and V2b by Western blot analysis in cells co-transfected with cDNAs encoding V2a fused to GFP or the HA (hematoglutinin) tagged V2b. We found that the protein expression of V2a was the same at increasing concentrations of V2b cDNA employed in the transfections (Fig 7, inset A and B). As expected, the protein expression of V2b increased in cells co-transfected with a fix amount of V2a cDNA and increasing amounts of V2b cDNA. To determine whether the decrease in the [3H]AVP binding was due to a change in the number of receptors or to a change in the binding affinity of receptors, we biotin labeled the surface proteins and carried out Western blots of avidin-agarose precipitates (Fig. 7, inset C). We demonstrated that cells transfected with equal amounts of cDNAs, encoding the V2a and V2b showed a remarkably reduction of the biotin-labeled surface receptors (Fig. 7, inset C, lane3). This finding is in good agreement with the [3H]AVP binding experiments (Fig. 7, bar 3) and further support the concept that the expression of the V2b splice variant down-regulates the cell surface expression of the V2a receptor, most likely through retention in the ER/Golgi complex by receptor oligomerization (Sarmiento et al., 2004). On this basis, the differential expression of the splice variants may have a major role on the AVP signaling in Purkinje cells of the cerebellum.
Figure 7. The V2b splice variant down-regulated the surface expression of V2a receptor.
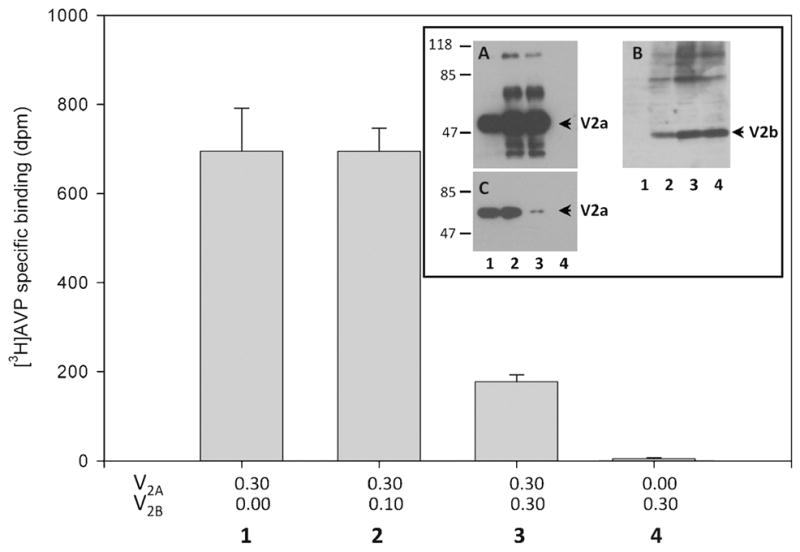
CHO K1 cells transfected with increasing amounts of cDNA encoding V2b (μg) and a constant amount of cDNA encoding V2a (bars/lanes 1–3). Cells transfected with V2b alone did not show [3H] AVP specific binding (bar 4). The inset shows Western blots of transfected cell extracts using anti-GFP to detect V2a tagged with GFP (A) and anti-HA to detect V2b tagged with HA (B). The V2a protein expression does not significantly change in cells transfected with increasing amounts of V2b cDNA (A); the expression of V2b increased as the amount of V2b cDNA employed for transfections (B). Western blot analysis of biotin-labeled proteins precipitated with neutravidin agarose beads and stained with anti-GFP antibodies (C). The numbers (1–4) correspond to the bar and lanes (inset) and indicate the amount of cDNAs (μg) employed in the transfections. Data are the average of three experiments.
DISCUSSION
The main finding of this work is that the functional splice variant V2a and the truncated receptor variant V2b are preferentially expressed in Purkinje cells in the cerebellum. During post-natal development of the rat cerebellum the expression levels of the splice variants were similar: the ratios of V2a/V2b were near 1. However, the cellular distributions of both splice variants were developmentally regulated; at P1 the variants were preferentially expressed in the external granule cell layer and descending granule cells, whereas at later stages of development the expression of the splice variants were preferentially expressed in the Purkinje cells. In addition, the V2 mRNA and also both isoforms are expressed in Bergmann fiber at P15. These findings suggests that the neuroblastic external granule cells and Bergmann fibers transiently express both splice variants, similarly to the transient expression of the V2 vasopressin receptor during development of the hippocampus (Kato et al., 1995) and other regions of the brain (Tribollet et al., 1991a; Hirasawa et al., 1994b). Similar transient expression has been reported in the Bergmann glia for other receptors such the GABAA receptors (Muller et al., 1994). The transient expression of the vasopressin receptor in the external granules suggests that vasopressin might play a role in the molecular mechanism of migration of the cerebellar granule cells. Likewise, the expression in the Bergmann fibers suggests that AVP might participate in the dendritogenesis and synaptogenesis of Purkinje cells.
Our in situ hybridization studies are in good agreement with earlier experiments identifying V2 receptor mRNA in the granular layer of the developing and adult cerebella (Kato et al., 1995). Our work further showed the subcellular distribution of the V2 splice variants in Purkinje cells: both variants were mainly distributed in the cell somata and dendrites.
The mechanisms underlying the down-regulation of surface V2a receptors demonstrated in this work appears to involve the intracellular retention of V2a mediated by oligomerization with V2b, which is retained in the ER/Golgi (Sarmiento et al 2004), thus precluding trafficking of the receptor to the plasma membrane. The similar expression of both splice variants in the cerebellum strongly suggests a novel post-translational regulation of the surface expression of the V2 receptors, which should fine tune the response of AVP signaling in the cerebellum. The impact of changes in the density of surface receptors is best illustrated by the dramatic changes of social behavior due to changes in the levels of expression of AVP-receptors in the brain (Lim et al., 2004; Lim et al., 2005).
Previous studies have detected AVP binding sites in the cerebellum (Pearlmutter et al., 1983; Brinton et al., 1984; Phillips et al., 1990; Tribollet et al., 1991b) but the subtype of receptor present and the precise cellular location are still elusive. Now, we have established the expression of V2 receptors in the cerebellum, by using multiple and sensitive approaches to detect the low levels of protein and message encoding this receptor. Moreover, we demonstrated the expression of both splice variants in the Purkinje cells and the transient expression in the external granules cells and Bergmann glia.
Overwhelming evidence indicates that vasopressin and its analogs impact on memory, learning functions and social behavior (de Wied et al., 1984; de Wied et al., 1987; de Wied et al., 1991; Insel and Shapiro, 1992; Bohus et al., 1993; Buwalda et al., 1993; de Wied et al., 1993; Young et al., 1997; Dantzer, 1998; Francis et al., 2002; Winslow and Insel, 2004) and control of motor activity (Maiti et al., 1986b; Balaban et al., 1988; Marchese et al., 1991; Willcox et al., 1992; Diamant et al., 1994; Croiset and De Wied, 1997; van Londen et al., 1998). Likewise, the vasopressin signaling system has been implicated in pathological states such as the barrel rotation and proconvulsive effects (Maiti et al., 1986b; Croiset and De Wied, 1997). Moreover, vasopressin-induced barrel rotation was abolished after chemical destruction of Purkinje cells (Maiti et al., 1986b). These findings are in good agreement with our observation that the V2 receptor system is located in Purkinje cells where it provides the anatomical substrate for these (patho)physiological observations.
Acknowledgments
This was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grants and 1060158 (CBG), the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie (WME), the National Institute of Health grant R01EY014218 and a grant from Welch Foundation (JN). We would like thank Professor Brian T. Pickering for critically reading the manuscript.
Abbreviations
- AVP
arginine vasopressin
- DDAVP
desmopressin
- SDS
sodium dodecyl sulphate
- CHO-K1
Chinesehamster ovary cells
- NBT
nitroblue tetrazolium chloride
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
References
- Balaban CD, Fredericks DA, Wurpel JN, Severs WB. Motor disturbances and neurotoxicity induced by centrally administered somatostatin and vasopressin in conscious rats: interactive effects of two neuropeptides. Brain Res. 1988;445:117–129. doi: 10.1016/0006-8993(88)91080-3. [DOI] [PubMed] [Google Scholar]
- Berson BS, Berntson GG, Zipf W, Torello MW, Kirk WT. Vasopressin-induced antinociception: an investigation into its physiological and hormonal basis. Endocrinology. 1983;113:337–343. doi: 10.1210/endo-113-1-337. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M. Vasopressin receptors. Trends Endocrinol Metab. 2000;11:406–410. doi: 10.1016/s1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- Bohus B, Borrell J, Koolhaas JM, Nyakas C, Buwalda B, Compaan JC, Roozendaal B. The neurohypophysial peptides, learning, and memory processing. Ann N Y Acad Sci. 1993;689:285–299. doi: 10.1111/j.1749-6632.1993.tb55554.x. [DOI] [PubMed] [Google Scholar]
- Brinton RE, Gee KW, Wamsley JK, Davis TP, Yamamura HI. Regional distribution of putative vasopressin receptors in rat brain and pituitary by quantitative autoradiography. Proc Natl Acad Sci U S A. 1984;81:7248–7252. doi: 10.1073/pnas.81.22.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Nyakas C, Koolhaas JM, Bohus B. Neuroendocrine and behavioral effects of vasopressin in resting and mild stress conditions. Physiol Behav. 1993;54:947–953. doi: 10.1016/0031-9384(93)90307-2. [DOI] [PubMed] [Google Scholar]
- Caffe AR, van Leeuwen FW, Buijs RM, van der Gugten J. Vasopressin and noradrenaline coexistence in the rat locus ceruleus: differential decreases of their levels in distant brain areas after thermal and neurotoxic lesions. Brain Res. 1988;459:386–390. doi: 10.1016/0006-8993(88)90657-9. [DOI] [PubMed] [Google Scholar]
- Cooper KE, Naylor AM, Veale WL. Evidence supporting a role for endogenous vasopressin in fever suppression in the rat. J Physiol. 1987;387:163–172. doi: 10.1113/jphysiol.1987.sp016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotte N, Balestre MN, Phalipou S, Hibert M, Manning M, Barberis C, Mouillac B. Identification of residues responsible for the selective binding of peptide antagonists and agonists in the V2 vasopressin receptor. J Biol Chem. 1998;273:29462–29468. doi: 10.1074/jbc.273.45.29462. [DOI] [PubMed] [Google Scholar]
- Croiset G, De Wied D. Proconvulsive effect of vasopressin; mediation by a putative V2 receptor subtype in the central nervous system. Brain Res. 1997;759:18–23. doi: 10.1016/s0006-8993(97)00070-x. [DOI] [PubMed] [Google Scholar]
- Choe Y, Son GH, Lee S, Park E, Moon Y, Kim K. Cell differentiation of gonadotropin-releasing hormone neurons and alternative RNA splicing of the gonadotropin-releasing hormone transcript. Neuroendocrinology. 2003;77:282–290. doi: 10.1159/000070886. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–414. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- de Wied D, Elands J, Kovacs G. Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor? Proc Natl Acad Sci U S A. 1991;88:1494–1498. doi: 10.1073/pnas.88.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wied D, Gaffori O, Burbach JP, Kovacs GL, van Ree JM. Structure activity relationship studies with C-terminal fragments of vasopressin and oxytocin on avoidance behaviors of rats. J Pharmacol Exp Ther. 1987;241:268–274. [PubMed] [Google Scholar]
- de Wied D, Gaffori O, van Ree JM, de Jong W. Central target for the behavioural effects of vasopressin neuropeptides. Nature. 1984;308:276–278. doi: 10.1038/308276a0. [DOI] [PubMed] [Google Scholar]
- Diamant M, Baars AM, Kovacs GL, De Wied D. Barrel rotation induced by central arginine8-vasopressin treatment: involvement of neurohypophyseal peptide receptors. Pharmacol Biochem Behav. 1994;47:27–32. doi: 10.1016/0091-3057(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Bures J, Landgraf R. Vasopressin administration via microdialysis into the septum interferes with the acquisition of spatial memory in rats. Neurosci Lett. 1992;142:69–72. doi: 10.1016/0304-3940(92)90622-e. [DOI] [PubMed] [Google Scholar]
- Firsov D, Mandon B, Morel A, Merot J, Le Maout S, Bellanger AC, de Rouffignac C, Elalouf JM, Buhler JM. Molecular analysis of vasopressin receptors in the rat nephron. Evidence for alternative splicing of the V2receptor. Pflugers Arch. 1994;429:79–89. doi: 10.1007/BF02584033. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons MD, Roberts MM, Sherman TG, Robinson AG. Models of neurohypophyseal homeostasis. Am J Physiol. 1992;262:R1121–1130. doi: 10.1152/ajpregu.1992.262.6.R1121. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez CB, Figueroa CD. Vasopressin and bradykinin receptors in the kidney: implications for tubular function. Biol Res. 1999;32:63–76. [PubMed] [Google Scholar]
- Gonzalez CB, Figueroa CD, Reyes CE, Caorsi CE, Troncoso S, Menzel D. Immunolocalization of V1 vasopressin receptors in the rat kidney using anti-receptor antibodies. Kidney international. 1997;52:1206–1215. doi: 10.1038/ki.1997.445. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Hermanson O, Blomqvist A. Distribution of preprovasopressin mRNA in the rat central nervous system. J Comp Neurol. 1999;411:181–200. [PubMed] [Google Scholar]
- Hashimoto H, Fukui K, Noto T, Nakajima T, Kato N. Distribution of vasopressin and oxytocin in rat brain. Endocrinol Jpn. 1985;32:89–97. doi: 10.1507/endocrj1954.32.89. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Ang VT, Jenkins JS. Localization of vasopressin in the rat brain. Brain Res. 1980;197:75–81. doi: 10.1016/0006-8993(80)90435-7. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Hashimoto K, Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur J Pharmacol. 1994a;267:71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Nakayama Y, Ishiharada N, Honda K, Saito R, Tsujimoto G, Takano Y, Kamiya H. Evidence for the existence of vasopressin V2 receptor mRNA in rat hippocampus. Biochem Biophys Res Commun. 1994b;205:1702–1706. doi: 10.1006/bbrc.1994.2864. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Igarashi N, Hirasawa A, Tsujimoto G, Kobayashi M. Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation. 1995;59:163–169. doi: 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. Int J Dev Neurosci. 2005;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Maiti A, Shahid Salles K, Grassi S, Abood LG. Barrel rotation and prostration by vasopressin and nicotine in the vestibular cerebellum. Pharmacol Biochem Behav. 1986a;25:583–588. doi: 10.1016/0091-3057(86)90145-0. [DOI] [PubMed] [Google Scholar]
- Maiti A, Shahid Salles K, Grassi S, Abood LG. Behavior and receptor changes after kainate lesioning of nodular cerebellum. Pharmacol Biochem Behav. 1986b;25:589–594. doi: 10.1016/0091-3057(86)90146-2. [DOI] [PubMed] [Google Scholar]
- Marchese A, Mihic SJ, Wu PH, Kalant H. Arginine8-vasopressin potentiates the motor in coordinating effects of pentobarbital. Eur J Pharmacol. 1991;202:341–345. doi: 10.1016/0014-2999(91)90276-v. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Muller T, Fritschy JM, Grosche J, Pratt GD, Mohler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994;14:2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AM, Cooper KE, Veale WL. Vasopressin and fever: evidence supporting the existence of an endogenous antipyretic system in the brain. Can J Physiol Pharmacol. 1987;65:1333–1338. doi: 10.1139/y87-211. [DOI] [PubMed] [Google Scholar]
- Pearlmutter AF, Costantini MG, Loeser B. Characterization of 3H-AVP binding sites in particulate preparations of rat brain. Peptides. 1983;4:335–341. doi: 10.1016/0196-9781(83)90144-4. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Abrahams JM, Kelly JM, Mooser V, Trinder D, Johnston CI. Localization of vasopressin binding sites in rat tissues using specific V1 and V2 selective ligands. Endocrinology. 1990;126:1478–1484. doi: 10.1210/endo-126-3-1478. [DOI] [PubMed] [Google Scholar]
- Pickering BT, Swann RW, Gonzalez CB. Biosynthesis and processing of neurohypophysial hormones. Pharmacol Ther. 1983;22:143–161. doi: 10.1016/0163-7258(83)90057-8. [DOI] [PubMed] [Google Scholar]
- Sarmiento JM, Anazco CC, Campos DM, Prado GN, Navarro J, Gonzalez CB. Novel down-regulatory mechanism of the surface expression of the vasopressin V2 receptor by an alternative splice receptor variant. J Biol Chem. 2004;279:47017–47023. doi: 10.1074/jbc.M410011200. [DOI] [PubMed] [Google Scholar]
- Sarmiento JM, Ehrenfeld P, Anazco CC, Reyes CE, Troncoso S, Figueroa CD, Muller-Esterl W, Gonzalez CB. Differential distribution of the vasopressin V receptor along the rat nephron during renal ontogeny and maturation. Kidney international. 2005;68:487–496. doi: 10.1111/j.1523-1755.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- Sawyer WH, Manning M. The development of potent and specific vasopressin antagonists. Kidney Int Suppl. 1988;26:S34–37. [PubMed] [Google Scholar]
- Stebbins CL, Bonigut S, Liviakis LR, Munch PA. Vasopressin acts in the area postrema to attenuate the exercise pressor reflex in anesthetized cats. Am J Physiol. 1998;274:H2116–2122. doi: 10.1152/ajpheart.1998.274.6.H2116. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Ortiz-Acevedo A, Hill JM. Spinal vasopressin modulates the reflex cardiovascular response to static contraction. J Appl Physiol. 1992;72:731–738. doi: 10.1152/jappl.1992.72.2.731. [DOI] [PubMed] [Google Scholar]
- Szczepanska-Sadowska E. Interaction of vasopressin and angiotensin II in central control of blood pressure and thirst. Regul Pept. 1996;66:65–71. doi: 10.1016/0167-0115(96)00053-5. [DOI] [PubMed] [Google Scholar]
- Thurston CL, Campbell IG, Culhane ES, Carstens E, Watkins LR. Characterization of intrathecal vasopressin-induced antinociception, scratching behavior, and motor suppression. Peptides. 1992;13:17–25. doi: 10.1016/0196-9781(92)90135-p. [DOI] [PubMed] [Google Scholar]
- Tian Y, Sandberg K, Murase T, Baker EA, Speth RC, Verbalis JG. Vasopressin V2 receptor binding is down-regulated during renal escape from vasopressin-induced antidiuresis. Endocrinology. 2000;141:307–314. doi: 10.1210/endo.141.1.7256. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dreifuss JJ. Appearance and transient expression of vasopressin and oxytocin receptors in the rat brain. J Recept Res. 1991a;11:333–346. doi: 10.3109/10799899109066412. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dubois-Dauphin M, Dreifuss JJ. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res. 1991b;58:13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]
- van Londen L, Kerkhof GA, van den Berg F, Goekoop JG, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, de Wied D. Plasma arginine vasopressin and motor activity in major depression. Biol Psychiatry. 1998;43:196–204. doi: 10.1016/S0006-3223(97)80433-7. [DOI] [PubMed] [Google Scholar]
- Vawter MP, De Wied D, Van Ree JM. Vasopressin fragment, AVP-(4-8), improves long-term and short-term memory in the hole board search task. Neuropeptides. 1997;31:489–494. doi: 10.1016/s0143-4179(97)90044-5. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Poulin P, Veale WL, Pittman QJ. Vasopressin-induced motor effects: localization of a sensitive site in the amygdala. Brain Res. 1992;596:58–64. doi: 10.1016/0006-8993(92)91532-j. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Yue C, Mutsuga N, Scordalakes EM, Gainer H. Studies of oxytocin and vasopressin gene expression in the rat hypothalamus using exon- and intron-specific probes. American journal of physiology. 2006;290:R1233–1241. doi: 10.1152/ajpregu.00709.2005. [DOI] [PubMed] [Google Scholar]