Summary
Objectives
To evaluate the impact of Oportunidades, a large-scale, conditional cash transfer programme in Mexico, on birthweight. The programme provides cash transfers to low-income, rural households in Mexico, conditional on accepting nutritional supplements health education, and health care.
Methods
The primary analyses used retrospective reports from 840 women in poor rural communities participating in an effectiveness study and randomly assigned to incorporation into the programme in 1998 or 1999 across seven Mexican states. Pregnant women in participating households received nutrition supplements and health care, and accepted cash transfers. Using multivariate and instrumental variable analyses, we estimated the impact of the programme on birthweight in grams and low birthweight (<2500 g), receipt of any pre-natal care, and number of pre-natal visits.
Results
Oportunidades beneficiary status was associated with 127.3 g higher birthweight among participating women and a 4.6 percentage point reduction in low birthweight.
Conclusion
The Oportunidades conditional cash transfer programme improved birthweight outcomes. This finding is relevant to countries implementing conditional cash transfer programmes.
Keywords: conditional cash transfer, birthweight, Mexico, impact evaluation, human capital
Introduction
Reducing the incidence of low birthweight is a global health priority because of its consequences on neonatal, childhood, and adolescent morbidity and mortality (Institute of Medicine 1985, McCormick 1985; Ashworth 1998; Moore et al. 1999), and adult economic productivity (Prentice & Moore 2005; Alderman & Berhman 2006). More than 95% of the 20 million low birthweight infants born globally per year come from low-income populations (UNICEF & WHO 2004). Whereas pre-term birth accounts for the majority of low birthweight infants in high-income settings (Blondel et al. 2002), intrauterine growth restriction (IUGR) and a combination of IUGR and pre-term births promotes low birthweight among many poor populations (de Onis et al. 1998). Low-income populations generally have a relatively high prevalence of infectious, nutritional, maternal, and perinatal conditions, which could include low nutritional intake and pre-pregnancy body mass index, hypertensive disorders of pregnancy and untreated infections (Villar & Belizan 1982; Bergström 2003; Kramer 2003).
Recommended interventions to reduce low birthweight in less developed settings include improving maternal nutrition and increasing the use of pre-natal care (Kramer 1987; Merialdi et al. 2003; Bhutta et al. 2005). Under controlled conditions, nutritional supplements have proven efficacious in promoting higher birthweight (Christian et al. 2003; Cogswell et al. 2003). For pre-natal care, however, randomized controlled trials comparing a standard number vs. reduced number of goal-oriented pre-natal visits report few significant improvements in birth outcomes (Villar et al. 2001). Reliable evidence of the effectiveness of these strategies is needed to guide investments that aim to improve infant and child survival.
In 1997, Mexico introduced a large-scale conditional cash transfer programme (CCT) that aims, in part, to improve birth outcomes through better maternal nutrition and use of pre-natal care. The programme (originally called PROGRESA and now Oportunidades), uses cash transfers as incentives for parents to invest in their children's health and education so that they obtain the capabilities necessary to escape poverty when they reach adulthood. To improve reproductive health outcomes, Oportunidades' cash transfers to beneficiary households are conditioned, in part, on pregnant women completing a prescribed pre-natal care plan, obtaining nutritional supplements, and attending an educational programme about health and nutritional topics.
Across diverse settings, CCTs have been successful in increasing the use of health services as well as reducing child mortality, mortality, anaemia, and stunting (Bautista et al. 2004; Gertler 2004; Gertler & Fernald 2004; Maluccio & Flores 2004; Morris et al. 2004; Rivera et al. 2004; Barham 2005; Rawlings & Rubio 2005). Previous health evaluations of CCTs have focused on child health outcomes and service utilization. In this article, we evaluate whether Mexico's CCT programme had an impact on birthweight and pre-natal care utilization. Mexico is a good setting for this analysis as its CCT programme is the oldest and one of the largest programmes in existence. Despite efforts to reduce poverty and health disparities, Mexico's poor are characterized by conditions amenable to health interventions, including high rates of nutrition and vitamin-related deficiencies (Hernandez-Diaz et al. 1999; Jaime-Perez & Gomez-Almaguer 2002; Shamah-Levy et al. 2003; Villalpando et al. 2003), infectious diseases (Sanchez-Perez et al. 2002; Brentlinger et al. 2003), and preventable morbidity and mortality related to reproductive health (Calderon-Garciduenas et al. 2002; Palacio-Mejia et al. 2003; Frank et al. 2004, CONAPO 2007).
Methods
The programme
In 1997, Mexico introduced Oportunidades, a programme designed to break the intergenerational transmission of poverty, by providing incentives for parents to invest in the human capital of their children. Cash transfers are conditional on family members obtaining health and education services (Bautista et al. 2004; Gertler 2004; Rivera et al. 2004). Programme beneficiaries were phased-in based on federal resource availability, which allowed for an ethical evaluation of programme effectiveness. Coverage expanded from some 300 000 rural families in 1997 to approximately 2.6 million rural families in 2000. By 2007, the programme covered approximately five million low-income families (more than one in five of all families in Mexico) in both rural and urban settings (SEDESOL 2008).
The rural programme established eligibility in two stages: poor communities were first identified, and low-income households were identified within those communities (Skoufias et al. 1999). Poor communities were selected using a marginalization index constructed from census data measuring literacy, household infrastructure, and employment. Within poor communities, a socioeconomic survey was conducted to construct a proxy means test using data about socioeconomic characteristics, occupation, income, and disability; and access to health services. On average, 78% of the households in selected communities were classified as eligible for programme benefits, and 97% of eligible households with young children enrolled in the programme. Once enrolled, households received benefits for three years conditional on meeting programme requirements. To prevent migration into treatment communities, new households were unable to enrol until the next certification period.
Participating households receive cash transfers for health and education. The monthly health stipend is fixed at approximately US$ 15 per household per month. It is conditional on each family member obtaining regular clinic consultations, and attending pláticas (health education talks) and monthly meetings for the principal beneficiary, usually the mother in the household. Oportunidades required that households prove compliance via certification at public clinics and schools (Adato et al. 2000, SEDESOL 2003). The education transfer is based on school grade and sex. The maximum monthly benefit cap for health and education together equals approximately US$ 90 and US$ 160 for families with primary and high school children, respectively (Parker & Teruel 2005). Total transfers for health and education average 17–20% of pre-programme rural per capita household consumption (Gertler et al. 2004). Only 1% of households were denied the cash transfer due to non-compliance (Rivera et al. 2004).
The Oportunidades health requirements vary by age. For pregnant women, five pre-natal visits are required, with an emphasis on monitoring the pregnancy's progression; and the prevention, detection, and control of obstetric and perinatal risk factors. In addition to obtaining healthcare, milk-based nutritional supplements are recommended for pregnant and lactating women (Rosado et al. 2000).
Participating adults are required to attend monthly pláticas, which emphasize preventive care, sanitation, and hygiene. Specifically, pregnant women are required to attend meetings about what to expect from pre-natal care consultations, the clinical content of this care, maternal nutrition, and other reproductive health information.
Experimental design
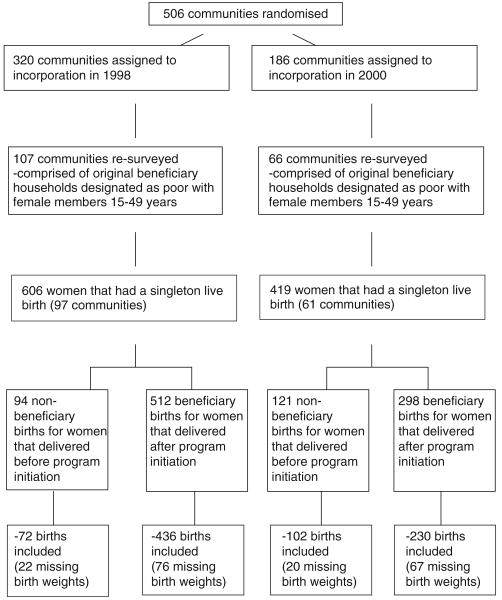
The government commissioned an independent evaluation of programme impact on health, nutrition and poverty outcomes. Planned as a randomized evaluation, it was based on a sample of 506 treatment communities, randomly selected using probabilities proportionate to the size of 6400 communities (Berhman & Todd 1999). Of the 506 experimental communities, 306 were randomly assigned to the treatment group, scheduled to receive benefits starting in April 1998. The remaining communities in the control group started to receive benefits in December of 1999. Participants were unaware of the timing of programme roll-out.
These analyses focus on pregnancy and birthweight; therefore, the study uses information from the fertility module about the date of birth and administrative records about the date of the first cash transfer received by beneficiary households. Beneficiary births are those births that occurred after the household received their first cash transfer. Non-beneficiary births are those that occurred among eligible women prior to receiving the first cash transfer.
Data processing
A fertility survey was fielded in 2003 to evaluate the programme's impact on reproductive health outcomes, using a subset of the original treatment and control communities, and a third group of women from communities that were not eligible for the intervention (CONAPO 2003). The survey used a two-stage stratified sampling design. Communities and households were randomly selected based on a probability sample proportionate to the number of women of reproductive age women (15–49 years). All eligible women were interviewed in selected households. Written consent for participation was obtained from the mother or household head. The project was approved by the Human Subjects and Ethics Committee of the National Institute of Public Health, Mexico. Among women identified for survey completion, 84% fully completed interviews. The most common reason cited for incompletion was not at home (5.1%); and 1.8% refused to be interviewed. Other reasons noted in the survey included permanent or temporary movements of the women targeted for interview. The sample includes women who experienced a singleton live birth between 1997 and 2003, were designated as poor and eligible for Oportunidades, and lived in the original treatment and control communities (Figure 1). With these limitations, the utilization analyses includes 1025 women. The main analyses about birthweight include 82% of these women (n = 840) that reported about birthweight (174 non-beneficiary and 666 beneficiary births).
Figure 1.
Flow diagram of study design and participants.
The main analyses use the dependent variable of birthweight reported by the mother. The survey relies on retrospective reports of birth outcomes. Evidence suggests that maternal reports are reliable for extended periods after delivery (O'Sullivan et al. 2000). Where available, the child's weight and birth date were checked with individual medical records. Birthweight is expressed as a continuous variable in grams and as a dichotomous variable of low birthweight (less than 2500 g). Dependent variables evaluating utilization include receipt of any pre-natal care, receipt of at least five pre-natal visits, and a continuous variable measuring the total number of pre-natal consultations. Obtaining five pre-natal care consultations is an Oportunidades' programme requirement.
From the fertility module, information was collected about maternal characteristics and birth histories, including maternal age, the number of prior pregnancies, prior miscarriage or abortion, the number of days after birth the infant was weighed, whether the mother smoked during pregnancy, and infant sex. Household and community socioeconomic and demographic baseline characteristics were collected from the 1997 census prior to the intervention. From census data, information included educational level and age of the head of household, maternal educational levels, age and number of all household members, indigenous speaking households, the number of large household assets (ownership of land, home ownership, refrigerator, gas heater, television, internal water in household, and electricity in household). Community factors included distance to the capital city, altitude, and the availability of a health centre, drainage system, and public telephone, and average male and female wages.
Analysis
Our primary analyses estimated programme impact on birthweight in grams and the probability of low birthweight. We also analysed programme impact on obtaining any pre-natal care, the minimum number of consultations required to receive Oportunidades benefits, and the total number of pre-natal visits. We used multivariate statistical methods that control for individual, household, and community covariates to reduce idiosyncratic variation and improve the power of the estimates. The continuous dependent variables were analysed using both community random effects and community fixed effects multivariate linear regression; the dichotomous dependent variables were analysed using community random effects and community fixed effects linear probability models as well as random effects logistic regression models. We found similar estimates across the statistical models, and report the random effects results. Both random and fixed effects take into account intra-cluster correlation that may exist because the interventions were randomized at the community level. The main analyses report the results for the coefficients measuring programme participation. Statistical analyses were done using stata (release stata 9.2; Stata Corp., College Station, TX, USA). Statistical significance was defined as P < 0.05.
Programme participation is measured in three ways to identify the overall programme impact and the relative importance of time on the programme and cash received. Overall programme impact is represented by a variable identifying beneficiary birth, defined as one that occurred after the household received their first cash transfer. Programme treatment intensity is defined as the number of months between the date of receiving the first cash transfer and the date of birth. Neither variable is likely to be correlated with household behaviours because the date of incorporation into the programme was randomly assigned (Berhman & Todd 1999). In addition, a previous study found no relationships between the programme and fertility decisions (Steklov et al. 2006).
Third, we identify the importance of cash received. Actual cash transfers are based, in part, on the households' decisions to send their children to school and compliance with programme requirements. These benefits were not allocated randomly and represent a source of bias. Instrumental variable analysis is a commonly used econometric method to remove the effects of hidden biases (Newhouse & McClellan 1998; Greenland 2000; Joffe & Midell 2006). We generated an instrument that operates through transfers received but is unlikely to be correlated with other behaviours of the household. The instrument used is potential monthly cash transfers per capita. Potential transfers are estimated by applying the programme rules to the household's pre-intervention demographic composition and children's school enrolment, assuming no school drop-outs or grade repetition. The instrumental variable analysis is similar to the ‘intent to treat’ analyses for randomized controlled trials because it assumes the absence of alternative pathways and effect modification. Potential cash transfers are used as an instrument for actual cash transfers in a two-staged least squares regression, and the coefficients produce an adjusted estimate on an absolute rather than relative scale.
The regression models were defined a priori based on well-established conceptual frameworks for the analysis of birthweight and its determinants (Rosenzweig & Schultz 1983; Mosley & Chen 1984; Kramer 1987). They include the following independent variables: maternal age, total prior pregnancies, prior miscarriage or abortion, whether the child was alive at the time of the survey, educational level of the household head of the mother (entered linearly and squared), age of the household head, whether the household spoke an indigenous language, possession of large assets, household size, the proportion of male and female children 0–5 and 6–17 years of age in the household, distance to the capital city (expressed as a logarithm), whether there was a public health centre in the community, and average male and female wage rates in the community. The household asset index was generated by summing up the individual items and expressing assets as a proportion of the total. Regressions explaining birthweight included additional covariates, such as whether the mother smoked during pregnancy, infant sex, the number of days after birth the infant was weighed, and altitude in meters. The tables report the overall programme impact. For programme intensity and cash transfers, the coefficients are multiplied by the average number of months on the programme and the average cash transfer received by beneficiaries, respectively.
Results
Table 1 compares the outcome variables, maternal and infant characteristics, and baseline household and community demographics and socioeconomics between non-beneficiary and beneficiary births. A total of 174 non-beneficiary and 666 beneficiary births were studied. The sampling strategy resulted in a well-balanced sample, as measured by only one significant difference at the 5% level for the 21 individual, household, and socioeconomic characteristics measured. Non-beneficiaries had more prior pregnancies (5.1) compared with 4.7 among beneficiaries.
Table 1.
Comparison of individual, household and community characteristics for non-beneficiary and beneficiary births
| Mean (standard deviation)* | ||||
|---|---|---|---|---|
| Variables | Non-beneficiaries | Beneficiaries | Difference | P-value |
| Maternal and infant characteristics† | ||||
| Maternal age (years) | 29.48 (6.38) | 29.22 (6.75) | −0.25 | 0.66 |
| Total prior pregnancies‡ | 5.05 (2.42) | 4.62 (2.59) | −0.43 | 0.04 |
| Prior miscarriage or abortion (%) | 8.05 | 6.61 | −1.44 | 0.49 |
| Mother smoked during pregnancy (%) | 4.60 | 4.80 | 0.20 | 0.89 |
| Days after birth weighed | 3.37 (7.81) | 2.48 (6.08) | −0.89 | 0.12 |
| Alive at time of interview (%) | 99.43 | 98.20 | −1.23 | 0.26 |
| Female (%) | 43.68 | 46.85 | 3.17 | 0.49 |
| Baseline household socioeconomics and demographics | ||||
| Household socioeconomic index (0–1) | 0.42 (0.18) | 0.41 (0.18) | −0.02 | 0.36 |
| Indigenous-speaking household (%) | 27.01 | 34.53 | 7.52 | 0.07 |
| Educational level of household head (years) | 3.70 (2.71) | 3.60 (2.57) | −0.10 | 0.73 |
| Age of household head (years) | 41.32 (8.91) | 40.17 (9.92) | 0.15 | 0.15 |
| Maternal educational level (years) | 4.18 (2.54) | 4.19 (2.73) | 0.01 | 0.95 |
| Household size | 6.51 (2.23) | 6.53 (2.43) | 0.03 | 0.91 |
| Males, 0–5 years in household (%) | 0.15 | 0.14 | −0.01 | 0.40 |
| Females, 0–5 years in household (%) | 0.16 | 0.14 | −0.02 | 0.15 |
| Males, 6–17 years in household (%) | 0.14 | 0.16 | 0.02 | 0.22 |
| Females, 6–17 years in household (%) | 0.16 | 0.14 | −0.01 | 0.25 |
| Baseline community characteristics | ||||
| Altitude (m) | 1255.43 (855.58) | 1333.69 (805.35) | 78.26 | 0.34 |
| Distance to urban centre (km) | 106.42 (43.94) | 107.91 (43.16) | 1.49 | 0.75 |
| Health centre in community (%) | 78.13 | 81.23 | 3.10 | 0.32 |
| Female wages, formal employment (pesos per month) | 163.38 (507.28) | 178.25 (576.46) | 14.87 | 0.72 |
| Male wages, formal employment (pesos per month) | 221.10 (1218.51) | 267.29 (1140.06) | 46.19 | 0.42 |
Unless otherwise indicated.
The number with data for the prenatal care visits is 804.
Differences significant at 5% level.
The first set of regressions evaluates programme impact on birthweight in grams and the odds of low birthweight (Table 2). In the unadjusted models, mean birthweight is 82 g higher for beneficiary births (P = 0.13). Including control variables that reduce residual variance, beneficiary status in the adjusted model predicts 127.3 g higher birthweight [95% confidence interval (CI): 21.3, 233.1; P = 0.02]. Separately, programme impact using the average beneficiary time on programme amounts to 68.3 g (P = 0.05), and programme impact from cash received amounts to 78.2 g (P ≤ 0.10). For low birthweight (Table 3), beneficiary status in the adjusted models predicts a 4.6 percentage point decrease in low birthweight and the average time on programme predicts a decline of 3.3 percentage points (P ≤ 0.05). The unadjusted model is not significant for low birthweight, but the coefficients are within the range of the CIs. We found no programme impact on pre-natal care-seeking, obtaining a minimum of five consultations, or the total number of consultations at conventional significance levels (Table 4).
Table 2.
Programme impact on birthweight in grams*
| Programme participation, model | Programme impact | P-value |
|---|---|---|
| Beneficiary at birth, unadjusted model | 81.98 | 0.13 |
| Beneficiary at birth, adjusted model† | 127.27 | 0.02 |
| Programme months, adjusted model | 68.26 | 0.05 |
| Cash transfer, instrumental variable model | 78.18 | 0.07 |
Number of observations is 840.
Adjusted and instrumental variable models include all maternal and infant, household and community variables listed in Table 1.
Table 3.
Programme impact on low birthweight*
| Programme participation variable, model† | Programme impact | P-value |
|---|---|---|
| Beneficiary at birth, unadjusted model | −0.031 | 0.18 |
| Beneficiary at birth, adjusted model | −0.046 | 0.05 |
| Programme months, adjusted model | −0.033 | 0.04 |
| Cash transfer, instrumental variable model | −0.036 | 0.06 |
Number of observations is 840.
Adjusted and instrumental variable models include all maternal and infant, household and community variables in Table 1.
Table 4.
Programme impact on prenatal care utilization*
| Got any prenatal care (=1) | Obtained five visits (=1) | Number of visits | ||||
|---|---|---|---|---|---|---|
| Programme participation variable, model† | Programme impact | P-value | Programme impact | P-value | Programme impact | P-value |
| Beneficiary at birth, unadjusted model | 0.0274 | 0.08 | 0.0342 | 0.36 | −0.2264 | 0.35 |
| Beneficiary at birth, adjusted model | 0.0250 | 0.12 | 0.0355 | 0.35 | −0.2034 | 0.42 |
| Programme months, adjusted model | 0.0173 | 0.10 | -0.0071 | 0.78 | −0.1039 | 0.53 |
| Cash transfer, instrumental variable model | 0.0235 | 0.06 | 0.0235 | 0.42 | −0.0022 | 0.99 |
Number of observations is 804.
Adjusted and instrumental variable models include maternal, household, and community variables listed in Table 1, relevant.
Discussion
We used retrospective reports from women who participated in a randomized effectiveness trial to examine the impact of Mexico's CCT programme on birthweight among poor rural women. Overall programme impact amounts to a 127.3 g increase in birthweight and a 4.6 percentage point reduction in the incidence of low birthweight. The magnitude of the results compares well with previous impact evaluations of this programme. Prior studies reported that children in participating households have a 25.3% reduction in illness episodes and the probability of anaemia (Gertler 2004), and an increase in age-adjusted height by 1.1 cm (Rivera et al. 2004). These large effects could be attributed to intervention population, which is marginalized, poor (less than 20th wealth percentile nationally), and characterized by high rates of modifiable risk factors that could plausibly be addressed by increased use of quality healthcare.
The magnitude of the results also compares well with findings from controlled trials. Protein energy or magnesium supplements during pregnancy reduce the risk of small for gestational age by 30% (Merialdi et al. 2003). Multiple micronutrient supplements resulted in reductions in the incidence of low birthweight (Christian et al. 2003; Zagré et al. 2007; SUMMIT et al. 2008); increases in birthweight of 64 g and 67 g (Christian et al. 2003; Zagré et al. 2007); and reductions in early infant mortality by 18–33% (SUMMIT et al. 2008). Cogswell et al. (2003) found that iron supplements resulted in higher birthweights (206 g) and declines in the incidence of low birthweight.
We consider three possible pathways for this impact. Improved birthweight could have resulted from improved maternal nutrition, higher health care utilization, or improvements in the quality of health care received. Accepting nutritional supplements was a programme requirement for pregnant and lactating women, and these supplements were designed to meet their nutritional needs. Studies have noted, however, that there were major problems related to compliance, leakage, and availability at health centers of nutritional supplements for the Oportunidades programme (Adato et al. 2000). Zarco et al. 2006 reported that participants initially experienced nausea, diarrhea, and vomiting from the supplements, which probably affected compliance. Efforts to minimize such side effects by diluting the supplement may have reduced its nutrient density. Substantial leakage may have occurred due to a culture of sharing food (Adato et al. 2000). All of these factors could have reduced the desired health impact of the supplements. However, better nutrition could have also resulted from increases in disposal income. Participating households consumed on average 75 cents of every peso from the transfer programme, which left increased disposable income for investments (Gertler et al. 2004). Previous studies documented that beneficiary households used the additional financial resources for purchasing more and more nutritious calories (Hoddinott & Skoufias 2003). Higher levels of cash are associated with improved child anthropometric outcomes, possibly attributable to food purchases or improvements in household sanitation or environment (Fernald et al. 2008). Better nutrition or sanitation could be one pathway contributing to the results.
Improved birth outcomes could have also resulted from higher health care utilization. Specifically for pregnant women, five pre-natal visits are required, with an emphasis on monitoring the pregnancy's progression; and the prevention, detection, and control of obstetric and perinatal risk factors. Bautista et al. 2004 reported that beneficiaries had higher rates of health service utilization; however, we found no differences in the odds of seeking pre-natal care or obtaining a higher number of consultations among women in this sample. It does not appear that the positive impact on birthweight, therefore, is attributable to increases in utilization resulting from the programme compliance requirements for pre-natal care.
Lastly, the programme impact on birthweight could have resulted from higher quality health care. The government had expressed an intention to increase supplies and human resources in anticipation of higher healthcare utilization in programme areas. However, a survey of 317 clinics conducted one year after programme implementation reported shortages of medical and support personnel, equipment, and drugs (Adato et al. 2000). At the same time, evidence suggests that beneficiaries did receive higher quality care (Barber & Gertler 2008). Given no evidence of supply-side improvements, this effect could be attributed to the programme's goal of promoting more informed and active consumers of healthcare. Future research about Oportunidades will disaggregate these pathways to explain how the programme resulted in better health outcomes among children and adults.
This study has several limitations. It is limited to rural areas and initial years of programme implementation. In using birthweight outcomes, the study assumes that infants who experience intrauterine growth restriction are smaller at birth. However, birthweight does not always capture growth anomalies and large infants can be growth restricted (Wilcox 2001). Increasing birthweight is desirable if it leads to positive long-term health and developmental outcomes. Because of the prevalence of infectious, maternal, and perinatal conditions, it is plausible that health care and nutrition address the conditions that promote low birthweight in this setting. This contrasts with high-income populations characterized by increasing rates of pre-term birth related to the use of assisted reproduction technology and obstetrical interventions – for which pre-natal nutritional, medical, and risk assessment procedures have limited impact (Lu et al. 2003).
The study relies on the accuracy of maternal reports. Studies about maternal recall of birth characteristics consistently report correlations between maternal recall and medical records for birthweight and/or gestational age at approximately 0.9 (Lumey et al. 1994; Yawn et al. 1998; McCormick & Brooks-Gunn 1999; Tomeo et al. 1999; Walton et al. 2000; Buka et al. 2004; Catov et al. 2006). Among studies from low-income populations, the results are similar, and demonstrate that mothers can accurately recall perinatal events. Correlations between maternal recall of birthweight and medical records ranged from 0.89 to 0.95 in Taiwan (Sou et al. 2006) and 0.89 to 0.96 in Israel (Gofin et al. 2000). Researchers in the Philippines reported specificity correlations of 0.8 to 0.9 for obstetrical complications reported by mothers and hospital records (Stewart & Festin 1995). Robles and Goldman (1999) compared birthweight data from health interview surveys with weighted estimates derived from delivery characteristics and maternal education. They conclude that survey data could underestimate the true incidence of low birthweight in a given country, and that most studies lack an objective standard of comparison. We are unaware of studies that have been conducted among the poor in rural Mexico about the accuracy of birthweight recall. However, this survey was designed by the Population Council in Mexico; in addition, household surveys such as the Demographic and Health Surveys conducted in low-income settings routinely use maternal reports.
The time interval is a factor in maternal recall, and there was a difference in the median time since birth between the groups in this study. We evaluated the presence of recall bias empirically with the data. Specifically, we estimated regression models for birthweight in grams for beneficiaries and non-beneficiaries with the explanatory variables as a set of dummy variables for child year of birth. We found no significant results for the year dummies, suggesting that time since birth did not affect recall bias in this study.
Women that reported birthweight in grams are associated with a higher number of household assets and maternal age. However, beneficiary status and time on the programme are not associated with the availability of birthweight data. As confirmed by the descriptive comparisons, missing birthweight observations do not affect the balance of characteristics between beneficiaries and non-beneficiaries for this sample.
Conclusions
Many governments have turned to CCT programmes as a means of improving the health and schooling of children born into poor families. By providing money directly to poor households, conditional cash transfers aim to better target the poor and overcome household financial constraints in accessing services. We find that the programme contributed to higher birthweight and lower incidence of low birthweight among beneficiary women. The study recognizes the problems with retrospective reports. However, the results contribute to a growing body of evidence that these programmes increase investments in children's health, and that these investments have paid off in terms of better health outcomes in early life. These findings may be applicable to other large-scale incentive based welfare programmes, which employ conditional cash transfers and health utilization requirements.
Acknowledgments
The authors are grateful for comments and assistance from Tania Barham, Becca Feldman, Juan Pablo Gutierrez and Marta Rubio. The authors remain responsible for all errors and omissions. This research was funded by grants from the National Institutes of Health Fogarty International Center TW006084 and National Institute of Child Health and Human Development.
References
- Adato M, Coady D, Ruel M. An Operations Evaluation of PROGRESA from the Perspective of Beneficiaries, Promotoras, Schools Directors, and Health Staff. International Food Policy Research Institute; Washington DC: 2000. [Google Scholar]
- Alderman H, Berhman JR. Reducing the incidence of low birthweight in low-income countries has substantial economic benefits. World Bank Research Observer. 2006;21:25–48. [Google Scholar]
- Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. European Journal of Clinical Nutrition. 1998;52(Suppl 1):S34–S41. [PubMed] [Google Scholar]
- Barber SL, Gertler PJ. Empowering women to obtain higher quality care: evidence from an evaluation of Mexico's conditional cash transfer program. Health Policy and Planning. 2008 doi: 10.1093/heapol/czn039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham T. Providing a Healthier Start to Life: The Impact of Conditional Cash Transfer Programs on Infant Mortality. Working paper, University of Colorado; Boulder: 2005. [Google Scholar]
- Bautista S, Bertozzi S, Gertler P, Gutierrez JP, Hernandez M. The Impact of Oportunidades on Health Status, Morbidity, and Service Utilization among Beneficiary Population: Short-term Results in Urban Areas and Medium-term Results in Rural Areas. National Institute of Public Health; Cuernavaca, Mexico: 2004. [Google Scholar]
- Bergström S. Infection-related morbidities in the mother, fetus, and neonate. Journal of Nutrition. 2003;133:1656S–1660S. doi: 10.1093/jn/133.5.1656S. [DOI] [PubMed] [Google Scholar]
- Berhman J, Todd P. A Report on the Sample Sizes Used for the Evaluation of the Education, Health, and Nutrition Program (PROGRESA) of Mexico. International Food Policy Research Institute; Washington DC: 1999. [Google Scholar]
- Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115:519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- Blondel B, Kogan MD, Alexander GR, et al. The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: an international study. American Journal of Public Health. 2002;92:1323–1330. doi: 10.2105/ajph.92.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentlinger PE, Capps L, Densen M. Hookworm infection and anemia in adult women in rural Chiapas, Mexico. Salud Pública de México. 2003;4:117–119. doi: 10.1590/s0036-36342003000200008. [DOI] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal pregnancy event: accuracy of maternal recall. Schizophrenia Research. 2004;71:417–426. doi: 10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas AL, Martinez-Salazar G, Fernandez-Diaz H, Cerda-Flores RM. Hospital maternal mortality: causes and consistency between clinical and autopsy diagnosis at the Northeastern Medical Center of the IMSS, Mexico. Ginecología Obstetricia de México. 2002;70:95–102. [PubMed] [Google Scholar]
- Catov JM, Newman AB, Kelsey SF, et al. Accuracy and reliability of maternal recall of infant birthweight among older women. Annals of Epidemiology. 2006;16:429–431. doi: 10.1016/j.annepidem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birthweight in rural Nepal: double blind randomized community trial. British Medical Journal. 2003;326:571–574. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell ME, Parvanta I, Ickes L, Yip R, Britternham GM. Iron supplementation during pregnancy, anemia, and birthweight: a randomized controlled trial. American Journal of Clinical Nutrition. 2003;78:773–781. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- CONAPO (National Population Council) Survey to Measure the Reproductive Health Impact of the Oportunidades Program 2003: The Sampling Design. Population Council; Mexico City: 2003. [Google Scholar]
- CONAPO (National Population Council) Indicators of Reproductive Health from the Mexican Republic (in Spanish) Population Council; Mexico City: 2007. [Google Scholar]
- Fernald LC, Gertler PJ, Neufeld LM. Role of cash in conditional cash transfer programmes for child health, growth, and development: an analysis of Mexico's Oportunidades. Lancet. 2008;371:828–837. doi: 10.1016/S0140-6736(08)60382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R, Pelcastre B, Salgado de Snyder VN, Frisbie WP, Potter JE, Bronfman-Pertzovsky MN. Low birthweight in Mexico: new evidence from a multi-site postpartum hospital survey. Salud Pública de México. 2004;46:23–31. doi: 10.1590/s0036-36342004000100004. [DOI] [PubMed] [Google Scholar]
- Gertler PJ. Do Conditional Cash Transfers improve child health? Evidence from PROGRESA's control randomized experiment. American Economic Review. 2004;94:336–341. doi: 10.1257/0002828041302109. [DOI] [PubMed] [Google Scholar]
- Gertler PJ, Fernald L. The Medium Term Impact of Oportunidades on Child Development in Rural Areas. National Institute of Public Health; Cuernavaca, Mexico: 2004. [Google Scholar]
- Gertler PJ, Martinez S, Rubio M. The Impact of Oportunidades on Micro-Enterprise and Agricultural Production Activities in Rural Mexico. Technical Document No 19. National Institute of Public Health; Cuernavaca, Mexico: 2004. [Google Scholar]
- Gofin R, Neumark YD, Adler B. Birthweight recall by mothers of Israeli children. Public Health. 2000;114:161–163. [PubMed] [Google Scholar]
- Greenland S. An introduction to instrumental variables for epidemiologists. International Journal of Epidemiology. 2000;29:722–729. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Peterson KE, Dixit S, et al. Association of maternal short stature with stunting in Mexican children: common genes vs common environment. European Journal of Clinical Nutrition. 1999;53:938–945. doi: 10.1038/sj.ejcn.1600876. [DOI] [PubMed] [Google Scholar]
- Hoddinott J, Skoufias E. The impact of Progresa on food consumption. Food and Nutrition Bulletin. 2003;24:379–380. doi: 10.1177/156482650302400410. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Preventing Low Birthweight. National Academy Press; Washington DC: 1985. [PubMed] [Google Scholar]
- Jaime-Perez JC, Gomez-Almaguer D. Iron stores in low-income pregnant women at term. Archives of Medical Research. 2002;33:81–84. doi: 10.1016/s0188-4409(01)00346-0. [DOI] [PubMed] [Google Scholar]
- Joffe M, Midell J. Complex causal process diagrams for analyzing the health impacts on policy interventions. American Journal of Public Health. 2006;96:473–479. doi: 10.2105/AJPH.2005.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birthweight: methodological assessment and meta-analysis. Bulletin of the World Health Organization. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. Journal of Nutrition. 2003;133:1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- Lu MC, Tache V, Alexander GR, Kotelchuck M, Halfon N. Preventing low birthweight: is prenatal care the answer? Journal of Maternal, Fetal, and Neonatal Medicine. 2003;13:362–380. doi: 10.1080/jmf.13.6.362.380. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Ravelli AC. Maternal recall of birthweights in adult children: validation by hospital and well baby clinic records. International Journal of Epidemiology. 1994;23:1006–1012. doi: 10.1093/ije/23.5.1006. [DOI] [PubMed] [Google Scholar]
- Maluccio JA, Flores R. Food Consumption and Nutrition Division Discussion Paper No 184. International Food Policy Research Institute; Washington DC: 2004. Impact Evaluation of a Conditional Cash Transfer Program: The Nicaraguan Red de Protección Social. [Google Scholar]
- McCormick M. The contribution of low birthweight to infant mortality and childhood morbidity. New England Journal of Medicine. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- McCormick MC, Brooks-Gunn J. Concurrent child health status and maternal recall of events in infancy. Pediatrics. 1999;104:1176–1181. [PubMed] [Google Scholar]
- Merialdi M, Carroli G, Villar J, et al. Nutritional interventions during pregnancy for the prevention or treatment of impaired fetal growth: an overview of randomized controlled trials. Journal of Nutrition. 2003;133(Suppl 2):1626S–1631S. doi: 10.1093/jn/133.5.1626S. [DOI] [PubMed] [Google Scholar]
- Moore SE, Cole TJ, Collinson AC, Poskitt EM, McGregor IA, Prentice AM. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. International Journal of Epidemiology. 1999;28:1088–1095. doi: 10.1093/ije/28.6.1088. [DOI] [PubMed] [Google Scholar]
- Morris S, Flores R, Olinto P, Medina J. Monetary incentives in primary health care and effects on use and coverage of preventive health care interventions in rural Honduras: cluster randomized trial. Lancet. 2004;364:2030–2037. doi: 10.1016/S0140-6736(04)17515-6. [DOI] [PubMed] [Google Scholar]
- Mosley J, Chen H. An analytical framework for the study of child survival in developing countries. Population and Development Review. 1984;10(Suppl):25–45. [Google Scholar]
- Newhouse J, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annual Review of Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- O'Sullivan JJ, Pearce MS, Parker S. Parental recall of birthweight: how accurate is it? Archives of Diseases in Childhood. 2000;82:202–203. doi: 10.1136/adc.82.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. European Journal of Clinical Nutrition. 1998;52(Suppl 1):S5–S15. [PubMed] [Google Scholar]
- Palacio-Mejia LS, Rangel-Gomez G, Hernandez-Avila M, Lazcano-Ponce E. Cervical cancer, a disease of poverty: mortality differences between urban and rural areas in Mexico. Salud Pública de México. 2003;45(Suppl 3):S315–S325. doi: 10.1590/s0036-36342003000900005. [DOI] [PubMed] [Google Scholar]
- Parker S, Teruel GM. Randomization and social program evaluation: the case of Progresa. Annals of the American Academy of Political and Social Science. 2005;599:199–219. [Google Scholar]
- Prentice M, Moore SE. Early programming of adult diseases in resource poor countries. Archives of Disease in Children. 2005;90:429–432. doi: 10.1136/adc.2004.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings LB, Rubio GM. Evaluating the impact of conditional cash transfer programs. World Bank Research Observer. 2005;20:29–55. [Google Scholar]
- Rivera JA, Sotres-Alvarez D, Habicht JP, Shamah T, Villalpando S. Impact of the Mexican program for education, health, and nutrition (Progresa) on rates of growth and anemia in infants and young children. A randomized effectiveness study. Journal of the American Medical Association. 2004;291:2563–2570. doi: 10.1001/jama.291.21.2563. [DOI] [PubMed] [Google Scholar]
- Robles A, Goldman N. Can accurate data on birthweight be obtained from health interview surveys? International Journal of Epidemiology. 1999;28:925–931. doi: 10.1093/ije/28.5.925. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Rivera J, Lopez G, Solano L. Development, production, and quality control of nutritional supplements for a national supplementation program in Mexico. Food and Nutrition Bulletin. 2000;21:30–34. [Google Scholar]
- Rosenzweig MR, Schultz TP. Estimating a household production function: heterogeneity, the demand for health inputs, and their effects on birthweight. Journal of Political Economy. 1983;91:723–746. [Google Scholar]
- Sanchez-Perez HJ, Hernan MA, Hernandez-Diaz S, Jansa JM, Halperin D, Ascherio A. Detection of pulmonary tuberculosis in Chiapas, Mexico. Annals of Epidemiology. 2002;12:166–172. doi: 10.1016/s1047-2797(01)00308-8. [DOI] [PubMed] [Google Scholar]
- SEDESOL (Ministry of Social Development) Agreement for Issue and Publication of the Operational Rules of the Oportunidades Program for Human Development for the Fiscal Year 2003. Government of Mexico; Mexico City: 2003. [Google Scholar]
- SEDESOL (Ministry of Social Development) Oportunidades Program for Human Development. Monitoring, Evaluation, and Management Indicators and Results of the Oportunidades Program by State. Government of Mexico; Mexico City: 2008. [Google Scholar]
- Shamah-Levy T, Villalpando S, Rivera JA, Mejia-Rodrigues R, Camacho-Cisneros M, Monterruibio EA. Anemia in Mexican Women: a public health problem. Salud Pública de México. 2003;45(Suppl 4):S499–S507. doi: 10.1590/s0036-36342003001000006. [DOI] [PubMed] [Google Scholar]
- Skoufias E, Davis B, Berhman J. An Evaluation of the Selection of Beneficiary Households in Progresa: Final Report. International Food Policy Research Institute; Washington DC: 1999. [Google Scholar]
- Sou SC, Chen WJ, Hsieh WS, Jeng SF. Severe obstetrical complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. Journal of Clinical Epidemiology. 2006;59:429–435. doi: 10.1016/j.jclinepi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Steklov G, Winters P, Todd J, Regalia F. Department of Economics Working Paper Series No 2006-1. American University; Washington DC: 2006. Demographic Externalities from Poverty Programs in Developing Countries: Experimental Evidence from Latin America. [Google Scholar]
- Stewart MK, Festin M. Validation study of women's reporting and recall of major obstetrical complications treated at the Philippine General Hospital. International Journal of Gynecology and Obstetrics. 1995;48(Suppl):S53–S66. doi: 10.1016/0020-7292(95)02320-c. [DOI] [PubMed] [Google Scholar]
- SUMMIT Study Group. Shankar AH, Jakari AB, et al. Effect of maternal micronutrient supplementation on fetal loss and infant death in Indonesia: a double blind cluster-randomised trial. Lancet. 2008;371:215–227. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- Tomeo CA, Rich-Edwards JM, Michels KB, Berkey CS, Hunter DJ, Frazier AL. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- United Nations Children's Fund & the World Health Organization. Low Birthweight: Country, Regional, and Global Estimates. UNICEF; New York: 2004. [Google Scholar]
- Villalpando S, Montalvo-Velarde I, Zambrano N, et al. Vitamins A, and C and folate status in Mexican children under 12 years and women 12–49 years: a probabilistic national survey. Salud Pública de México. 2003;45(Suppl 4):S508–S519. doi: 10.1590/s0036-36342003001000007. [DOI] [PubMed] [Google Scholar]
- Villar J, Belizan JM. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstetrics and Gynecological Survey. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- Villar J, Ba'aqeel H, Piaggio G, et al. WHO antenatal care randomised trial for the evaluation of a new model of routine antenatal care. Lancet. 2001;357:1551–1564. doi: 10.1016/s0140-6736(00)04722-x. [DOI] [PubMed] [Google Scholar]
- Walton KA, Murray LJ, Gallagher AM, Cran GW, Savage MJ, Boreham C. Parental recall of birthweight: a good proxy for recorded birthweight? European Journal of Epidemiology. 2000;16:793–796. doi: 10.1023/a:1007625030509. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance – and the unimportance – of birthweight. International Journal of Epidemiology. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Suman VJ, Jacobson SJ. Maternal recall of distant pregnancy events. Journal of Clinical Epidemiology. 1998;51:399–405. doi: 10.1016/s0895-4356(97)00304-1. [DOI] [PubMed] [Google Scholar]
- Zagré NM, Desplats G, Adou P, Mamdoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster randomized, double-blind, controlled programmatic study in Niger. Food and Nutrition Bulletin. 2007;28:317–327. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- Zarco A, Mora G, Pelcastre B, Flores M, Bronfman M. Acceptability of dietary supplements in the national Mexican program ‘Oportunidades’. Salud Pública de México. 2006;48:325–331. doi: 10.1590/s0036-36342006000400007. [DOI] [PubMed] [Google Scholar]