How did the study come about?
Mental illness makes a major contribution to the global burden of disease. The impact of disorders such as depression and anxiety has been heightened by the increasing success of public health measures in controlling physical diseases. Mental health disorders are severely impairing in their own right but may also exacerbate the disability resulting from physical disease. The situation will be worse globally as the pattern of morbidity seen in the developed world sweeps over low- and middle-income countries. Mental illness is commonly understood to result from complex interactions between vulnerability and stress, though such a model is uniquely difficult to study, particularly in longitudinal or life course designs. A considerable proportion of individuals who experience mental illness during their lives report the emergence of symptoms and impairment during the adolescent years.
Adolescence is a critical period of accelerated maturation. Individuals differ widely in their rate of physical, social, psychological and sexual development. Physiological changes occurring over the second decade of life include alterations in gonadal hormone levels as well as significant elevations in glucocortioids1 resulting in physical maturation. There are also changes in psychological functions associated with brain development such as cognitive control of emotions, greater reasoning skills and problem solving ability.2,3 These changes occur within the contexts of peer groups, school and family settings, each of which has been shown to have an important impact on individual development.
A striking feature of this period is the emergence of major mental illness, such as depressive, anxiety, eating and behaviour disorders and psychoses, some of which have their genesis earlier in childhood. Early onset carries considerable risk for continuity and recurrence into adult life.4,5 In particular, rates of depression and suicide rise alarmingly over the adolescent period. The co-occurrence of two or more diagnoses is the rule rather than the exception. Likewise, gender is of considerable importance as there are markedly emerging sex differences in the incidence of emotional disorders (females > males) in the adolescent period. In contrast, the known ratio of three males for every female in childhood conduct disorder contrasts with an almost equal sex ratio for cases beginning in the adolescent years.4,5
What do we know about the risk factors for these disorders?
There have been a number of large and influential studies characterizing selected risk factors in adolescence and determining their relative predictive effects for mental illness.4 These have shown a consistent pathogenic role for long-standing social adversities in a child's life (i.e. exposures lasting more than a few weeks at a significant level of intensity); the precise nature of which have yet to be fully characterized. There is also considerable evidence for complex and changing interrelationships over time between genetic and environmental influences on the onset and persistence of the majority of mental illnesses.6,7 The extent to which patterns of risk are gender differentiated or linkages along pathways to diagnostic outcomes are moderated by biological sex is not known. While much remains to be understood regarding the precise nature of the interplay between genes and environments (GxE interactions and G.E correlations), there is considerable interest in determining the intermediate biology that transforms a genetically and/or environmentally vulnerable individual into one whose maladaptive response to personal and social difficulties leads to psychiatric disorder.
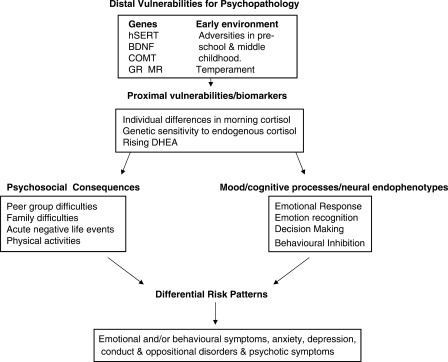
To date, none of the cohort studies that has focused on adolescent mental health has incorporated a comprehensive approach to characterize the range of causal pathways from genes through intermediate physiology and psychology to psychopathological outcomes. The closest comparison is with the important Dutch study, Tracking Adolescents’ Individual Lives Survey (TRAILS). This is a prospective population-based cohort study aimed at understanding the aetiology of mental health problems. This multidisciplinary study collected data from various sources (parent, child, teacher, peers) via multiple methods (self-report, interview, neurocognitive tests and biological measures) and followed participants from pre-adolescence, (aged 10–12 years) to young adulthood (aged 24 years).5 The ROOTS cohort has added a unique component in its theoretical framework (Figure 1), which proposes measurable biological mechanisms arising from the interplay between genes and environment, from infancy to adolescence, to predict the onset of psychiatric episodes through the second and third decades of life.
Figure 1.
Theoretical framework
What is being measured and why: the scope of ROOTS measurements?
The ROOTS study adopts a standard longitudinal design. The objectives are to evaluate the relative contributions of defined genetic, physiological, psychological and social variables, occurring at different stages of child development, to the overall risk for psychopathology during adolescence. In order to formulate specific developmentally sensitive hypotheses, we have selected detailed measures of risk from both within and external to the adolescent, in a repeat measures design with three waves of data collection (baseline, 18- and 36-month follow-ups). The primary intention is to delineate the genetically sensitive intermediate biology (endophenotypes and biomarkers) that precedes, correlates with and/or predicts dimensional risk markers for psychiatric disorders according to current clinical phenotypes. We also expect that genetic and environmental factors may vary in their effects at different points in time. For example, environmental factors have been shown to be significant during adolescence for predicting substance misuse disorders, whereas genetic factors become progressively more important through early and middle adulthood.6
Our theory proposes two sets of risk processes, distal and proximal, which render individuals at differential levels of vulnerability for subsequent psychopathology. Distal vulnerabilities (DV) arise from genetic variations and early childhood factors that influence individual differences in endophenotype severity and prodromal dimensions of moods, feelings and behaviour. Proximal vulnerabilities (PV), occurring during adolescence, include rising cortisol and DHEA and level of cognitive control of emotions influencing individual differences in stress response behaviour.
We do not propose that we know, or can measure, all possible DV factors. Rather, informed by prior studies, we have selected those (genes and environments) we consider the strongest markers for DV. We have selected four physiological systems in which genetic polymorphisms of susceptibility genes have been associated with adverse social environments, abnormal psychology and/or mental illness. These are polymorphisms in brain-derived neurotrophic factor (BDNF), serotonin (5HTTLPR, 5HT1a, 5HT2c), selected glucocorticoids (NR3C1) and mineralocorticoid nuclear receptors (NR3C2), COMT (Catechol-O-methyl transferase) and monoamine oxidase (MAO-A). The full list of selected genes is outlined in Table 1.
Table 1.
Genotyping of the ROOTS cohort
| dbSNP | Gene symbol | Description |
|---|---|---|
| rs988748 | BDNF | Brain-derived neurotrophic factor |
| rs12273363 | BDNF | Brain-derived neurotrophic factor |
| rs6265 | BDNF, BDNFOS | Brain-derived neurotrophic factor, brain-derived neurotrophic factor opposite strand |
| rs6352 | SLC6A4 | Solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 |
| rs1799921 | HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A |
| rs1800041 | HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A |
| rs6295 | HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A |
| rs6304 | HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A |
| rs1805055 | HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A |
| rs6314 | HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A |
| rs6311 | HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A |
| rs6318 | HTR2C | 5-hydroxytryptamine (serotonin) receptor 2C |
| rs3813929 | HTR2C | 5-hydroxytryptamine (serotonin) receptor 2C |
| rs7488262 | TPH2 | Tryptophan hydroxylase 2 |
| rs34115267 | TPH2 | Tryptophan hydroxylase 2 |
| rs1386494 | TPH2 | Tryptophan hydroxylase 2 |
| rs1803986 | MAOA | Monoamine oxidase A |
| rs1800466 | MAOA | Monoamine oxidase A |
| rs1799835 | MAOA | Monoamine oxidase A |
| rs2289658 | NTRK2 | Neurotrophic tyrosine kinase, receptor, type 2 |
| rs4680 | COMT, ARVCF | Catechol-O-methyltransferase, armadillo repeat gene deletes in velocardiofacial syndrome |
| rs2241165 | GAD1 | Glutamate decarboxylase 1 (brain, 67 kDa) |
| rs6196 | NR3C1 | Nuclear receptor subfamily 3, group C, member 1 (glucocorticoid) |
| rs5522 | NR3C2 | Nuclear receptor subfamily 3, group C, member 2 (mineralocorticoid) |
The second component of our DV model involves measuring the adolescent's exposure to adversities. We have undertaken retrospective investigations of childhood experience using a new semi-structured interview (described in detail below) conducted with the primary care-giver. This interview assesses adversities over the child's lifetime. We will be able to determine the nature of the measured DV by combining selected genetic factors (hSERT, BDNF, GR/MR, COMT) i.e. particular polymorphisms, with patterns of adverse experiences including family discord, exposure to maltreatment and specific life events and difficulties such as accidents and illnesses and being taken into care.
Our criteria for selecting our PV factors are similar to those we used to identify DV. Within the adolescent, these include physical measures, hormone measures (cortisol and DHEA), psychological and behavioural measures and current mental well-being including the presence of current and recent psychiatric disorder. We also evaluated various psychosocial experiences occurring in peer group or family domains of the adolescent.
There is a complex relationship between measured cortisol and its interpretation as a DV or a PV. Animal studies suggest there may be epigenetic effects on the glucocorticoid receptor as one result of an adverse early social environment with subsequent effects on the hypothalamic–pituitary-axis and hence circulating cortisol levels.7–10 Prospective studies on both adolescents and adult women show that higher morning cortisol levels are associated with the subsequent onset of affective disorders.11–13 In contrast, conduct disorders are associated with cortisol hypo-reactivity to stress, but show no differences in basal morning cortisol.14 These distinctions in the HPA axis may indicate discrete pathophysiological processes for emotional and behavioural disorders, respectively. One of the goals of this study is to define more precisely how these hormonal factors interact with genes and social environments, to determine psychopathological outcome.
In addition, we tested whether neuropsychological characteristics were potential consequences of specific patterns of DV or PV factors or both. Thus, at the age of 16 years, 280 participants took part in an in-depth investigation of frontal executive functioning. They were selected on the basis of genetic and/or environmental risk for psychopathology. We focused on assessing ‘top down’ processes of decision making, behavioural inhibition and reversal learning (i.e. cognitive flexibility when exposed to unexpected and sudden changes in a recently learned computerized task). Deficits in these skills are associated with impairments in emotion processing15 and may form part of an endophenotypic profile for a range of mood-related psychiatric disorders such as depression,16 anxiety17 and obsessional disorders.18 Executive dysfunctions have also been described in child conduct disorders19 but their precise role is unclear. The general objective of this investigation is to understand how far these deficits contribute individually to patterns of risk processes, and how they interact. More specifically it is not known if, in normal adolescents, these neuropsychological features relate to distal genetic (e.g. allelic variation in 5HTTLPR) or environmental (e.g. childhood family discord or morning cortisol levels) vulnerabilities or arise in relation to more PV (e.g. recent life events and difficulties or variation cortisol reactivity).
Who is in the sample?
We attempted to recruit a broad range of young people from the County of Cambridgeshire. To that end, we recruited from a wide geographical area extending 30 miles north, 20 miles south and 20 miles west of Cambridge as well as from the city and surrounding villages (Figure 2).
Figure 2.
Two ROOTS participating schools
We approached 27 secondary schools (25 state and 2 private schools), requesting permission both to recruit eligible students via the school and interview consenting students in school during the school day. Eligible students were those aged between 14 and 14 years 11 months during the allotted 2-week interview period in each school. Eighteen schools agreed to take part and 3762 students were invited. Written, informed consent (from both the teenager and a parent) was required.
Our initial recruitment material, rather formal information letters and consent forms, yielded a consent rate of only 18% from the first four schools. We redesigned our literature to take the form of a short invitation letter, coloured leaflet and small consent postcard. We also gave presentations in schools at year-group assemblies accompanied by Q&A sessions. The consent rate rose to 38% in schools 6–19. School five straddled the old and new approaches in that they received the original literature but also received the school assemblies. Recruitment here was 32%. Overall, consents were received from 1238 (33%), 675 girls (54.5%) and 563 (45.5%) boys.
We used the ACORN categories developed by CACI (http://www.caci.co.uk) and derived from post-code data as a proxy measure of social and economic class. ACORN generates five categories, which are further divided into 17 groups and 56 types. We have used the five primary categories only. Table 2 compares the ACORN categories of the ROOTS sample (both initial invitations and subsequent consents) to both local Cambridgeshire and wider UK figures provided by CACI.
Table 2.
ROOTS sample by ACORN category and comparison with wider populations
| A ACORN category | B ROOTS: invited x ACORN category (data on 3487/3762) n (%) | C Cambridgeshire (data supplied by CACI) (%) | D UK (data supplied by CACI) (%) | E ROOTS: consents x ACORN category (data on 1231/1238) n (%) | F ROOTS: consents within ACORN category percentage of column B |
|---|---|---|---|---|---|
| 1. Wealthy Achiever | 1652 (47.4) | 41.2 | 25.4 | 665 (54) | 40 |
| 2. Urban Prosperity | 197 (5.6) | 11.1 | 11.5 | 81 (6.6) | 41 |
| 3. Comfortably off | 917 (26.3) | 30.1 | 27.4 | 302 (24.5) | 33 |
| 4. Moderate means | 298 (8.5) | 6.6 | 13.8 | 59 (4.8) | 20 |
| 5. Hard-pressed | 423 (12.1) | 10.9 | 21.2 | 124 (10.1) | 29 |
Table 2 shows the proportion of participants within the ROOTS sample, by ACORN category. Compared with UK figures, ROOTS/Cambridgeshire samples have twice as many ‘wealthy achievers’ but only half the number of ‘moderate means’ or ‘hard-pressed’ families.
How often have they been followed up?
The sample was re-assessed at 18 and 36 months after initial assessment. Our data therefore span adolescence from 14 to nearly 18 years of age. This maximizes the opportunity for detection of developmental changes in the measured variables, as this age range will include many who pass from early to completed puberty. Initial data collection commenced in April 2005 and continued until mid-December 2006. The first postal questionnaire follow-up at 18 months commenced in November 2006 and was completed in May 2008. The final data collection phase began in February 2008 and is scheduled for completion in December 2009.
What has been measured—how and when?
Data from both parent and child have been collected at each wave of measurement, using both self-report and semi-structured interview measures. On receipt of written, informed consent from both child and a parent/carer, questionnaire packs were posted home, independently, to both (we requested the mother participate where possible). Sample containers for saliva collection were also sent to students’ homes with instructions and a ‘spit diary’ for recording details of collection. Adolescents were asked to provide saliva samples morning and evening for three consecutive school days. Within 2 weeks, students were interviewed individually at school and asked to provide another saliva sample for DNA extraction. Mothers were interviewed at home within 6 months of completing their questionnaires. At interview, both mother and student completed further self-report measures. A full description of measures at each time period is provided in Tables 3 and 4.
Table 3.
ROOTS psychosocial measures at three time points in adolescents
| Self-reports |
Interview assessment |
Salivary sampling |
|||||
|---|---|---|---|---|---|---|---|
| Timing | Clinical symptoms | Cognitive evaluations | Lifestyle | Mental state | Physical state | Endocrinology | SNPS |
| At entry | Mood and feelings; revised manifest anxiety scale; short Leyton obsessional inventory; behaviour symptoms | Rosenberg self-esteem scale; mood-related ruminative response style; depressed states questionnaire; personal mastery | Eating, smoking and alcohol habits; self-harming behaviour; pastimes/hobbies; friendship satisfaction; family function; recent life events and difficulties | K-SADS-PL | Height; weight; body composition; waist circumference; continuous 7-day pedometer assessment | Cortisol DHEA | See separate table |
| 18 months | Mood and feelings; revised manifest anxiety scale; behaviour symptoms | Mood-related ruminative response style; personal mastery | Friendship satisfaction; life events | None | None | None | None |
| 36 months | As for entry plus psychological distress (K-10); schizotypal symptoms (brief SSI) | As for entry plus well-being | As for entry plus bullying | K-SADS PL; psychotic symptoms (PLIKS) | Height; weight; body composition | None | None |
Table 4.
ROOTS psychosocial measures at three time points in parents
| Self-reports |
Interview assessment |
||||
|---|---|---|---|---|---|
| Timing | Family characteristics | Cognitive evaluation of adolescent | Lifestyle | Mental state | Family environment and health |
| At entry | Demographics; ethnicity; maternal education; lifetime mental and physical diagnosis of each family member | Social and emotional awareness; temperament | Friendship satisfaction; family function; recent life events/difficulties | MINI-International Neuropsychiatric Interview (MINI) | Early life experiences (family discord, abuse, health problems and diagnoses, financial difficulties, separations, neglect, loss, homelessness, parenting style, school and friendship difficulties). Obstetric history, birth weight, developmental milestones |
| 18 months | Social and emotional awareness | Life events and difficulties; parents' height/weight | None | None | |
| 36 months | Demographics; lifetime mental and physical diagnosis of each family member; mothers’ psychological distress and schizotypal symptoms | As for entry | Family function | None | None |
At the first follow-up phase a selection of the baseline self-report questionnaires were repeated and posted home, to both adolescents and parents/carers. The final follow-up phase, 3 years after initial interviews, consists of a repeat K-SADS PL interview and repeated self-report measures extended to include a short screen for psychotic symptoms, a measure of psychological distress, a bullying questionnaire and a schizotypal symptom measure (Tables 3 and 4).
The Cambridge early experience interview
The Cambridge early experience interview (CAMEEI) was developed as a user-friendly, non-judgemental, semi-structured measure of childhood experience that would extend the scope and quality of existing instruments.11,20–22 This semi-structured interview is conducted with the child's primary care-giver. CAMEEI involves counts of life experiences, child's age at occurrence, duration, and an interviewer assessment of their practical impact on the daily life of the family. Pre-interview mothers completed a three-section timeline: pre-school years, primary school years and secondary school years, on which they were requested to record any events, positive or negative, they felt had been important in the life of the child. These timelines became the starting point for the interview. Events were recorded in each time period throughout the life of the child. In this way we hope to characterize not only the number, type and level of impact of events and difficulties, but also describe a developmental gradient of social risk from birth through infancy, the pre-school years and early childhood. Inter-rater reliability with 48 mothers on a number of core items has been highly satisfactory (kappa ranges from 0.7 to 0.9).
Retention in the study
We have actively sought to retain the interest and compliance of our young sample. We have a distinctive ROOTS logo, which appears on all correspondence and gives the study a strong identity in the community. We have maintained contact with participating families and schools via customized birthday and Christmas cards and a termly newsletter. The ROOTS website, http://www.roots.group.cam.ac.uk, on which we post-commentary, selected observations and points of interest, will serve as a repository for reporting findings.
Of the original 1238 consents, 53 families withdrew at the first data collection point (either before during or immediately after the parent or proband interview). A further 44 parents withdrew but gave permission for their offspring to remain in the study, and three mothers could not be contacted for the interview or did not attend their appointment (but did not actively withdraw). Therefore 1185 students (95.5%) and 1141 parents (92%) progressed.
At the 18-month follow-up stage 1141 parent and 1185 student questionnaires were posted. Of those, 867 (76%) were returned from parents and 877 (74%) from teenagers. Only 11 student/parent pairs (<1%) actively withdrew from the study at this stage. Therefore, we expect 1174 students (99%) and 1130 parents (99%) to be available for follow-up into the third phase, which is currently underway.
To date, 757 17-year-olds have been invited to final interview: only 24 (3%) have refused, 488 (65%) have completed their assessments, a further 228 (30%) have agreed and 17 (2%) are proving difficult to trace. This final assessment stage is due for completion in December 2009.
Collaborations
The ROOTS study has two major collaborators.
The MRC Epidemiology Unit, Institute of Metabolic Sciences (http://www.mrc-epid.cam.ac.uk) took detailed physical measures (height, weight and waist circumference) and recorded levels of physical activity using sub-maximal step tests and Actiheart monitors (work for 4 days), on over 900 14-year olds. The activity monitors were repeated on 250 participants aged 17 years. This collaboration provides a unique opportunity to examine the associations between physical activity and social and emotional well-being over time.
The MRC Cognitive Brain Sciences Unit (http://www.mrc-cbu.cam.ac.uk) is to undertake neuroimaging, on a selected sub-sample of ROOTS adolescents. The aims of this neurocognitive sub-study are: (i) to establish population-based data on the structure and function of the normal developing brain between later adolescence and early adult life; and (ii) to undertake a genetically and environmentally sensitive study of emotion regulation and response for both emotional and behavioural disorders. We plan to study those with and without the s/s variant of the 5-HTT and who have or have not experienced early-life adversity. We will determine, in those with both the s/s gene variant and child adversity exposure compared with those with one or neither vulnerability, if there is: (a) a heightened neural response to negatively valenced feedback and stimuli, in the amygdala and ventral anterior cingulate cortices; and (b) reduced grey matter volume in cortical regions important for cognitive flexibility and emotion regulation. This study will therefore provide valuable information on the neural systems that subserve psychological vulnerability in those with selected genetic and/or early adversities.
What are the main strengths and weaknesses?
There are a number of strengths. The current study is the first to take physiological measures of cortisol and DHEA over the adolescent period. These hormones have effects on mood and emotion and levels vary with maturation and with social stress. Relating them to genetic and early-life experiences over time is a unique component of this programme.
Large-scale studies suffer the risk of measuring only ‘surface’ phenotypes but we have improved on previous longitudinal studies by embedding quasi-experimental methods in a design that is both genetically and environmentally sensitive.
The retrospective nature of the assessment of early social adversity is a limitation. Birth cohort studies avoid recall bias, but are costly in terms of time and money. Such studies have made major contributions to the field, but rarely penetrate to the neurobiological level during the period of risk as ROOTS aims to do.
A second limitation is the low acceptance rate into the study of those invited, although this is consistent with levels of recruitment to other prospective cohort studies in this region of the UK.23
A further characteristic is the relatively prosperous socio-economic profile of the population. There are few urban areas compared with the population of the UK as a whole, although schools in some of our centres outside Cambridge serve considerably deprived neighbourhoods. Thus, the study sampling frame is unlike that used in studies from inner cities with high levels of economic and social deprivation. Strictly speaking, findings from ROOTS should not be generalized to such populations but, by the same token, neither should findings from inner city studies be applied to largely sub-urban and rural populations such as ours. Nevertheless, our own view is that the developmental mechanisms underlying psychopathology will not be fundamentally different at the individual level in contrasting parts of the UK, even though the prevalence and nature of psycho-social adversity may differ.
Access to data: where can I find out more?
After the primary hypotheses of the main study and the collaborative extensions have been completed, it is intended to archive the ROOTS datasets to enable access by other researchers. Research groups, UK or international, wishing to register an interest in using aspects of the data should prepare a short, outline research proposal and submit it to the Executive Group (comprising ROOTS PIs, study administrators, and extension project collaborators).
Funding
The Wellcome Trust (Grant no. 074296). NIHR Collaboration for Leadership in Applied Health Research & Care (CLAHRC, partial) for Cambridgeshire & Peterborough.
Acknowledgements
We thank Helen Shires, Sarah Cleary and Jayne Wright for hormone assays and DNA extraction and Dr Maria Ban for genotyping the serotonin transport (SLC6A4) and monoamine oxidase A (MAOA). Other genetic variants were typed by Geneservices (geneservice.co.uk).
Conflict of interest: None declared.
References
- 1.Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci. 2004;1021:110–23. doi: 10.1196/annals.1308.012. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry Allied Disciplines. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 4.Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry. 2005;44:972–86. doi: 10.1097/01.chi.0000172552.41596.6f. [DOI] [PubMed] [Google Scholar]
- 5.Oldehinkel AJ, Verhulst FC, Ormel J. Low heart rate: a marker of stress resilience. The TRAILS study. Biol Psychiatry. 2008;63:1141–46. doi: 10.1016/j.biopsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch General Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 9.Weaver IC, D'Alessio AC, Brown SE, et al. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 11.Goodyer IM, Herbert J, Tamplin A, Altham PM. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- 12.Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry. 2007;62:40–6. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Harris TO, Borsanyi S, Messari S, et al. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiatry. 2000;177:505–10. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- 14.Fairchild G, van Goozen SH, Stollery SJ, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roiser JP, Levy J, Fromm SJ, et al. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33:1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavares JV, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychol Med. 2003;33:959–67. doi: 10.1017/s0033291703008432. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JS, Erickson K, Luckenbaugh DA, et al. Differential performance on tasks of affective processing and decision-making in patients with Panic Disorder and Panic Disorder with comorbid Major Depressive Disorder. J Affect Disord. 2006;95:165–71. doi: 10.1016/j.jad.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(Pt 12):3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 19.Hill J. Biological, psychological and social processes in the conduct disorders. J Child Psychol Psychiatry Allied Disciplines. 2002;43:133–64. doi: 10.1111/1469-7610.00007. [DOI] [PubMed] [Google Scholar]
- 20.Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry. 2007;64:577–84. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- 21.Sandberg S, Rutter M, Giles S, et al. Assessment of psychosocial experiences in childhood: methodological issues and some illustrative findings. J Child Psychol Psychiatry Allied Disciplines. 1993;34:879–97. doi: 10.1111/j.1469-7610.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 22.Williamson DE, Birmaher B, Ryan ND, et al. The stressful life events schedule for children and adolescents: development and validation. Psychiatry Res. 2003;119:225–41. doi: 10.1016/s0165-1781(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 23.Surtees PG, Wainwright NW, Brayne C. Psychosocial aetiology of chronic disease: a pragmatic approach to the assessment of lifetime affective morbidity in an EPIC component study. J Epidemiol Commun Health. 2000;54:114–22. doi: 10.1136/jech.54.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]