Abstract
Background In African settings with poor access to health care, surveillance and surveys of disease burden are often done through home visits. The optimal recall period to capture data on symptoms and health utilization is unknown.
Methods We collected illness data among 53 000 people during fortnightly home visits in rural and urban Kenya. Dates of cough, fever and diarrhoea in the past 2 weeks and health-seeking behaviour were recorded. Incidence rates were modelled using Poisson regression for data collected from 1 July 2006 to 30 June 2007.
Results Incidence rates were higher in days 0–6 before the home visit than in days 7–13 before the home visit for all three symptoms, for the rural and urban sites, for children and adults, for self- and proxy-reported symptoms and for severe and non-severe illness in children. Recall decay was steeper in the rural than the urban sites, and for proxy- than self-reported symptoms. The daily prevalence of symptoms fell <80% of the maximum prevalence when asking about symptoms >3 days before the home visit for children and >4 days for persons ≥5 years of age. Recall of previously documented clinic visits, and prescriptions of antimalarials and antibiotics also declined by ∼7, 15 and 23% per week, respectively, in children aged <5 years, and 6, 20 and 16%, respectively, in older persons (P < 0.0001 for each decline).
Conclusions A 2-week recall period underestimates true disease rates and health-care utilization. Shorter recall periods of 3 days in children and 4 days in adults would likely yield more accurate data.
Keywords: Africa, diarrhoea, fever, memory recall, population surveillance, respiratory infections
Introduction
Defining the disease burden of major infectious diseases in developing countries is important for public health policymakers when allocating limited resources. Most commonly, incidence and prevalence of disease are defined at health facilities. However, in sub-Saharan Africa, health facility utilization is limited. The majority of cases of infectious diseases in Kenya do not present at health facilities.1,2 Because of underutilization of health facilities, alternative approaches have been employed to define disease burden in developing country settings. One method is cross-sectional health utilization surveys performed at the community level to estimate rates and health seeking for various diseases. Another method is to make regular home visits and ask about symptoms of recent illness. Although more resource intensive, home-based surveillance yields longitudinal data with the added benefits of being able to evaluate seasonality, to define both the incidence and prevalence and to evaluate the impact of interventions on disease occurrence.3,4
An important consideration in carrying out home-based surveillance for infectious diseases is the optimal recall period for illness. A 2-week recall has been used in the majority of studies and surveys, including Demographic and Health Surveys (DHSs) undertaken every 5 years in many developing countries.2,3,5 A 2-week interval of recall, however, seems to be one chosen more as a compromise between logistics, cost and data quality, rather than one based on accuracy alone.5 Better definition of the effect of recall time on the reporting of symptoms might lead to more reliable estimates of true disease burden and health-utilization patterns.
Since 2005, the Centers for Disease Control and Prevention (CDC) and the Kenya Medical Research Institute (KEMRI) have maintained population-based surveillance for major infectious disease syndromes in defined populations of rural western Kenya and an urban informal settlement in Nairobi. The objectives of the surveillance are to define disease burden, describe epidemiologic patterns of disease and evaluate the health impact of interventions over time in these populations. To achieve this, disease is characterized in the clinic, as well as through home visits. Field workers make home visits every 2 weeks and ask about recent symptoms. These longitudinal home visits allowed us to evaluate the effect of recall period on reporting of symptoms, clinic visitation and drug use. This information on recall is important in interpreting the surveillance data.
Materials and methods
Surveillance sites
The CDC’s International Emerging Infections Program, in collaboration with KEMRI, has conducted population-based, morbidity surveillance since late 2005 at two sites in Kenya. Asembo is a rural location in Bondo District of western Kenya along Lake Victoria. The surveillance population numbers approximately 25 000 in 33 villages. All study participants must have resided permanently in the area for 4 calendar months and have been registered into the KEMRI/CDC Demographic Surveillance System (DSS).6,7 The population is predominantly subsistence farmers and fishermen belonging to the Luo ethnic group. Houses are widely dispersed in a bushy landscape cultivated with small fields. The area comprises ∼100 km2 with an overall population density of about 325 persons per square kilometre. Malaria transmission is endemic and occurs year round.7 The area had a child (<5 years of age) mortality ratio of 227 per 1000 live births in 2002.6 Asembo has high HIV seroprevalence rates—11% in men and 21% in women aged 13–34 years in 2003.8
The other site, with approximately 28 000 participants, is in Gatwikira and Soweto villages in the Kibera informal settlement of Nairobi, one of the largest contiguous urban slums in Africa. The surveillance area comprises 0.42 km2 and has a population density of about 65 000 persons per square kilometre. Most employed residents are casual labourers, servants, security guards or small-business merchants within the city. The area has a maze-like array of semi-permanent housing, with dirt paths between the dwellings and open sewers. Malaria is not endemic due to the high altitude, although cases of malaria are frequent among people arriving from other parts of Kenya. HIV prevalence among adults is ∼15% (KEMRI/CDC data). As with the Asembo site, participants must have resided in Kibera for at least 4 months prior to enrolment.
In both surveillance sites, participants can access free health care at centrally located clinics staffed mostly by study-supported and trained personnel.
Population-based surveillance methods
Community interviewers visit enrolled households every 2 weeks to inquire about illnesses during the past 2 weeks. Community interviewers are high-school graduates without specific health-related training; however, they undergo intensive protocol-specific training. They ask each participant a screening question, ‘Have you had fever, cough or difficulty in breathing, diarrhoea, yellow eyes or any other illness or injury in the past 2 weeks?’. Participants who answer affirmatively to the screening question are given a more extensive questionnaire about their illness. For key symptoms, including cough, fever and diarrhoea, participants are asked to provide the exact days in the past 2 weeks when they had those symptoms. Other symptoms are categorized as present or absent. For persons >5 years of age, direct interviews with the participant are attempted. If not at home or unable to answer questions for any reason, a proxy who is able to answer illness questions about the participant is sought. For children <5 years of age, the mother, or other knowledgeable caretaker, is interviewed. Data are collected using personal digital assistants (PDAs), backed up daily and uploaded onto a computer every 2 weeks. PDAs are programmed in Visual Basic dot-Net and data are stored in SQL databases. Regular quality control checks are performed by data management staff.
Data analysis
For data collected from 1 July 2006 to 30 June 2007, we modelled incidence rates of illness for fever, diarrhoea and cough, using Poisson regression (PROC GENMOD, SAS version 9.1, SAS Institute, Cary, NC, USA). We controlled for clustering of symptoms at the household level using generalizing estimating equations (GEEs). Incidence rates were calculated as the number of new episodes of a syndrome per person-year. We used a symptom-free interval to define new episodes of the same syndrome. For diarrhoea, we used a diarrhoea-free interval of 3 days to define new episodes.9–11 For cough and fever, we used a cough- or fever-free interval of 7 days to define new episodes.12 We calculated the denominator using the person-time contribution of all study participants from the fortnightly visits. Only days in which a symptom could be recorded if present were included in the denominator. For instance, if a person was away at the time of the fortnightly visit and no suitable proxy could be found, those 14 days were not included in the denominator. Likewise, if home visits were made >14 days apart, the intervening days in which no data on symptoms were collected were not included. Moreover, only days in which a new episode could be counted were included in the denominator; therefore, subsequent days of an episode after the initial day and days in the symptom-free interval were excluded from the denominator.13 Separate Poisson regression models were fit for children <5 years and persons ≥5 years of age. Variables for disease (fever, diarrhoea, cough), site (Asembo, Kibera), week (days 0–6 and days 7–13 before interview), site-by-week interaction, site-by-disease interaction and 3-way site-by-week-by-disease interaction, were included in the model to allow for estimation and comparison of incidence rates for each combination of disease, site and week. Incidence rate ratios (RRs) and 95% confidence intervals (CIs) comparing days 0–6 with days 7–13 before the interview date were calculated. Additional models were fit for children with inclusion of a variable for severe/non-severe illness, and for persons ≥5 years of age with inclusion of a variable for self- vs proxy-report of symptoms.
We compared the recall effect upon severe illnesses in children to explore whether people tended to better recall symptoms of severe illnesses. Severe illnesses were defined for children <5 years of age as those that included a danger sign, or meeting the severe pneumonia or severe dehydration criteria, from WHO’s Integrated Management of Childhood Illness (IMCI) algorithm.14 Similar indicators of severity based on symptoms were not available for adults, so severity was not assessed in adults. In adults, we assessed whether recall decay was affected if symptoms were reported by the participant him/herself, or by a proxy.
We assessed the percentage of persons with each symptom (daily prevalence) on each day of the 14-day recall period for home visits. We arbitrarily considered days that were ≥80% of the maximum daily prevalence rate (day 1 before interview date) as days in which recall of symptoms was adequate.
We evaluated whether there was recall decay in reporting of visiting the clinic and of taking anti-malarial and antibiotic medications using data from Asembo. To do this, we limited the analysis to those individuals with a documented visit to the surveillance referral clinic, Lwak Hospital, and to those who were prescribed and given an anti-malarial and antibiotic at Lwak Hospital. We looked at the subsequent home visit, which could be from 0 to 13 days after the clinic visit. At the home visit, interviewers asked whether the participant had visited the clinic and received anti-malarial and antibiotic medications in the past 2 weeks. We evaluated whether there was a decline in the percentage of persons reporting clinic visits or medication prescription according to the number of days the home visit followed the clinic visit with a linear test for trend using log-binomial regression (PROC GENMOD, SAS version 9.1). Day 0, when the clinic and home visits were on the same day, was excluded from analysis because the clinic visit might have followed the home visit on that day and so would not have been reported.
The head of the household gave informed consent for home visits. The protocol and consent forms were reviewed and approved by the Ethical Review Boards of KEMRI (SSC# 932) and the Institutional Review Board of CDC (IRB # 4566).
Results
The incidence rates were higher in days 0–6 than in days 7–13 before the home visit (Table 1). This finding was consistent for all three symptoms, for the rural and urban sites and for children and adults. The decay in recall between days 0–6 and days 7–13 before home visit was steeper in the rural than the urban site for all symptoms and for both children <5 years and persons ≥5 years of age, ranging from 20 to 91% more recall decay in the rural site (Table 1). Higher incidence was documented in days 0–6 than days 7–13 before home visit in persons ≥5 years of age for both self-reported symptoms (RR 1.67; 95% CI 1.55–1.79) and proxy-reported symptoms (RR 1.84; 95% CI 1.72–1.97). Recall decay was 10% lower for self-reported symptoms (Table 2). Higher incidence was documented in days 0–6 than days 7–13 before home visit in children <5 years of age for both non-severe illness (RR 2.08; 95% CI 1.93–2.25) and severe illness (RR 2.04; 95% CI 1.82–2.28), although there was no difference between the amount of recall decay in severe and non-severe illness (Table 2).
Table 1.
Incidence rates and Rate Ratios (RRs) comparing the week before home visit (days 0–6) with 2 weeks before home visit (days 7–13)a
| Asembo |
Kibera |
||||||
|---|---|---|---|---|---|---|---|
| Incidence, days 0–6, number of episodes person-year (95% CI) | Incidence, days 7–13, number of episodes/ person-year (95% CI) | RR comparing days 0–6 with days 7–13b (95% CI) | Incidence, days 0–6, number of episodes/ person-year (95% CI) | Incidence, days 7–13, number of episodes/ person-year (95% CI) | RR comparing days 0–6 with days 7–13b (95% CI) | Ratio of RRs comparing days 0–6 with days 7–13, Asembo vs Kiberab (95% CI) | |
| Persons ≥5 years of age | |||||||
| Fever | 3.79 (3.70–3.89) | 1.56 (1.52–1.60) | 2.53 (2.45–2.62) | 0.58 (0.56–0.61) | 0.33 (0.31–0.35) | 1.84 (1.72–1.97) | 1.37 (1.28–1.48) |
| Diarrhoea | 0.46 (0.44–0.48) | 0.23 (0.21–0.24) | 2.13 (2.00–2.27) | 0.15 (0.13–0.16) | 0.086 (0.077–0.095) | 1.77 (1.56–2.01) | 1.20 (1.05–1.38) |
| Cough | 4.08 (3.97–4.18) | 1.99 (1.93–2.04) | 2.14 (2.07–2.21) | 0.84 (0.81–0.87) | 0.58 (0.56–0.61) | 1.49 (1.41–1.57) | 1.44 (1.35–1.53) |
| Children <5 years of age | |||||||
| Fever | 9.76 (9.38–10.15) | 2.76 (2.63–2.88) | 3.54 (3.36–3.73) | 2.38 (2.25–2.50) | 1.15 (1.07–1.22) | 2.07 (1.92–2.24) | 1.71 (1.56–1.88) |
| Diarrhoea | 1.72 (1.61–1.84) | 0.72 (0.66–0.78) | 2.39 (2.20–2.59) | 1.46 (1.37–1.56) | 0.79 (0.73–0.86) | 1.84 (1.68–2.02) | 1.30 (1.15–1.47) |
| Cough | 9.63 (9.24–10.03) | 2.79 (2.66–2.93) | 3.45 (3.27–3.64) | 3.20 (3.05–3.36) | 1.77 (1.67–1.88) | 1.81 (1.69–1.93) | 1.91 (1.75–2.08) |
aAsembo, rural western Kenya and Kibera, Urban Nairobi. Data from 1 July 2006 to 30 June 2007.
bModel for persons ≥5 years controls for proxy vs self-report.
Table 2.
Evaluation of specific factors related to recall decaya
| Interaction variables (second variable listed is referent) | Children <5 years of age Ratio of RRs comparing days 0–6 with days 7–13 before home visit (95% CI) | Persons ≥5 years of age Ratio of RRs comparing days 0–6 with days 7–13 before home visit (95% CI) |
|---|---|---|
| Syndromesb in Asembo | ||
| Diarrhoea vs fever | 0.67 (0.62–0.73) | 0.84 (0.79–0.90) |
| Cough vs fever | 0.97 (0.92–1.03) | 0.84 (0.82–0.87) |
| Cough vs diarrhoea | 1.45 (1.32–1.58) | 1.00 (0.94–1.07) |
| Syndromes in Kibera | ||
| Diarrhoea vs fever | 0.89 (0.80–0.98) | 0.96 (0.84–1.10) |
| Cough vs fever | 0.87 (0.80–0.95) | 0.81 (0.75–0.87) |
| Cough vs diarrhoea | 0.98 (0.89–1.09) | 0.84 (0.73–0.96) |
| Self vs proxy | Not applicable | 0.90 (0.87–0.94) |
| Severe vs non-severe illness | 0.98 (0.89–1.07) | Not applicable |
aAsembo, rural western Kenya and Kibera, Urban Nairobi. Data from 1 July 2006 to 30 June 2007. Factors were evaluated as interaction terms in Poisson regression model.
bSyndromes are shown separately for Asembo and Kibera due to significant interaction between syndrome and site in the model.
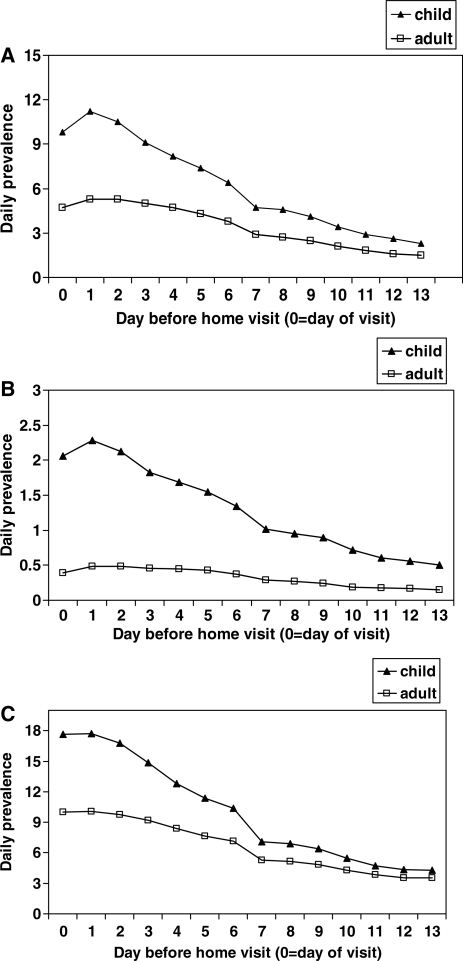
Daily prevalence of symptoms increased slightly from the day of the visit to 1 day before the visit (Figure 1). This is likely because the day of the visit was not a complete 24-h day of observation. After day 1, the daily prevalence decreased slightly during the next few days, more so in children than adults (Figure 2). There was a steeper decline in the daily prevalence of all symptoms in the week before the home visit among children <5 years of age than among older children and adults, after which the daily prevalence continued to decline, but less steeply, over the following week in both age groups. Daily prevalence that was >80% of the maximum daily prevalence, on day 1 before the home visit, was observed in the 3 days before the home visit in children and in the 4 days before the home visit in persons ≥5 years of age (Figure 2). A similar decay in the daily prevalence of reported symptoms was found in the urban site (data not shown).
Figure 1.
Daily prevalence of (A) fever, (B) diarrhoea (C) and cough calculated as percentage of persons reporting symptoms on each day in the 2 weeks prior to home visit, Asembo, western Kenya. Data from 1 July 2006 to 30 June 2007.
Figure 2.
Relative symptom prevalence of fever, diarrhoea and cough compared with day 1 as reference, Asembo, western Kenya. Data from 1 July 2006 to 30 June 2007. Line demarcates 80% of maximum prevalence.
There was a decrease in the percentage of persons at the home visit who reported making a clinic visit and taking anti-malarial and antibiotic medications with increasing number of days from the clinic visit (Table 3). This decline was not as steep as that observed with recall of symptoms. From the first to the second week after the clinic visit, there was a reduction in recall of clinic visits, anti-malarial use and antibiotic use of 7% (95% CI 5–9), 15% (95% CI 11–20) and 23% (95% CI 14–32), respectively, for children <5 years of age; for persons ≥5 years of age, the reduction in recall was 6% (95% CI 5–7), 20% (95% CI 15–25) and 16% (95% CI 10–22), respectively.
Table 3.
Recall at the home visit following documented clinic visits at Lwak Hospitala
|
n (%) reporting a clinic visit to Lwak in past 2 weeks |
n (%) reporting receiving antimalarialsb in the past 2 weeks |
n (%) reporting receiving an antibioticb in the past 2 weeks |
||||
|---|---|---|---|---|---|---|
| Day after clinic visit that home visited | Children <5 yearsc of age | Persons ≥5 yearsc of age | Children <5 yearsc of age | Persons ≥5 yearsc of age | Children <5 yearsc of age | Persons ≥5 yearsc of age |
| 0d | 56 (35) | 310 (49) | 11 (12) | 21 (9) | 15 (27) | 49 (16) |
| 1 | 139 (94) | 531 (96) | 59 (77) | 129 (69) | 35 (65) | 132 (52) |
| 2 | 137 (98) | 440 (96) | 61 (86) | 120 (76) | 35 (65) | 137 (56) |
| 3 | 152 (99) | 449 (96) | 73 (84) | 122 (73) | 46 (71) | 124 (54) |
| 4 | 125 (96) | 433 (96) | 74 (85) | 110 (71) | 36 (71) | 107 (49) |
| 5 | 142 (97) | 424 (94) | 59 (83) | 102 (65) | 35 (64) | 99 (45) |
| 6 | 156 (96) | 490 (94) | 81 (83) | 137 (64) | 43 (67) | 142 (52) |
| 7 | 170 (98) | 561 (94) | 78 (74) | 169 (66) | 48 (74) | 168 (55) |
| 8 | 130 (94) | 432 (93) | 76 (87) | 113 (60) | 32 (55) | 117 (54) |
| 9 | 112 (91) | 359 (90) | 71 (85) | 97 (52) | 27 (51) | 94 (43) |
| 10 | 142 (89) | 374 (89) | 78 (77) | 104 (56) | 34 (48) | 98 (39) |
| 11 | 100 (86) | 309 (87) | 56 (68) | 76 (51) | 25 (42) | 79 (38) |
| 12 | 101 (84) | 298 (87) | 58 (64) | 77 (50) | 25 (45) | 80 (37) |
| 13 | 137 (86) | 441 (84) | 68 (51) | 125 (50) | 25 (42) | 114 (35) |
aClinic visitation, antimalarial, and antibiotic use based on the day after documented clinic visit at Lwak Hospital that the home visit was made, Asembo, rural western Kenya. Data from 1 July 2006 to 30 June 2007.
bFor anti-malarial and antibiotic recall, only those individuals with a known prescription given at Lwak hospital were included in the denominator.
cFor all six columns, the linear test for trend had a P-value <0.0001 for a decrease in recall from days 1–13; day 0 was excluded (see ‘Methods’ section).
dDay 0 is when the home visit is on the same day as the clinic visit.
Discussion
Our study demonstrated recall decay of symptoms to be a robust phenomenon occurring at multiple levels—for both children and adults, for three key symptoms, for self-reported and proxy-reported symptoms in adults, for severe and non-severe illness in children and in rural and urban African settings. This finding suggests that rates of diseases identified in different studies might vary as a result of not only differing demographics, environmental conditions and case definitions, but also differences in the recall period used.3,4
Previous studies on recall decay focused on children in single study sites and all but one on single syndromes.15–19 In Bangladesh, there was a 34% decrease in diarrhoea episodes among toddlers reported 48 h prior to the interview time.15 In India, the proportion of infants in whom diarrhoea was reported was similar up until day 3 prior to the interview date, thereafter showing a steady decline with a 15, 26 and 45% decrease in the proportion of children with diarrhoea reported on days 3, 6 and 7–13, respectively, before the interview date.19 Under-reporting of diarrhoea episodes has also been shown to occur if the recall period is >2–3 days in DHS surveys that ask about diarrhoea in the past 2 weeks.16 A study from the Gambia that carried out weekly visits showed a ∼50% decrease in the symptoms of diarrhoea, fever and respiratory illness among infants between the day of the visit and 8 days before.17 Another study of maternal recall of symptoms of acute respiratory illness showed that the specificity of the symptoms recalled was better 2 weeks after the diagnosis than 4 weeks after.18
The findings of our study have implications for designing studies to define the rate of disease using surveillance methods based on recall of symptoms. The period of recall should probably not extend back >3 days for children and >4 days for adults to achieve a rate that is at least 80% of the possible maximum rate. To achieve complete ascertainment of symptoms during longer time periods, more frequent visits than fortnightly or weekly visits should be considered. More frequent household visits have been made in some studies, but are resource intensive and likely to lead to participant fatigue over time.3,11,20–22
An alternative strategy to continuous morbidity surveillance for defining disease rates is to record symptoms in only a fraction of days.23,24 Sampling at 7- or 14-day intervals, asking about symptoms 24–48 h before the visit, was shown to result in a relatively small loss of precision when estimating longitudinal prevalence of diarrhoeal disease, requiring only small increases in sample size.24 Our data suggest that the precision of such intermittent sampling strategies might be increased by using 3–4-day recall periods without compromising accuracy too much. However, the interval of sampling was sensitive to the duration of the disease episodes, and so intermittent sampling might apply more to measurements of longitudinal prevalence than incidence.23,24 If periods of recall >3–4 days are considered, mnemonics such as symptom diaries might be employed to minimize recall decay, although the compliance and accuracy with such devices in populations with low literacy have been questioned.17,25
To our knowledge, this is the first report of recall decay for clinic visitation and drug use. This finding has implications for surveys assessing health-seeking patterns and drug use in communities, such as the DHSs, which ask about clinic use and medications in the past 2 weeks. If a recall period of >1 week is used, clinic utilization and drug use will likely be underestimated. Recall of antibiotic use was lower than anti-malarial use likely because anti-malarials are more recognizable by name than are antibiotics in rural western Kenya.
Our study evaluated the impact of several different factors on symptom recall that shed light on how human memory of symptoms works. First, we showed that proxy-reported symptoms have steeper recall decay than self-reported symptoms, which would be expected since individuals remember their own experiences better than other people do.26 Secondly, we found that people in the urban setting seemed to remember their symptoms better than people in the rural setting. The reason for this is not clear, but it could be related to the urban population being more educated and aware of dates due to salaried employment, compared with the rural setting where most persons are subsistence farmers. Alternatively, because rates of reported disease are higher in the rural setting, recall of separate episodes of similar illness might be more difficult, a phenomenon that has been shown.26 Our study did not support the so-called ‘salient principle’, whereby more severe symptoms are remembered longer than milder symptoms, which has been observed before, although the finding is not consistent across studies.5,17 Lastly, our data show that recall seemed to drop precipitously 1 week before home visit, suggesting that recall of symptoms might be packaged into week blocks, rather than in days.
Symptom recall by participants might have been biased by several aspects of human memory.26 First, ‘telescoping’ of memory, in which events are remembered to have occurred more recently than they actually did, could have occurred.26,27 It is possible that symptoms that occurred 7–13 days before the home visit might have been recalled as having occurred during days 0–6 before home visit, thereby falsely elevating the rates calculated for the last week. This phenomenon of telescoping has been documented with events occurring over longer periods of recall of months or years and might not tend to occur as markedly in a 2-week recall period, particularly during the past 3–4 days. Secondly, people tend not to remember dates as well as events in an autobiographical sequence.26 Therefore, by asking participants to remember symptoms in relation to a personal event, such as a market day or a church day, rather than in a 2-week period, might have led to greater accuracy of recall, particularly for less recent symptoms. Lastly, participants might have tended to over-report symptoms because they wanted to be ‘helpful’ interviewees, whereas on the other hand they might have under-reported symptoms due to interview fatigue during longitudinal surveillance.26,28 Although both of these occurrences might have yielded inaccurate rates of disease, they would likely not have affected this analysis of recall as they would have been unlikely to have been differential with respect to symptom recall period.
In conclusion, because of under-utilization of health facilities, defining disease burden in developing country settings can be more complete when asking persons to recall symptoms of recent illnesses during home visits. Recall of symptoms is greatly influenced by the time period of recall. Our data suggest that the period of recall should not exceed 3 days in children and 4 days in adults to identify at least 80% of the maximum rate of disease, particularly for longitudinal studies involving repetitive visits. Limitations of symptom recall should be considered in the context of the logistics and cost of designing a surveillance system to define the burden of disease in developing countries.
Funding
Core funding for the International Emerging Infections Programme, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Conflict of interest: None declared.
KEY MESSAGES.
During home visits, 2-week recall periods of symptoms underestimated true disease incidence, as suggested by higher incidence of disease reported in Days 0–6 before home visit than in Days 7–13 before home visit.
The findings were consistent for all symptoms (cough, fever and diarrhoea), age group, site (urban and rural), severity of illness and proxy- and self-reported symptoms.
Daily prevalence of symptoms reported at home visits decreased <80% of the maximum recall when asking about symptoms >3 days before the home visit for children and >4 days for persons ≥5 years of age.
Reporting of documented clinic visitation, anti-malarial and antibiotic use also decreased as the number of days between the clinic visit/drug prescription and home visit increased.
Acknowledgements
The authors would like to acknowledge the Kenya Medical Research Institute for making this work possible and permission of the acting Director to publish this work. They also thank the leaders and people of Asembo and Kibera for their support and participation in this project. They extend their particular thanks for the contributions to this study made by George Aol, Barrack Aura, George Okoth, James Ndirangu and the entire staff of data collectors and managers for Asembo and Kibera. This material was partly presented at the 57th annual meeting of the American Society for Tropical Medicine and Hygiene, New Orleans, LA, USA, 7–11 December 2008. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centres for Disease Control and Prevention.
References
- 1.Amin AA, Marsh V, Noor AM, Ochola SA, Snow RW. The use of formal and informal curative services in the management of paediatric fevers in four districts in Kenya. Trop Med Int Health. 2003;8:1143–52. doi: 10.1046/j.1360-2276.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Central Bureau of Statistics (CBS), Kenya Ministry of Health, ORC Macro. Kenya Demographic and Health Survey 2003: Key Findings. USA: Calverton, MD; 2004. [Google Scholar]
- 3.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 4.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Kroeger A. Health interview surveys in developing countries: a review of the methods and results. Int J Epidemiol. 1983;12:465–81. doi: 10.1093/ije/12.4.465. [DOI] [PubMed] [Google Scholar]
- 6.Adazu K, Lindblade KA, Rosen DH, et al. Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg. 2005;73:1151–58. [PubMed] [Google Scholar]
- 7.Phillips-Howard PA, Nahlen BL, Wannemuehler KA, et al. Impact of permethrin-treated bed nets on the incidence of sick child visits to peripheral health facilities. Am J Trop Med Hyg. 2003;68:38–43. [PubMed] [Google Scholar]
- 8.Amornkul PN, Vandenhoudt H, Nasokho P, et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in Rural Western Kenya. PLoS One. 2009;4:e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baqui AH, Black RE, Yunus M, Hoque AR, Chowdhury HR, Sack RB. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol. 1991;20:1057–63. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 10.Morris SS, Cousens SN, Lanata CF, Kirkwood BR. Diarrhoea—defining the episode. Int J Epidemiol. 1994;23:617–23. doi: 10.1093/ije/23.3.617. [DOI] [PubMed] [Google Scholar]
- 11.Wright JA, Gundry SW, Conroy R, et al. Defining episodes of diarrhoea: results from a three-country study in Sub-Saharan Africa. J Health Popul Nutr. 2006;24:8–16. [PubMed] [Google Scholar]
- 12.Zaman K, Baqui AH, Yunus M, et al. Acute respiratory infections in children: a community-based longitudinal study in rural Bangladesh. J Trop Pediatr. 1997;43:133–37. doi: 10.1093/tropej/43.3.133. [DOI] [PubMed] [Google Scholar]
- 13.Rothman K, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 14.World Health Organization (WHO) Integrated Management of Childhood Illness. Geneva: WHO; 2008. [PubMed] [Google Scholar]
- 15.Alam N, Henry FJ, Rahaman MM. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18:697–700. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 16.Boerma JT, Black RE, Sommerfelt AE, Rutstein SO, Bicego GT. Accuracy and completeness of mothers' recall of diarrhoea occurrence in pre-school children in demographic and health surveys. Int J Epidemiol. 1991;20:1073–80. doi: 10.1093/ije/20.4.1073. [DOI] [PubMed] [Google Scholar]
- 17.Byass P, Hanlon PW. Daily morbidity records: recall and reliability. Int J Epidemiol. 1994;23:757–63. doi: 10.1093/ije/23.4.757. [DOI] [PubMed] [Google Scholar]
- 18.Harrison LH, Moursi S, Guinena AH, et al. Maternal reporting of acute respiratory infection in Egypt. Int J Epidemiol. 1995;24:1058–63. doi: 10.1093/ije/24.5.1058. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan R, Venkatarao T, Koya PK, Kamaraj P. Influence of recall period on estimates of diarrhoea morbidity in infants in rural Tamilnadu. Indian J Public Health. 1999;43:136–9. [PubMed] [Google Scholar]
- 20.Genser B, Strina A, Teles CA, Prado MS, Barreto ML. Risk factors for childhood diarrhea incidence: dynamic analysis of a longitudinal study. Epidemiology. 2006;17:658–67. doi: 10.1097/01.ede.0000239728.75215.86. [DOI] [PubMed] [Google Scholar]
- 21.Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–33. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 22.Strina A, Cairncross S, Barreto ML, Larrea C, Prado MS. Childhood diarrhea and observed hygiene behavior in Salvador, Brazil. Am J Epidemiol. 2003;157:1032–38. doi: 10.1093/aje/kwg075. [DOI] [PubMed] [Google Scholar]
- 23.Morris SS, Santos CA, Barreto ML, et al. Measuring the burden of common morbidities: sampling disease experience versus continuous surveillance. Am J Epidemiol. 1998;147:1087–92. doi: 10.1093/oxfordjournals.aje.a009403. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt WP, Luby SP, Genser B, Barreto ML, Clasen T. Estimating the longitudinal prevalence of diarrhea and other episodic diseases: continuous versus intermittent surveillance. Epidemiology. 2007;18:537–43. doi: 10.1097/EDE.0b013e318093f3ce. [DOI] [PubMed] [Google Scholar]
- 25.Goldman N, Vaughan B, Pebley AR. The use of calendars to measure child illness in health interview surveys. Int J Epidemiol. 1998;27:505–12. doi: 10.1093/ije/27.3.505. [DOI] [PubMed] [Google Scholar]
- 26.Bradburn NM, Rips LJ, Shevell SK. Answering autobiographical questions: the impact of memory and inference on surveys. Science. 1987;236:157–61. doi: 10.1126/science.3563494. [DOI] [PubMed] [Google Scholar]
- 27.Sudman S, Bradburn NM. Effects of time and memory factors on response in surveys. J Am Stat Assn. 1973;68:805–15. [Google Scholar]