Summary
Rapidly induced, specific antibodies generated in extrafollicular foci are important components of early immune protection to influenza virus. The signal(s) that prompt B cells to participate in extrafollicular rather than germinal center responses are incompletely understood. To study the regulation of early B cell differentiation events following influenza infection, we exploited earlier findings of a strong contribution of C12 idiotype-expressing B cells to the primary hemagglutinin (HA)-specific response against influenza A/PR/8/34. Using an idiotype-specific mAb to C12 and labeled-HA, in conjunction with multicolor flow cytometry, we followed the fate of C12Id-expressing influenza HA-specific B cells in wildtype BALB/c mice, requiring neither genetic manipulation nor adoptive cell transfer. Our studies demonstrate that HA-specific C12Id+ B cells are phenotypically indistinguishable from follicular B cells. While they induced both extrafollicular and germinal center responses, extrafollicular responses were strongly predominant. Provision of increased HA-specific T cell help increased the magnitude of the extrafollicular response, but did not shift the C12Id+ response towards germinal center formation. Collectively the data are consistent with the hypothesis that B cell fate-determination following activation is a stochastic process in which infection-induced innate signals might drive the preferential expansion of the early extrafollicular response.
Introduction
Influenza virus infection-induced anti-viral antibodies can contribute to survival from primary and secondary infection [1-7]. Rapid B cell responses in the local respiratory tract draining mediastinal lymph node (MedLN) are induced as early as 48-72 h after infection [8]. They contribute to viral clearance during primary infection by neutralizing the virus and reducing influenza virus spread [2, 5]. Therefore the events that govern early B cell activation following influenza virus infection are crucial for ameliorating disease outcome. The mechanisms underlying early B cell activation, however, are incompletely understood.
Rapid antibody production originates from extrafollicular foci developing at the edges of the T and B cell zones in secondary lymphoid tissues following antigen exposure. These responses are thought to generate primarily short-lived plasma cells [9]. Rapid antibody production at extrafollicular sites is attributed to T cell-independent as well as T-dependent responses [10, 11]. In contrast, the slower intrafollicular germinal center reactions require cognate CD4 T cell - B cell interactions [12, 13]. They are regarded as the birthplace of long-lived humoral immunity, providing both memory B cells and long-lived plasma cells [11, 13]. Both extra and intra-follicular responses develop strongly in the regional lymph nodes following influenza virus infection [14].
The selection events that underlie the establishment of extrafollicular versus germinal center B cell responses are important events in the initiation of the adaptive immune response. They coordinate the formation of crucial rapidly protective responses, while ensuring long-term protection from re-infection [11]. There is evidence that rapid (antiviral) antibody production can be provided by distinct B cell subsets [1, 11, 15-19]. Marginal Zone (MZ) B cells are one such subset. They can respond to blood-borne antigens through rapid production of antibodies at extrafollicular sites [17, 18]. In the mouse these B cells are only found in the spleen, however, and not in lymph nodes [20, 21]. Thus, MZ B cells are unlikely to play a role in the response to influenza virus infections, as respiratory tract draining MedLN are the main sites of the initial influenza virus-induced B cell response [14]. Whether there are other subsets in the lymph nodes that act as functional equivalents to splenic MZ B cells is currently unknown.
Recently, BCR affinity-guided selection events have been implicated as a factor that could determine the B cell fate following protein immunization [22]. Paus and colleagues [22] used an elegant adoptive cell transfer approach with transgenic hen egg lysozyme (HEL)-specific B cells to provide evidence that BCR affinity thresholds exist that steer B cells towards a particular response. In that study high-affinity B cell-antigen interaction resulted in predominantly extrafollicular foci responses, whereas HEL-specific B cells binding antigen with weaker overall affinities were predominately selected into the germinal center response. These data are consistent with a study on Vesicular Stomatitis Virus (VSV) infection-induced B cell responses, in which Roost et al observed no improvement on the overall antibody-affinity during the course of VSV infection and showed that early-induced virus-specific antibodies are of relatively high affinities [23]. Massive and rapidly induced differentiation without memory formation of C12 idiotype-expressing HA-specific antibodies to influenza A/PR/8, was proposed also as a possible mechanism to explain the strong contribution of these antibodies to the primary but not secondary response in BALB/c mice following immunization with this virus [24].
In apparent contrast, results from immunization studies with the hapten (4-hydroxy-3-nitrophenyl) acetyl (NP) suggested a stochastic model, in which a particular B cell is recruited equally to develop into either extrafollicular or germinal center responses [25, 26].
It is difficult to analyze B cell fate-decisions in vivo due to the lack of known unique characteristics of B cells that give rise to extrafollicular foci and germinal centers, respectively. Our aim was to establish a system with which to follow the contributions of a naturally occurring, antigen-specific B cell population that could help to elucidate early B cell selection events following influenza virus infection.
Earlier immunization studies with influenza A/Puerto Rico/34/8 (A/PR8) revealed a particularly strong, virus neutralizing and protective [2] early-induced response encoded by the C12 idiotype (C12Id) to one of the four major antigenic sites on HA1, the Cb site, in BALB/c mice [27]. Following immunization these C12Id+ HA-specific antibodies were shown to dominate the early HA-specific serum IgG response, but were absent from secondary responses [24, 27]. In contrast to another extensively studied idiotype-restricted response (C4) specific for the antigenic site Sb, which showed extensive mutations following immunization with influenza A/PR8 [28-31], sequence analysis of over 50 HA-specific hybridomas generated following primary immunization indicated that C12Id+ antibodies are exclusively germline encoded [27]. C12Id antibodies utilize a single Vκ-gene (Vk4/5 – VkC12), together with one of two closely related VH-genes from the J558 family (VHC12.1 and VHC12.2). In contrast to their similar V-gene usage, these antibodies use any of the four Jk and JH genes, respectively and at least three distinct D genes. Thus, HA-specific C12Id+ antibodies are diverse in CDR3 region lengths and sequence, while sharing fine specificity for the Cb site [27].
Using labeled influenza A/PR8 HA [32] and a mAb to C12Id [24] we followed C12Id+ HA-specific B cells in the context of the polyclonal B cell response to influenza virus infection in wildtype mice. The current study identifies HA-specific C12Id+ B cells as conventional follicular B cells that initiate both extra- and intra-follicular B cell responses, although with a strong bias towards the extrafollicular response type. This bias was not overcome with increased availability of T cell help, suggesting that infection-induced innate signals might drive the preponderance of extrafollicular responses during early infection.
Results
Influenza virus infection-induced C12Id+ B cell responses follow distinct kinetics compared to the overall antiviral humoral response
To establish a non-transgenic model system with which to follow early virus-specific antibody responses to influenza virus infection in the context of a complete, polyclonal B cell response, we focused on the HA-specific response to influenza A/PR8. Previous immunization studies had shown that a particular idiotype, C12, generates a large fraction of the virus-specific early response to influenza A/PR8 in BALB/c mice [24, 27] and an anti-C12 idiotype mAb had previously been generated [24].
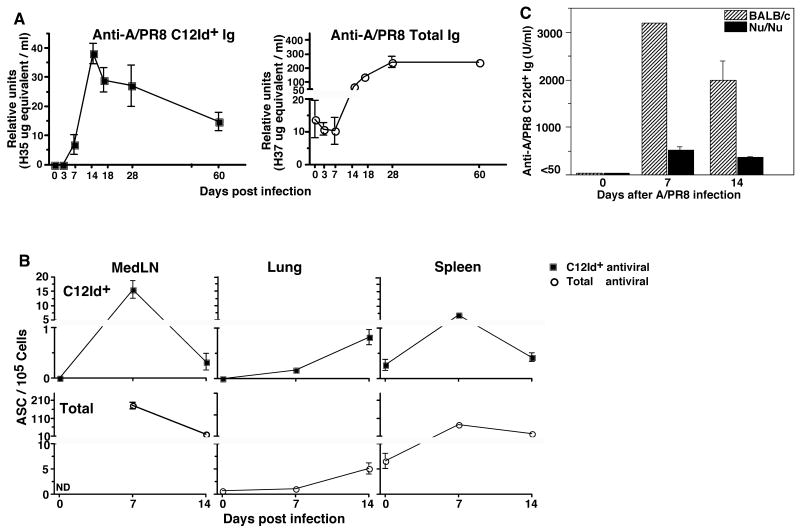
Infection of BALB/c mice with influenza A/PR8 showed that C12Id-expressing virus-specific serum antibody responses peaked rapidly, at around two-weeks after infection, consistent with the earlier immunization studies [24]. The C12Id response peak preceded the overall virus-specific antibody response peak by roughly two weeks (Fig. 1A). In contrast to the C12Id-encoded responses induced by immunization, which rapidly disappeared [24], antiviral C12Id serum antibodies were still measurable by day 60 following infection, albeit at levels reduced from their peak. The overall anti-viral serum antibody response reached plateau levels about one month after infection, after which time they were maintained over the lifetime of the mouse (Fig 1A, right panel and data not shown).
Figure 1. T-dependent rapid antibody responses encoded by C12Id are generated predominately in the regional lymph nodes following influenza infection.
(A) Shown are mean ± SD relative units Ig/ml serum of virus-specific C12Id+ (left panel, closed squares) and total antiviral (right panel, open circles) antibodies as determined by ELISA of four individual mice bled at indicated time-points before and after infection with influenza virus A/PR8. Relative units were calculated by comparison to binding of a virus-specific C12Id-expressing and non-C12Id-expressing mAb, respectively. (B) Shown are mean frequencies of virus-specific C12Id+ (upper panel) and virus-specific total Ig-secreting cells as determined by ELISPOT assays set up in duplicate of mediastinal lymph nodes (MedLN), lung tissue, and spleen from individual mice (n = 4) at indicated time points before and after infection. (C) Sera from groups (n = 4) of BALB/c (striped bars) and T cell-deficient (nu/nu) (filled bars) mice were taken before and at the indicated times after intranasal infection with influenza A/PR8. Virus-specific C12Id+ antibody levels were determined by ELISA and expressed as relative units (mean ± SD) calculated by comparison to a standard hyperimmune serum.
ELISPOT analysis on respiratory tract draining MedLN, spleen and lung identified the regional lymph nodes as the major site of early C12Id+ antibody production (Fig 1B). In contrast to the antibody responses in secondary lymphoid organs, the antibody secreting cells in the lung tissue indicated a steady accumulation. The rapid increase then decline of C12Id+ virus-specific serum antibodies could not be explained by T cell-independent B cell activation. T cell-deficient nude mice showed greatly reduced antiviral C12Id+ serum antibody titers compared to wild type BALB/c mice (Fig. 1C). While the C12Id-encoded response was greatly diminished, however, it was still measurable and followed kinetics similar to responses in wild type mice. Together, the virus-specific C12Id+ responses showed response kinetics distinct from those of the overall infection-induced humoral responses (Fig. 1A). The magnitude of this C12Id response suggested that we could follow C12Id+ B cells elaborating this response as prototypic “early” responders in the context of non-genetically manipulated wildtype BALB/c mice.
C12Id+ B cells display a follicular B cell phenotype in regional lymph nodes
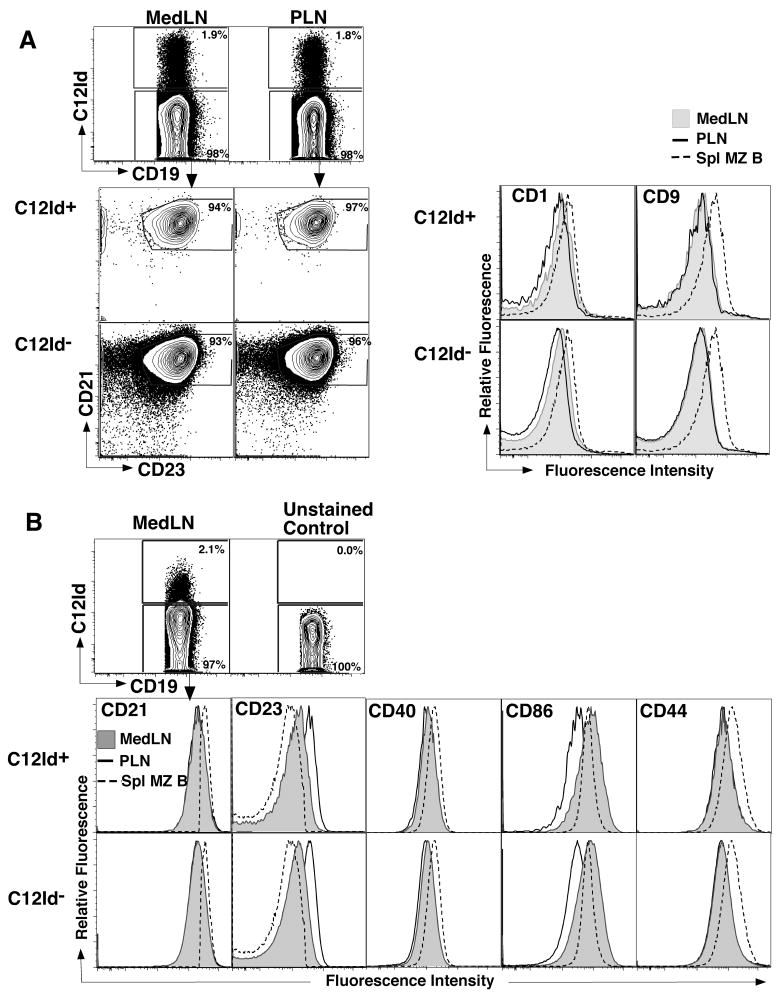
To study the characteristics of the rapidly differentiating C12Id+ B cells, we focused on regional lymph nodes, the site of strongest antibody production (Fig 1B). C12Id-expressing B cells were easily identified in MedLN and peripheral lymph nodes (pooled inguinal and axillaries) of non-infected mice, where they represented a relatively high frequency of B cells (between 1-2% of B cells, Fig. 2A and data not shown). Their frequencies in MedLN of non-infected mice were not significantly different from those in peripheral lymph nodes. As MedLN are extremely small in non-infected mice, we therefore used the peripheral lymph nodes as control for all remaining studies. The relatively high frequency of C12Id+ B cells is consistent with previous findings of high titers non-HA-specific C12Id-encoded antibodies in BALB/c mice prior to infection ([24] and data not shown). Importantly, none of the C12Id-encoded serum antibodies are virus-specific, unless mice are infected or immunized with A/PR8 (Figure 1A and data not shown). Thus C12Id-expressing B cells comprise a population of cells with heterogeneous specificities. HA-specific C12Id+ B cells do not undergo differentiation to antibody secreting cells prior to infection and therefore HA-specific C12Id+ antibodies are not part of the natural antibody repertoire to influenza virus in non-influenza infected mice, which we showed previously to be generated by B-1 cells [33].
Figure 2. C12Id+ lymph node B cells display a follicular B cell phenotype.
(A) Nine-color FACS analysis was conducted on pooled peripheral (PLN) and MedLN of non-infected BALB/c mice to determine the phenotype of C12Id+ lymph node B cells. Shown are 5% contour plots and histograms of CD19+ B cells following exclusion of CD3+ auto-fluorescent and dead cells. Numbers indicate frequencies of cells in the depicted gates. MedLN (grey filled) and PLN (solid line, no fill) B cells were further separated into C12Id+ (upper panel) and C12Id- (lower panel), and expression of CD21, CD23, CD1 and CD9 was determined. Splenic CD19+ CD23low/-, CD21hi marginal zone (MZ) B cells (dashed line, no fill) served as controls. (B) Activation marker expression on C12Id expressing and non-expressing B cells was determined on MedLN B cells at day 4 of influenza virus A/PR8 infection. Shown are 5% contour plots to depict staining of C12Id (top) and overlay histogram profiles (bottom) of C12Id+ (upper) and C12Id- (lower panels) B cells (solid line, filled histograms) identified as shown in (A). Expression levels of indicated markers were compared to that of splenic CD19+ CD21hi CD23low/- marginal zone B cells (dashed line, no fill) and PLN (solid line, no fill) B cells from uninfected mice.
B cells associated with rapid differentiation to antibody-forming cells are often attributed to certain B cell subsets, such as B-1 cells and splenic marginal zone B cells [11, 19, 34]. To determine the phenotype of C12Id B cells prior to infection, we compared C12Id+ and C12Id- lymph node B cells by flow cytometry. C12Id+ lymph node B cells were indistinguishable from the other lymph node B cells by phenotype, displaying a homogenous CD23+ CD21intm follicular B cell phenotype (Fig. 2A). They also expressed similar levels of the activation markers CD40, CD86 and CD44 on day 4 after infection with influenza A/PR8 compared to the other B cell populations in the MedLN (Fig. 2B). This is consistent with our earlier findings that most regional lymph node B cells from mice early after infection show type I IFN-mediated induction of CD86 and a decrease in CD23 expression [8, 35]. Thus, the C12Id+ B cells are similar in their levels or types of activation compared to the other lymph node B cells.
All C12Id+ and C12Id- B cells from peripheral and regional lymph nodes expressed lower levels of CD1 and CD9 compared to splenic CD23lo/- CD21hi marginal zone B cells (Fig. 2A, right panels) and similar levels compared to splenic follicular B cells (data not shown). Both regional lymph node C12Id+ and C12Id- B cells, showed slightly higher expression of CD1 compared to B cells in peripheral lymph nodes (Fig 2A, right panel). We conclude that C12Id lymph node B cells do not belong to a previously identified CD1hi follicular B cell subset [36]. Instead, and despite their rapid responses, they are phenotypically indistinguishable from other follicular B cells.
C12Id+ B cells participate mainly in extrafollicular foci responses
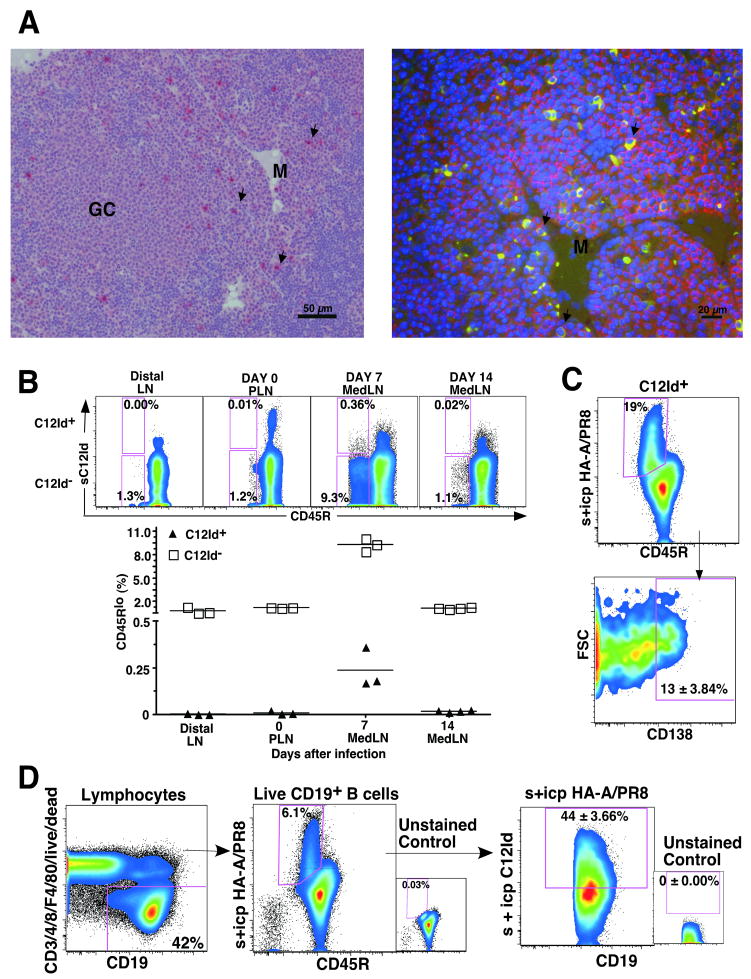
To determine the distribution of the C12Id+ B cells within the activated regional lymph node, we performed immunohistochemistry and double immunofluoresence staining using anti-C12Id and anti- CD138 (Syndecan) on MedLN harvested on day 10 after influenza infection. Large C12Id+ B cells with morphological appearance of plasma cells were found predominantly in the medullary cords. Their plasma cell phenotype was confirmed by staining for CD138 (Fig 3A). Extrafollicular foci responses in lymph nodes are found in the medullary areas [11], thus indicating that C12Id B cells rapidly differentiate via the extrafollicular pathway of B cell activation. This is also consistent with previous reports showing that this pathway is responsible for much of the early antibody response to pathogens [11, 37].
Figure 3. C12Id+ B cells generate strong extrafollicular foci responses.
(A) Immunohistochemistry (left) and double immunofluorescence (right) was done to identify C12Id antibody-expressing cells in MedLN collected from BALB/c mice on day 10 after infection with influenza A/PR8. Immunohistochemistry identifies the accumulation of C12Id+ cells (red, arrows) in the medullary cords (M) but not in germinal centers (GC) (Bar = 50 μm). Double immunofluorescence shows double-positive C12Id+ (green cytoplasmic staining) and CD138+ positive (red staining and arrows) cells in the medulla (M) of the MedLN (Bar = 20 μm). (B) Shown are pseudocolor FACS plots depicting CD45R (B220) and C12Id-expression on MedLN B cells collected at indicated times after infection, after gating on live (propidium iodideneg), non-dump (CD3/4/8/F4/80neg) CD19+ CD45R+/low cells (not shown). Scatter plot shows frequencies of C12Id+ (triangle) and C12Id- (square) cells among CD45Rlow lymph node (LN) B cells from BALB/c mice before and after infection (n = 3 - 4). Distal lymph nodes are PLN (inguinal and axillary) from day 7 and 14-infected mice. Each symbol represents data from an individual mouse. Horizontal line indicates the data mean. (C) Analysis of CD138 expression of HA-(A/PR8) specific C12Id+ plasmablasts in MedLN of mice infected for 7 days with influenza A/PR8 and treated in vivo with 0.25mg Brefeldin A for 6h prior to tissue collection. Shown are pseudo-color plots from a representative 5-color flow cytometric analysis of CD19+ C12Id+ B cells, identified by lack of expression for CD3, 4, 8, and F4/80 and expression of CD19 and C12Id (not shown). Virus-specific cells identified by binding to HA showed reduced expression of CD45R (B220) (upper panel, box). A subset of these cells was CD138+ (syndecan; lower panel). Number Indicates mean frequencies ± SD CD138+ cells among C12Id+ HA+ B cells from 4 individual mice. (D) Shown are pseudo-color FACS plots for gating of CD19+ (left panel) surface and intracytoplasmic HA-A/PR8 binding (s+ icp) (middle panel) cells that express the C12Id (right panel) in MedLN of 7 day influenza virus A/PR8-infected mice. Number in right panel indicates the mean frequency ± SD of C12Id+ HA-specific cells analyzed in MedLN of four individual mice.
Next, we performed FACS analysis on resting and non-infected peripheral lymph nodes and compared the frequency and phenotype of C12Id+ and C12Id- B cells to that of MedLN from day 7 and day 14 infected mice. Compared to peripheral lymph nodes from the same mice, or from non-infected control mice, we noted a strong, transient increase in CD19+ C12Id+ and C12Id- B cells that had down-regulated CD45R (B220) by day 7 of infection (Fig. 3B). Both, B220lo C12Id+ and C12Id- B cells were greatly reduced again by day 14 of infection, a kinetic that correlates with the early peak then reduction of antibody-secreting MedLN C12Id+ and C12Id- B cells measured by ELISPOT (Fig. 1B). Indeed FACS-purification of the B220lo B cells revealed that they are the main source of antibody-secreting foci after influenza infection (data not shown) and likely represent extrafollicular foci-derived plasmablasts. This is consistent with reports by others in immunization model systems that showed that extrafollicular foci-associated plasmablasts reduce expression of B220 [9].
Since staining for C12Id alone is not sufficient to follow virus-specific B cells, we identified the HA-specific C12Id+ B cells in the MedLN by multicolor flow cytometry using biotinylated influenza A/PR8 HA, as previously detailed [32], in conjunction with staining for C12Id (Fig 3C and 3D). To maximize identification of all C12Id+ virus-specific B cells, including those secreting antibodies in vivo and potentially expressing low levels of surface Ig, we treated mice with Brefeldin A for 6h prior to tissue collection followed by intracytoplasmic staining for immunoglobulin, analogous to studies analyzing in vivo cytokine secretion by CD8+ T cells [38]. The analysis showed that MedLN CD19+ HA-specific B cells expressing C12Id had a phenotype similar to that of extrafollicular-derived plasmablasts [9]: They are high in FSC, express relatively high levels of intracytoplasmic immunoglobulin and low levels of CD45R (Figure 3B and 3C). About 13% of the HA-specific B220lo C12Id+ B cells expressed the plasma cell marker CD138 on day 7 after influenza infection, indicating their terminal differentiation (Figure 3C).
Antibodies against HA are the major component of the antiviral humoral response induced during primary influenza virus infection [14]. By quantifying the HA-specific B cells by flow cytometry, we further show that in the MedLN about 40% of virus-HA-specific cells express C12Id on day 7 of infection (Fig. 3D). This confirms earlier antibody-measurements after immunization with A/PR8 [24] and demonstrates at the cellular level that the C12-encoded response is a major component of the early antiviral B cell response to influenza A/PR8 in BALB/c mice. The fast response kinetics of the early antiviral response can be attributed to the preferential involvement of HA-specific C12Id+ B cells in extrafollicular plasmablast growth and differentiation.
C12Id B cells can form germinal centers
While some studies indicated that B cells that form extrafollicular foci also participate in germinal center reactions [39, 40], others had concluded that precursors of extrafollicular foci are distinct by phenotype [41] of affinity for antigen [22] from those that give rise to germinal center responses. The above studies demonstrated strong extrafollicular foci-development by HA-specific C12Id+ B cells. C12Id-encoded virus-specific serum antibodies, however, were detectable for at least two months after infection, thus appeared relatively long-lived (Fig 1A). Given that serum antibodies have a half-life of only a few days in vivo [42, 43] and that extrafollicular foci responses are thought to only generate short-lived responses [9, 11], we examined next whether C12Id+ B cells participate also in germinal center reactions, i.e. structures known to provide long-lived immunity.
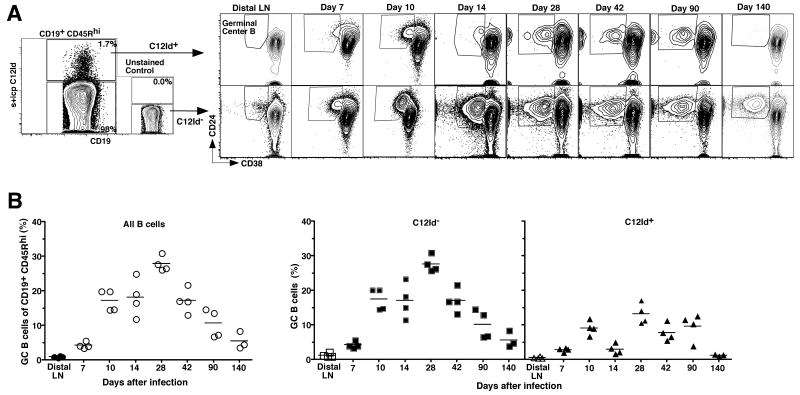
Germinal center development in MedLN was first measurable by day 7 of infection, peaked around day 28, and then remained present for at least 140 days (Fig. 4). C12Id+ B cells with a phenotype consistent of germinal center B cells (CD45Rhi CD38lo CD24hi Fig. 4) and PNAhi (data not shown) were observed by day 10 of infection. In contrast to the C12Id- responders that showed a time-dependent rise then cessation in the frequencies of germinal center B cells, however, C12Id+ germinal center B cell frequencies lacked consistent waxing and waning. Instead they were present only in small frequencies and with irregular kinetics. The relative frequencies of germinal center B cells among the C12Id non-expressers exceeded the frequency of C12Id+ cells at all times after infection (Fig.4). Given that the virus is cleared from the mice within 7 to10 days [2], germinal center formation was surprisingly long-lived in the regional lymph nodes (still present at low frequencies nearly 5 months after infection). This is consistent with reports on the late induction of influenza-specific memory CD4 T cells from antigen-pools that persist long after influenza virus clearance [44] and suggests that such antigen-pools must be present in the B cell follicles of the regional lymph nodes.
Figure 4. C12Id+ B cells can form germinal centers.
(A) Analysis of germinal center formation among C12Id+ and C12Id- MedLNs of mice infected for 140 days with influenza A/PR8. Shown are 5% contour plots with outliers including an unstained control (left panels) from a representative 7-color flow cytometric analysis of C12Id+ and C12Id- live B cells identified by lack of expression for CD3, 4, 8 and F4/80, expression of CD19 and high expression of CD45R (not shown). Germinal center B cells among C12Id+ and C12Id- cells were identified as CD38low CD24high (boxed, right panels). Numbers indicate frequencies of cells in the depicted gates. Each plot on the right represents results from one of 4 mice per time point. Results are summarized in (B). Scatter plots show frequencies of germinal center cells among all (left panel), C12Id- (middle) and C12Id+ (right) B cells. Frequencies of germinal center B cells in peripheral (distal) lymph nodes taken from day 7 and 14 infected mice are shown for comparison. Each symbol represents data from an individual mouse. Mean values for each time point are indicated by a horizontal line.
Importantly, the data demonstrate that while C12Id+ B cells participate vigorously in extrafollicular foci responses, they do form germinal centers, albeit at low frequencies and with irregular kinetics. Thus, a population of B cells expressing the same idiotype and recognizing the same epitope on influenza A/PR8 HA is able to initiate both extrafollicular foci and germinal center responses following influenza virus infection.
The presence of CD4+ helper T cells does not alter the virus-specific C12Id B cell response quality
Our studies in T-deficient mice indicated a strong enhancement, but not total dependence of virus-specific C12Id antibody formation on T cell help (Fig. 1B). Work by others had shown that extrafollicular foci form even in the absence of T cells. In contrast, germinal center formation is dependent entirely on T cells [12, 13]. We next aimed to determine whether an increased availability of T cell help could shift the balance of extrafollicular over germinal center responses towards the latter response. For that we adoptively transferred 2.3 × 106 TS-1 transgenic CD4 T cells 12 h prior to infection, roughly 40% of which expressed the clonotypic transgenic TCR specific for influenza HA from A/PR8 ([45] and data not shown). The increased availability of T cell help in the recipients significantly enhanced the frequencies of virus-specific C12Id+ antibody-secreting cells at day 7 of infection (Fig. 5A). Given that C12Id+ germinal centers are not visible prior to day 7 of infection (Fig. 3A and 4B), this indicated that the presence of helper T cells enhances the extrafollicular-derived C12Id+ antibody responses. Transfer of polyclonal CD4 T cells also seem to enhance these responses, although these differences did not reach statistical significance (p = 0.1; Fig. 5A).
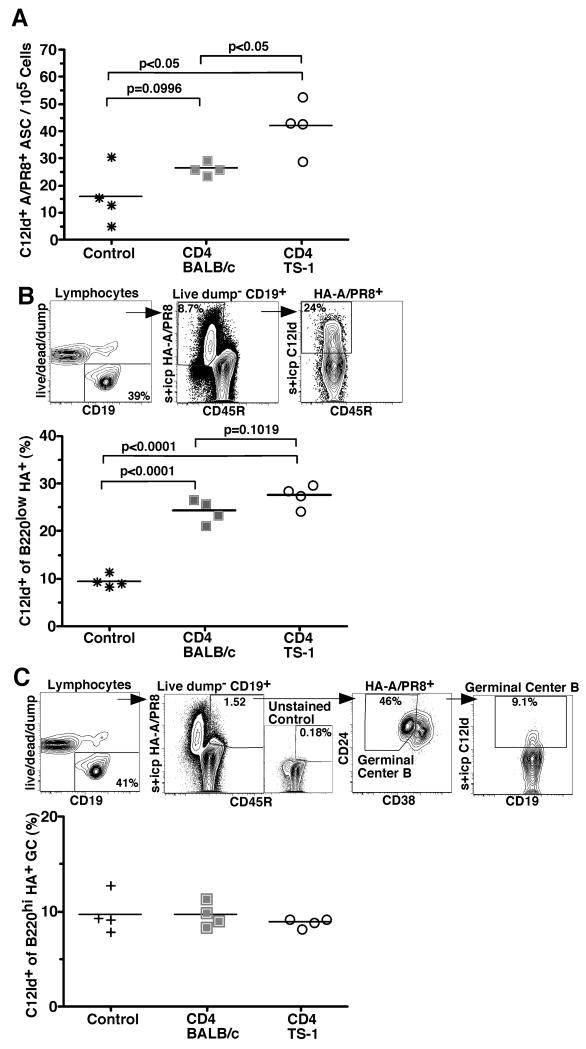
Figure 5. CD4 T cells enhance the magnitude but not the quality of the C12Id+ response.
(A) Shown is a scatter plot indicating frequencies of virus-specific C12Id+ antibody-secreting cells in MedLN of day 7 influenza virus A/PR8-infected BALB/c mice that received 2.3 × 106 CD4 T cells from either wild type (closed squares) or TCR-transgenic TS-1 mice (open circles), or PBS alone (control, stars) 12h prior to infection as assed by ELISPOT. (B) Shown is the FACS-gating strategy to determine the frequencies of C12Id+ plasma blasts in MedLN of mice as in (A). Live MedLN B cells were identified by lymphocyte FSC/SSC (not shown) lack of expression of CD3, 4, 8, F4/80 (dump), and expression of CD19. Cells were further gated on staining for CD45R (B220) and binding to HA-A/PR8 (surface and intracytoplasmic (s + icp)). Virus-specific C12Id+ plasmablasts were identified as B220lo HA+ surface and/or intracytoplasmic (s+icp) C12Id positive. Numbers indicate frequencies of cells in the depicted gates. Results are summarized in the scatter plot below. Each symbol represents results from one animal. Horizontal lines indicate mean frequencies. (C) Shown is the FACS-gating strategy to determine frequencies of C12Id+ germinal center B cells in MedLN from individual mice of groups similar to those shown for A and B, expect that controls did not receive PBS prior to infection. HA-specific C12Id+ germinal center B cells were identified as live, non-dump, CD19+ (left panel), HA+ CD45R (B220)high (second from left), CD24+ CD38lo (third) and C12Id+ (s+icp, right panel). FACS analysis gates were set based on control stains that lacked only the one marker of interest (shown for HA-stain and data not shown). Numbers indicate frequencies of cells in the depicted gates. Scatter plots summarize the results. Each symbol represents data from individual animals and horizontal line shows mean of the group. Results are from one of two independent experiments that yielded similar results. Statistical significance was calculated using the Student's t test.
Consistent with these findings, frequencies of HA-A/PR8-specific B220lo C12Id+ plasma blasts were higher in TS-1 helper T cell recipients compared to control mice that did not receive any CD4 T cells (Fig 5B). Transfer of polyclonal T cells also significantly enhanced the frequencies of the C12Id+ virus-specific cells (Fig. 5B). Whether this is due to the activation of T cells in the isolation process, or non-cognate interaction between B cells and CD4 T cells that could enhance extrafollicular responses, remains to be studied. Importantly, virus-specific germinal center B cell frequencies were unaltered by the transfer of specific or non-specific CD4 T cells (Fig 5C). Thus, the presence of helper CD4 T cells can enhance the magnitude of the extrafollicular B cell response but cannot shift the quality of the C12Id+ B cell response towards increased germinal center formation.
Discussion
Exploiting work by others that previously identified influenza A/PR8 HA-specific antibodies of the C12Id as a major component of the early B cell response to influenza [24, 27], and building on our more recent work identifying influenza HA-specific B cells by flow cytometry [32], we studied the fate of HA-specific B cells following influenza virus infection in genetically non-manipulated BALB/c mice. Our studies identify follicular B cells in the regional lymph nodes of infected mice as the cell population responsible for much of the early-induced C12Id+ antibody response via their rapid induction of extrafollicular foci. C12Id-expressing B cells also initiated germinal center responses, albeit to a lesser degree and with delayed and irregular kinetics. Increased CD4 T cell help enhanced the magnitude of the C12-initiated extrafollicular responses. Importantly, it did not shift the response quality towards increased germinal center formation. Together our studies indicate the presence of as yet unknown, presumably innate, signals that cause the expansion but not the initiation of extrafollicular over intrafollicular B cell responses.
Characterization of the early-responding C12Id+ HA-specific B cells failed to provide evidence for a phenotypically distinct B cell population in the regional lymph nodes that could give rise preferentially or exclusively to early antibody-forming foci, as suggested in earlier studies [41]. C12Id-expressing B cells in the local lymph nodes displayed a homogeneous CD23+ CD21intm follicular B cell phenotype, and despite their rapid responses, they expressed similar levels of activation markers as C12Id non-expressing B cells at that location (Fig 2). The early responding C12Id+ HA-specific B cells were also not significantly different with regard to expression of CD24, one of the markers identified by Linton et al [41] to correspond with extrafollicular foci development, although we measured CD24 expression with an anti-CD24 mAb different from that used by that earlier study (data not shown). Thus, lymph nodes seem to lack a specific resident B cell subpopulation comparable to the marginal zone B cell population in the spleen that can facilitate rapid responses to early blood-borne infection. Instead, follicular (C12Id+ HA-specific) B cells provide this rapid, strong (Fig. 1) and protective [2] extrafollicular B cell response (Fig. 3).
Various models have been proposed to explain the regulation of extra versus intra-follicular B cell responses [22, 26, 39-41, 46]. The influenza virus model system described here and available tools to study the C12Id-specific responses provide an excellent opportunity to further analyze this important differentiation process in vivo in the context of an infection. Our study identifies C12Id-expressing HA-specific B cells as predominant contributors to a strong extrafollicular B cell response in regional MedLN, the site of much of the early antibody response to this virus. Predominant participation of the C12Id-expressing HA-specific cells in extrafollicular responses is consistent with earlier findings that failed to find any mutated C12Id-sequences among HA-specific B cells [27], i.e. these cells showed no signs of affinity maturation. However, we show here that while C12Id+ cells vigorously participate in extrafollicular foci responses (Fig. 3), they can also initiate germinal centers (Fig. 4). While we cannot completely rule out that C12Id+ B cells that mutated their BCR might have lost the idiotope that allowed us to stain these cells for FACS analysis, the earlier extensive sequence studies on B cells from mice at various times after immunization (26), make this trivial explanation, somewhat unlikely.
Given the recent studies by Pauls et al [22] that implicated BCR-affinity for antigen in the selection process, our studies might suggest that high-affinity interactions with antigen are a necessary, but likely not sufficient, signal for extrafollicular foci development as C12Id+ HA-specific cells are able to also initiate germinal centers (Fig. 3 and 4). Failure to expand this germinal center response during early infection, rather than an inability to initiate it, might result in the irregular kinetics and small frequencies of C12Id+ germinal center B cells observed here (Fig. 4). This interpretation is not only consistent with the presented data, but also with earlier studies using the NP system, which demonstrated that extrafollicular foci and germinal center B cells can have a common precursor [25, 26, 39].
The vigorous infection-induced extrafollicular foci response is likely supported and modulated by external signals. CD4 T cells strongly increase the magnitude of the response (Fig. 1B and Fig. 5), but they do not appear to modulate B cell fate-decisions, as addition of T cell help increased the extrafollicular response without affecting germinal center responses (Fig. 5). Since transfer of non-virus-specific CD4 T cells alone affected extrafollicular foci size (Fig. 5), the C12Id B cell responses might be affected through secreted T cell products rather than cognate T-B interactions. IFN-γ could be one candidate, as we showed previously that in vivo blockade of IFN-γ significantly reduced the early antigen-specific IgG2a response following influenza virus infection [47]. Kim and coworkers showed that increased IL-12 production by Dendritic cells (DC) that lacked the Fc-receptor γ chain, leads to preferential generation of short-lived plasma cells and ablated germinal center responses [48]. Furthermore, our group and others have shown that type I IFN or TLR mediated signals [8, 35, 49, 50] can positively regulate the magnitude and quality of B cell responses [51, 52], supporting the notion that the local environment with its infection-induced signals might play an important role in shaping the B cell response at that location.
Taken together, we would argue that our data are most consistent with a model in which a stochastic process underlies the activation and differentiation of virus-specific B cell towards extra- versus intra-follicular responses. While the magnitude of the extrafollicular response type can be enhanced by helper T cells, T cells do not direct the preferential development of one over the other B cell differentiation pathway. Since C12Id+ B cells have a follicular B cell phenotype, arguing against the presence of a specific subset of rapidly responding lymph node B cells, it is likely that the presence of infection-induced innate signals drives strong extrafollicular foci responses early after infection. Identification of these signals could be of great value for the design of vaccines aiming to provide rapid immune protection. This non-transgenic infectious disease model now allows for a systematic analysis of short and long-term effects of innate signals on extrafollicular and germinal center responses.
Materials And Methods
Mice and virus
8- to 12-wk-old female BALB/c mice (Harley Sprague Dawley) and T cell-deficient BALB/C nude mice (Jackson Labs) were purchased and kept in filter top cages under conventional housing conditions. TS-1 mice, which express a transgenic TCR-α/β specific for I-Ed-restricted MHCII peptide 111-119 from influenza A/PR8 hemagglutinin [45], originally kindly provided by A. Caton (The Wistar Institute, Philadelphia), were bred and kept under the same housing conditions. Mice were infected intranasally under isoflurane anesthesia with a sublethal dose corresponding to 20 plaque-forming units (PFU) of A/PR/8 (H1N1) in 40 μl of PBS per mouse. Virus was grown in embryonated hen eggs and PFU were established as outlined [32]. For detection of in vivo antibody-secreting cells, Brefeldin-A was purchased from Sigma-Aldrich, pre-diluted to 5 mg / ml in DMSO, then further diluted to 0.25 mg / 200 μl in PBS and injected i.p. 6 hours prior to tissue collection. Sera for ELISA were collected from mice via tail vein bleeds. All experiments were performed according to protocols approved by the UC Davis Animal Use and Care Committee.
Cell preparation and flow cytometry
Lymph node, spleen, and lung tissue cell preparations were generated as previously described [8, 53]. Live cells were counted using a hematocytometer and trypan blue exclusion. Cell suspensions were stained as described previously [53] and surface stained with the following conjugated antibodies at previously determined optimal concentrations: CD4/8/F4/80-Pacific Blue (GK1.5/53.6.7/F4/80), CD38-FITC (clone 90), HA-A/PR8-biotin (as described [32]), CD1d-Cy5PE (1B1), CD21-Cy55PE (7G6), CD24-Cy55PE (30F.1), and CD23-allophycocyanin (B3.B4) were generated in-house following published protocols (www.drmr.com). C12Id-QDOT605 (23-1 Id [24]) was generated using the QDOT antibody conjugation kit (Invitrogen). Commercial reagents used were: CD9-biotin, CD3-Pacific Blue (BD Bioscience), CD40-FITC, CD86-PE, CD44-Cy5PE, SA-Cy7PE (all eBioscience), CD3-allophycocyanin-Alexa750, CD19-Cy5.5allophycocyanin (both Invitrogen), and anti-biotin-PE (Miltenyi Biotec). Live/dead fixable violet staining kit (Invitrogen) was used to discriminate dead cells. For intracytoplasmic C12Id and HA staining cells were fixed for 30 min. on ice using Cytofix/Cytoperm (BD Bioscience), followed by washing and intracytoplasmic staining for 30 min. at room temperature in Perm/Wash solution (BD Bioscience). Data acquisition was done using a FACSAria (BD Bioscience) set-up for 13-color analysis [53]. Data analysis was conducted using FlowJo software (kind gift from Adam Triester, TreeStar.Inc).
Immunohistochemistry and immunofluorescence
MedLN were fixed in 10% phosphate buffered formaldehyde solution for 24 hours and subsequently embedded in paraffin. 4 μm sections were cut using a microtome (Leica). The antigen was retrieved using 10mM heated citrate buffer (pH 6). Slides were stained overnight at room temperature with biotinylated rat anti-mouse C12 Id and stained for 1h with biotinylated anti-rat antibody (InnoGenex). For immunohistochemistry staining was revealed with ExtrAvidin Phosphatase (Sigma) for 30 min., followed by incubation with NovaRed substrate (Vector). The slide was counterstained with Mayer's hematoxylin and cover slipped with Permount (Fisher Scientific). Slides for immunofluorescence-staining were incubated with the same anti-mouse C12Id antibody for 1h, then secondary anti-rat antibody (InnoGenex) for 1h in the dark followed by SA-488 (Invitrogen). After washing, slides were incubated with streptavidin/biotin block (Vector) and the second primary antibody (biotin-conjugated rat anti-mouse CD138 (Syndecan-1), clone: 281-2, BD) was added for 2 hours in the dark. After washing, SA-Alexa 568 along with DAPI (both Invitrogen) were added and incubated for 1 hour each in the dark. Slides were cover-slipped with an antifade mounting media (ProLong Antifade Kit (P-7481) Invitrogen). Images were captured on a Zeiss Axioskop and prepared with Adobe Photoshop.
Magnetic cell separation and cell transfer
For CD4 T cell enrichment, single cell suspensions from peripheral lymph nodes of TCR-transgenic TS-1 and control BALB/c mice were stained with biotinylated antibodies to CD8, CD11b, CD19, GR-1 (all in-house generated) and CD49b (BD bioscience), followed by anti-biotin antibodies coupled to MACS beads (Miltenyi Biotec) and isolated by autoMACS (Miltenyi Biotec). CD4 T cell purities were > 96% as determined by staining with anti-CD4 and anti-CD3 antibodies. Recipient BALB/c mice received 2.3 × 106 CD4 T cells from either BALB/c or TS-1 donors 12h prior to infection.
ELISPOT and ELISA
Cell suspensions in medium (RPMI 1640, 292ug/ml L-Glutamine, 100μg/ml Penicillin/Streptomycin, 10% heat inactivated fetal calf serum, 0.03M 2-ME) were placed in duplicates at 106 cells / well into ELISPOT plates (MultiScreen HA Filtration; Millipore) coated with sucrose-density gradient-purified influenza A/PR8. 2-fold serial dilutions in medium were performed. Virus-specific ELISPOT assay were done as described previously [32] revealing with either Ig (H+L)-biotin (Southern Biotech) or with anti-C12Id-biotin (23-1 Id). Mean spot counts ± SD/106 input cells were calculated from all wells with countable spots.
Virus-specific ELISA was done as previously described [32]. For C12Id virus-specific ELISA 3% phosphate buffered PFA solution (pH 7.2) was used following serum incubation to crosslink antigen-antibody complexes and enhance sensitivity of the assay [24]. Relative virus-specific Ig units were calculated by comparison to a standard hyperimmune serum [47]. Relative virus-specific antibody concentrations were calculated from a standard virus-specific IgG C4Id+ mAb (clone H37-41-7) or a virus-specific IgG C12Id+ mAb (clone H35-C12.6.2) both purified from tissue-culture supernatant by protein G affinity chromatography. One relative unit was arbitrarily defined as equivalent to binding of 1 μg/ml of the relevant mAb. ELISA plates were measured on a SpectraMax M5 (Molecular Devices) ELISA reader, and data were analyzed using Soft MaxPro software (Molecular Devices).
Statistical Analysis
Statistical analysis was done using a two-tailed un-paired Student's t test with the help of Prism 4 software (GraphPad Software, San Diego, CA, USA). Data were regarded as statistically significant at ρ < 0.05.
Acknowledgments
We would like to thank Abby Spinner for help with the FACS Aria, Stefan Tunev (UC Davis) for extensive help with immunohistochemistry and immunofluorescence, Dr. Michael McChesney (UC Davis) for critical reading of the manuscript, and Walter Gerhard (The Wistar Institute) for critical reagents, advice, insight and inspiration throughout these studies. This work was supported by a grant from the NIH/NIAID AI051354 (to N. B.) and NIH training grant support to K.R. (T32-A160555 and T32 HL07013-31A1). The authors declare no financial or commercial conflict of interest.
References
- 1.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 3.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopf M, Brombacher F, Bachmann MF. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur J Immunol. 2002;32:2229–2236. doi: 10.1002/1521-4141(200208)32:8<2229::AID-IMMU2229>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol. 2005;79:5943–5951. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topham DJ, Doherty PC. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, Doherty PC, Turner SJ. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci U S A. 2003;100:7235–7240. doi: 10.1073/pnas.1232449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 9.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 10.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 12.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 13.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24:39–65. doi: 10.1615/critrevimmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 14.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108:431–439. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgarth N, Choi YS, Rothaeusler K, Yang Y, Herzenberg LA. B cell lineage contributions to antiviral host responses. Curr Top Microbiol Immunol. 2008;319:41–61. doi: 10.1007/978-3-540-73900-5_3. [DOI] [PubMed] [Google Scholar]
- 16.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLennan ICM, Gray D, Kumararatne DS, Bazin H. The lymphocytes of splenic marginal zones: a distinct B-cell lineage. Immunology Today. 1982;3:305–307. doi: 10.1016/0167-5699(82)90032-9. [DOI] [PubMed] [Google Scholar]
- 18.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraal G. Antigens take the shuttle. Nat Immunol. 2008;9:11–12. doi: 10.1038/ni0108-11. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan IC. B cells: the follicular dimension of the marginal zone. Immunol Cell Biol. 2008;86:219–220. doi: 10.1038/icb.2008.2. [DOI] [PubMed] [Google Scholar]
- 22.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roost HP, Bachmann MF, Haag A, Kalinke U, Pliska V, Hengartner H, Zinkernagel RM. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc Natl Acad Sci U S A. 1995;92:1257–1261. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavaler J, Caton AJ, Staudt LM, Gerhard W. A B cell population that dominates the primary response to influenza virus hemagglutinin does not participate in the memory response. Eur J Immunol. 1991;21:2687–2695. doi: 10.1002/eji.1830211107. [DOI] [PubMed] [Google Scholar]
- 25.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol. 1998;161:5373–5381. [PubMed] [Google Scholar]
- 27.Kavaler J, Caton AJ, Staudt LM, Schwartz D, Gerhard W. A set of closely related antibodies dominates the primary antibody response to the antigenic site CB of the A/PR/8/34 influenza virus hemagglutinin. J Immunol. 1990;145:2312–2321. [PubMed] [Google Scholar]
- 28.Carmack CE, Camper SA, Mackle JJ, Gerhard WU, Weigert MG. Influence of a V kappa 8 L chain transgene on endogenous rearrangements and the immune response to the HA(Sb) determinant on influenza virus. J Immunol. 1991;147:2024–2033. [PubMed] [Google Scholar]
- 29.Clarke SH, Staudt LM, Kavaler J, Schwartz D, Gerhard WU, Weigert MG. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. J Immunol. 1990;144:2795–2801. [PubMed] [Google Scholar]
- 30.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKean D, Huppi K, Bell M, Staudt L, Gerhard W, Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984;81:3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doucett VP, Gerhard W, Owler K, Curry D, Brown L, Baumgarth N. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J Immunol Methods. 2005;303:40–52. doi: 10.1016/j.jim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 35.Chang WL, Coro ES, Rau FC, Xiao Y, Erle DJ, Baumgarth N. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 36.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 37.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 39.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson AR, Zitron IM, McMichael AJ. Clones of B lymphocytes: their natural selection and expansion. Fed Proc. 1976;35:2195–2201. [PubMed] [Google Scholar]
- 41.Linton PL, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 42.Fahey JL, Sell S. The Immunoglobulins of Mice. V. The Metabolic (Catabolic) Properties of Five Immunoglobulin Classes. J Exp Med. 1965;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 44.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacLennan IC, Liu YJ, Oldfield S, Zhang J, Lane PJ. The evolution of B-cell clones. Curr Top Microbiol Immunol. 1990;159:37–63. doi: 10.1007/978-3-642-75244-5_3. [DOI] [PubMed] [Google Scholar]
- 47.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SJ, Caton M, Wang C, Khalil M, Zhou ZJ, Hardin J, Diamond B. Increased IL-12 inhibits B cells' differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. J Exp Med. 2008;205:2437–2448. doi: 10.1084/jem.20070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 52.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 53.Rothaeusler K, Baumgarth N. Evaluation of intranuclear BrdU detection procedures for use in multicolor flow cytometry. Cytometry A. 2006;69:249–259. doi: 10.1002/cyto.a.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]