Abstract
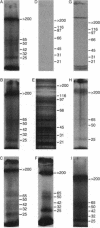
The endoperoxides are a new class of antimalarial agents, of which artemisinin (qinghaosu) is the prototype. We have previously shown that artemisinin is capable of alkylating proteins in model reactions. In the present study, we showed that when Plasmodium falciparum-infected erythrocytes are treated with a radiolabeled antimalarial endoperoxide, either arteether, dihydroartemisinin, or Ro 42-1611 (arteflene), the radioactivity is largely coverted into a form which can be extracted with sodium dodecyl sulfate (SDS). Autoradiograms of SDS-polyacrylamide gels showed that six malarial proteins are radioactively labeled by the three endoperoxides. This labeling occurs at physiological concentrations of drug and is not stage nor strain specific. The labeled proteins were not the most abundant proteins seen on Coomassie-stained gels. No proteins were labeled when uninfected erythrocytes were treated with these drugs, nor when infected erythrocytes were treated with the inactive analog deoxyarteether. Thus, the antimalarial endoperoxides appear to react with specific malarial proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brossi A., Venugopalan B., Dominguez Gerpe L., Yeh H. J., Flippen-Anderson J. L., Buchs P., Luo X. D., Milhous W., Peters W. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J Med Chem. 1988 Mar;31(3):645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- Caspers P., Etlinger H., Matile H., Pink J. R., Stüber D., Takács B. A Plasmodium falciparum malaria vaccine candidate which contains epitopes from the circumsporozoite protein and a blood stage antigen, 5.1. Mol Biochem Parasitol. 1991 Aug;47(2):143–150. doi: 10.1016/0166-6851(91)90173-4. [DOI] [PubMed] [Google Scholar]
- Crandall I., Sherman I. W. Plasmodium falciparum (human malaria)-induced modifications in human erythrocyte band 3 protein. Parasitology. 1991 Jun;102(Pt 3):335–340. doi: 10.1017/s0031182000064271. [DOI] [PubMed] [Google Scholar]
- Fairfield A. S., Eaton J. W., Meshnick S. R. Superoxide dismutase and catalase in the murine malaria, Plasmodium berghei: content and subcellular distribution. Arch Biochem Biophys. 1986 Nov 1;250(2):526–529. doi: 10.1016/0003-9861(86)90758-7. [DOI] [PubMed] [Google Scholar]
- Fairfield A. S., Meshnick S. R., Eaton J. W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983 Aug 19;221(4612):764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F., Cerami A., Henderson G. B. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie P., Roth E. F., Jr, Oppenheim J., Vanderberg J. P. Biochemical characterization of Plasmodium falciparum hemozoin. Am J Trop Med Hyg. 1990 Dec;43(6):584–596. doi: 10.4269/ajtmh.1990.43.584. [DOI] [PubMed] [Google Scholar]
- Gu H. M., Warhurst D. C., Peters W. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1984;78(2):265–270. doi: 10.1016/0035-9203(84)90296-7. [DOI] [PubMed] [Google Scholar]
- Hien T. T., White N. J. Qinghaosu. Lancet. 1993 Mar 6;341(8845):603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- Holder A. A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- Hong Y. L., Yang Y. Z., Meshnick S. R. The interaction of artemisinin with malarial hemozoin. Mol Biochem Parasitol. 1994 Jan;63(1):121–128. doi: 10.1016/0166-6851(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Klayman D. L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985 May 31;228(4703):1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Krungkrai S. R., Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans R Soc Trop Med Hyg. 1987;81(5):710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander O. A., Ager A. L., Jr, Morris V. C., May R. G. Qinghaosu, dietary vitamin E, selenium, and cod-liver oil: effect on the susceptibility of mice to the malarial parasite Plasmodium yoelii. Am J Clin Nutr. 1989 Aug;50(2):346–352. doi: 10.1093/ajcn/50.2.346. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Tsang T. W., Lin F. B., Pan H. Z., Chang C. N., Kuypers F., Chiu D., Lubin B. Activated oxygen mediates the antimalarial activity of qinghaosu. Prog Clin Biol Res. 1989;313:95–104. [PubMed] [Google Scholar]
- Meshnick S. R., Yang Y. Z., Lima V., Kuypers F., Kamchonwongpaisan S., Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob Agents Chemother. 1993 May;37(5):1108–1114. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner G. H., Oh C. H., Wang D., Gerena L., Milhous W. K., Meshnick S. R., Asawamahasadka W. Mechanism-based design, synthesis, and in vitro antimalarial testing of new 4-methylated trioxanes structurally related to artemisinin: the importance of a carbon-centered radical for antimalarial activity. J Med Chem. 1994 Apr 29;37(9):1256–1258. doi: 10.1021/jm00035a003. [DOI] [PubMed] [Google Scholar]
- Smythe J. A., Peterson M. G., Coppel R. L., Saul A. J., Kemp D. J., Anders R. F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Mar;39(2):227–234. doi: 10.1016/0166-6851(90)90061-p. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Asawamahasakda W., Meshnick S. R. Alkylation of human albumin by the antimalarial artemisinin. Biochem Pharmacol. 1993 Jul 20;46(2):336–339. doi: 10.1016/0006-2952(93)90425-v. [DOI] [PubMed] [Google Scholar]
- Zhang F., Gosser D. K., Jr, Meshnick S. R. Hemin-catalyzed decomposition of artemisinin (qinghaosu). Biochem Pharmacol. 1992 Apr 15;43(8):1805–1809. doi: 10.1016/0006-2952(92)90713-s. [DOI] [PubMed] [Google Scholar]