Abstract
Background and Objective:
The deleterious health consequences of smoking are even more severe for women, yet ironically, they have more difficulty quitting than men. Identifying relapse predictors for women and implementing strategies to increase their chances of successfully quitting and remaining abstinent are important goals. Clinicians and researchers suggest that women could achieve greater success in smoking cessation interventions if the initial quit attempt coincided with the follicular phase (i.e., preovulatory phase) of their menstrual cycle (MC) rather than the luteal phase (i.e., premenstrual). However, no experimental data have been published to support this claim. Our objective was to determine whether MC phase affected smoking status in premenopausal female smokers participating in a smoking cessation treatment trial.
Methods:
Data from 102 treatment-seeking smokers who participated in an 8-week nicotine replacement therapy (NRT) plus behavioral intervention smoking cessation study were examined retrospectively. NRT began the day subjects attempted to quit smoking (quit date). For analyses, smokers were grouped according to sex, and women were subdivided by MC phase at quit date into follicular (FF, days 1–14, n = 16) and luteal (LF, days 15–30, n = 21) groups.
Results:
Smoking status was examined on the third day after the quit date (day 3) and at 1 week posttreatment (week 9). On day 3, 52% of LFs reported smoking compared with 19% of FFs (p < 0.04), and at week 9, 71% of LFs reported smoking compared with 31% of FFs (p < 0.02). In a comparison group of men (n = 65), 25% were smoking at day 3 and 68% at week 9. Self-report at week 9 was verified by urine cotinine levels.
Conclusions:
These data support the supposition that better treatment outcomes can be achieved by scheduling quit dates to coincide with the follicular phase of the MC in female smokers.
INTRODUCTION
OVER THE PAST SEVERAL DECADES, we have gained extensive knowledge about the devastating health consequences of cigarette smoking, underscoring the importance of identifying relapse predictors and implementing strategies to increase smoking cessation success rates. For numerous reasons, this is particularly important for women. For example, from 1990 to 2003, the number of new lung cancer cases in women in the United States increased by 600%, while the number of new lung cancer cases in men remained the same (Women and Lung Cancer: www.plwc.org). Smoking is the primary cause of this increase, causing 27,000 more female fatalities per year than breast cancer.1 Further, the Surgeon General's Women and Smoking report (2001) concluded that other serious health consequences directly related to smoking are greater in women, partly due to the detrimental effects of smoking on unborn children.1 One factor that may affect smoking cessation success is the menstrual cycle (MC) phase at the start of treatment. It has been proposed, but not systematically examined, that women who begin treatment in the luteal or premenstrual phase will do poorly in treatment compared with women who begin treatment in the follicular or preovulatory phase.
An estimated 70%–85% of women experience symptoms of premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) at some point during their reproductive years.2-4 Symptoms are most prominent after ovulation and may vary in severity throughout the premenstrual phase and the first few days of menstruation.2 PMS is characterized by both emotional and physical symptoms, such as anxiety, impulsivity, depression, irritability, bloating, and headaches, whereas PMDD comprises mainly emotional symptoms that are similar to but more severe than those of PMS.3,4
Although the data are sparse and inconsistent, the effects of MC phase on female smoking behavior have been examined previously. It has been shown that women in the luteal phase of their cycle smoke more5-7 and experience higher craving for cigarettes.8 We observed that premenstrual women report more intense craving after exposure to smoking-related stimuli (e.g., videos) than women in their preovulatory phase.9 Some studies have reported that women smoke more cigarettes per day during the luteal phase of the cycle,6,7 although not all studies report this.10,11 Further, abstaining from smoking during the premenstrual phase appears to increase the discomfort associated with quitting compared with abstaining in other phases.5,8,12-16 As many premenstrual symptoms are symptoms that also characterize cigarette/nicotine withdrawal,17 however, attributing symptoms to one syndrome or the other in past studies has proved unproductive.
Findings demonstrate that several aspects of addictive behavior and its underlying physiology differ between men and women.18-21 The MC, which is controlled by hormonal fluctuations, importantly affects female behavior and may lie at the core of these differences. The present study is based on the hypothesis that women who begin smoking cessation treatment during the luteal phase will have greater difficulty achieving or maintaining abstinence than women beginning in the follicular phase. This study is fundamental to determine whether or not MC phase is a potential determinant of relapse vulnerability. Thus, we grouped subjects participating in a nicotine replacement therapy (NRT) study by sex and then in women by MC phase. End points used were smoking status on day 3 of treatment and at 1 week after treatment.
MATERIALS AND METHODS
Subjects
For this study, 102 smokers were selected from 208 subjects participating in an NRT plus behavioral treatment smoking cessation study at The University of Pennsylvania Treatment Research Center. All subjects received the same NRT regimen and differing levels of behavioral intervention. Clinical findings on the effectiveness of the behavioral treatment have been reported elsewhere.22,23 Subjects were recruited through a variety of sources, including university campus notices, community posters, local newspaper advertisements, and word of mouth.
Exclusion criteria for NRT study
Primary exclusion criteria for the NRT study were as follows: medical or other conditions contraindicating transdermal nicotine use, psychosis or other current severe mental disorders, and cognitive dysfunction and nonnicotine drug or alcohol abuse during the previous 6 months. Pregnancy status was confirmed by human chorionic gonadotropin-β subunit (hCG-β subunit) blood testing. Psychiatric health and drug dependence were assessed using the Structured Clinical Interview for DSM IV and the Addiction Severity Index Interview.24,25 Written consent was obtained from each participant prior to initiation of study procedures. The Institutional Review Board of the University of Pennsylvania approved the study.
Study-specific exclusion criteria
To effectively examine the effects of MC phase on smoking status, it was necessary to further restrict the eligibility requirements of the primary NRT study. Only subjects between the ages of 18 and 47 were included to minimize the possible confound introduced by perimenopausal women and to equate average age among the three groups. Further, women were excluded if they were taking hormonal preparations (including birth control pills), had an average cycle length outside the range of 26–30 days, or were currently experiencing MC difficulties. The additional criteria created three groups consisting of 65 men, 16 women who began treatment in the follicular phase (FFs), and 21 women beginning treatment in the luteal phase (LFs). The 102 subjects who met all study criteria averaged 35.6 years of age (SD = 7.8), smoked on average 26.5 (SD = 9.8) cigarettes per day, and smoked for an average of 18.0 years (SD = 7.8). Subjects were 72% white, 22% black, 4% Asian, 1% Hispanic, and 1% other. Subjects had an average of 15.1 years of education (SD = 3.1).
Data used to identify the two major phases of the MC were collected during the physical examination. Information obtained included self-reported MC length, regularity and characteristics of cycle, method of birth control, and the date of the first day of the last menses. Women were divided into two groups, FF and LF. The follicular phase (preovulatory) was defined as a 14-day period beginning on the first day of menses. The luteal phase (premenstrual) was defined as beginning on the 15th day after the 1st day of menses and ending on the 30th day.26
Assessments
Subjects' sociodemographic and smoking characteristics were obtained from a center-developed smoking history questionnaire. Self-reported smoking status at day 3 (i.e., 3 days after the quit date and prior to behavioral treatment) and at week 9 (i.e., 1 week posttreatment) were used as end points. Because subjects were being administered transdermal nicotine replacement, urine cotinine levels were not used as an indicator of smoking status at day 3. Cotinines were used to verify self-reported smoking status at week 9 after the nicotine absorbed through the patch was eliminated from the body. Cotinines were measured by gas chromatography/mass spectrometry (GC/MS).
RESULTS
One-way ANOVAS did not show differences among FFs, LFs, and men in age, education, number of cigarettes smoked per day, number of years smoking, or nicotine dependence as measured by The Fagerström Tolerance/Dependence Questionnaire.27 Chi-square analyses showed no differences between groups in race (Table 1). As chi-square analysis indicated no significant effect of one of three levels of behavioral intervention (low, medium, or high) on point prevalence abstinence at week 9, behavioral treatment conditions were collapsed for all the remaining analyses. Missing data (resulting from missed appointments) were conservatively treated as nonabstinent.
Table 1.
Demographics of Study Subjectsa
| Number of subjects |
Men 65 |
FFs 16 |
LFs 21 |
|---|---|---|---|
| Age, years | 35.6 ± 7.8b | 33.8 ± 8.9 | 36.8 ± 6.6 |
| Education, years | 15.1 ± 3.3 | 14.3 ± 2.6 | 15.4 ± 2.9 |
| Years smoking | 18.1 ± 7.8 | 16.2 ± 9.2 | 19.1 ± 6.8 |
| Cigarettes per day | 27.5 ± 10.5 | 24.6 ± 8.1 | 24.6 ± 8.6 |
| Dependence (FTND)c | 6.6 ± 1.6 | 6.5 ± 1.5 | 7.2 ± 1.3 |
| Race | |||
| White | 76.9% | 68.7% | 61.9% |
| Black | 15.4% | 25.0% | 38.1% |
| Other | 7.7% | 6.4% | 0.0% |
No significant differences were found between groups.
Values are average ± SD.
FTND, Fagerström Test for Nicotine Dependence.
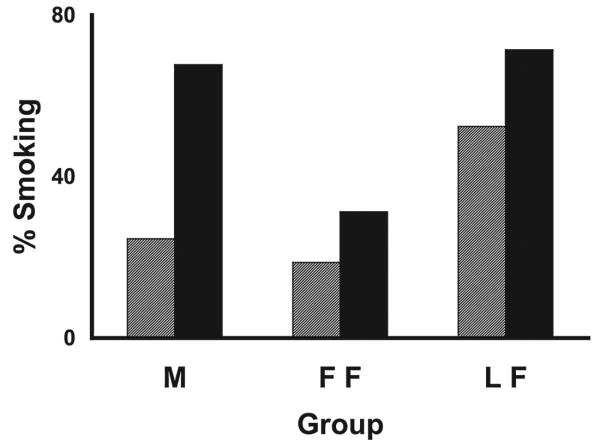
Self-reported smoking status was not different between men (n = 65) and women (n = 37) at day 3 or week 9. However, significant differences in smoking status were observed at both time points and over the course of treatment in women beginning treatment in the luteal phase (LF, n = 21) vs. those beginning in the follicular phase (FF, n = 16) (Fig. 1).
FIG. 1.
Smoking status at day 3 and week 9. At day 3 (hatched bars), 19% of follicular women (FF) and 52% of luteal women (LF) were smoking (p < 0.04). At week 9 (black bars), 31% of FF and 71% of LF were smoking (p < 0.02). Males subjects, included as a comparison group, were smoking 25% and 68% at day 3 and week 9, respectively.
At day 3, 52% of LFs reported smoking compared with 19% of FFs (p = 0.04, OR 4.77, 95% CI 1.04, 21.79) and compared with 25% of men (p = 0.02, OR 3.37, 95% CI 1.21, 9.39). There was no significant difference between FFs and men (p = 0.62, OR 1.42, 95% CI 0.36, 5.60). Generally, LFs were unable to quit or lapsed rapidly, whereas FFs and men were abstinent at day 3.
At week 9, the proportion of LFs who continued to smoke was significantly higher than that of FFs (71% vs. 31%, p < 0.02, OR 5.50, 95% CI 1.33, 22.73). The proportion of LFs and men nonabstinent was not different (71% vs. 68%, p = 0.75, OR 1.19, 95% CI 0.41, 3.51). The proportion of men who were smoking compared with nonabstinent FFs was significant (p = 0.01, OR 4.61, 95% CI 1.42, 14.97). At week 9, LFs did not recover from their initial inability to abstain or remain abstinent, FFs remained abstinent, and the proportion of men who lapsed approached that of LFs.
DISCUSSION
As expected, the success rate of females in the follicular phase (FFs) of the MC was significantly higher than that of females in the luteal phase (LFs). This difference was observed at the first data collection point, day 3, after all subjects were administered identical NRT medication but prior to the initiation of one of three levels of behavioral intervention. Thus, these results are independent of behavioral treatment intensity. The higher success rate in FFs in comparison to LFs and men persisted 1 week after treatment. In other words, the initial propensity of LFs to lapse/relapse was sustained even as they progressed through other stages of their cycle throughout treatment.
How could MC phase at the start of treatment influence women participant's behavior throughout? As stated, LFs either did not quit or relapsed early in treatment and failed to gain or regain abstinence over the course of treatment. It is conceivable that the combined discomfort of premenstrual and withdrawal symptoms at the time of quitting conferred a dual vulnerability, making initial abstinence more difficult and causing greater negative emotional reactions to their inability to abstain. Thus, LFs' initial lapse early in treatment was perpetuated. The results presented here emphasize the potential importance of MC phase at initial quit attempt in guiding treatment outcome.
A second possibility for the high and early relapse rate in LFs may reflect differences between the groups in cue responsivity. In an earlier reoprt, FFs had less craving to smoking stimuli than LFs, whereas male cue reactivity was not different from that of LFs.9 As these are the first studies to examine MC phase effects on smoking behavior that included a male control group, additional studies are necessary to determine if the effect is related to cue reactivity itself or to the effect of MC phase on cue reactivity.
As this is a retrospective study, it has its limitations. In this study, women were not stratified by MC phase at quit date, which could cause a selection bias. Although a possible caveat, the decision to quit may have been made when women were in a different phase from the one occurring at the quit date, as the screening process leading up to quit date varied from 2 days to 2 weeks across women. Regardless, the possibility of selection bias encourages future study wherein women are stratified according to MC phase. Another limitation to this study is the small sample size. Half the women participating in the original NRT study did not meet criteria to study MC phase, as many were close to menopause, using birth control methods, or had irregular menses. Future larger studies are necessary before firm conclusions on the role of MC phase in smoking cessation can be drawn. A third possible limitation is the slightly higher dependence scores and years smoking in the LFs compared with the FFs. However, as there were no significant differences in years smoking, cigarettes per day, or dependence (as measured by the FTND) between the groups, it is unlikely that these differences affected the results of this study.
MC phase was determined from the subject's self-report of the first day of the last menses. Physiological measurements of phase determination, considered by some as the paradigmatic tests of reliability, were not obtained in the original study. However, the only published study directly comparing several physiological measures with self-report found that none of them, including the gold standard of phase determination, anatomical definition by transvaginal ultrasound, were superior to self-report: “In all subjects, ultrasound confirmed expected cycle phase as predicted by self-report. …”12(p 235)
These findings should be taken in the context of the significant health problem of smoking and its effects on women. The reality is that the number of adolescent and adult females who smoke is increasing. Women smokers face increased smoking-related deleterious health consequences compared with men, including increased risk of lung cancer. These factors emphasize the importance of identifying relapse predictors and revealing their underlying mechanisms. Understanding the role of MC phase in psychological and physiological nicotine withdrawal will aid in the development of effective smoking cessation strategies. The preliminary findings reported here warrant the attention of researchers and clinicians alike. Researchers may consider collecting and analyzing prospective data on premenstrual severity and self-reported and physiological determinants of phase. Clinicians may consider arranging quit dates to coincide with the follicular phase, as this approach is without risk and may increase women's chances of successfully quitting smoking.
ACKNOWLEDGMENTS
We thank Peter Gariti, Ph.D., who conducted the initial NRT trial and provided access to the data.
This work was supported by NIDA grants 5T32-DA-07241 and RO1 10241.
REFERENCES
- 1.Women and smoking: A report of the Surgeon General. United States Department of Health and Human Services; Washington, DC: 2001. Available at www.cdc.gov/tobacco/data_statistics/sgr/sgr_2001/index.htm#full. [Google Scholar]
- 2.Van Keep PA, Lehert P. The premenstrual syndrome: An epidemiological and statistical exercise. In: Van Keep PA, Utian WH, editors. The premenstrual syndrome. MIT Press; Lancaster, England: 1981. p. 31. [Google Scholar]
- 3.Endicott J, Amsterdam J, Eriksson E, et al. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- 4.Ling FW. Recognizing and treating premenstrual dysphoric disorder in the obstetric, gynecologic, and primary care practices. J Clin Psych. 2000;61(Suppl 12):9. [PubMed] [Google Scholar]
- 5.Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- 6.Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: Results from single smoking sessions and the natural environment. Psychoneuroendocrinology. 2000;25:677. doi: 10.1016/s0306-4530(00)00018-4. [DOI] [PubMed] [Google Scholar]
- 7.DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav. 1995;20:335. doi: 10.1016/0306-4603(94)00070-f. [DOI] [PubMed] [Google Scholar]
- 8.Pomerleau CS, Garcia AW, Pomerleau OF, Cameron OG. The effects of menstrual cycle phase and nicotine abstinence on nicotine intake and on biochemical and subjective measures in women smokers: A preliminary report. Psychoneuroendocrinology. 1992;17:627. doi: 10.1016/0306-4530(92)90021-x. [DOI] [PubMed] [Google Scholar]
- 9.Franklin TR, Napier K, Ehrman R, Gariti P, O'Brien CP, Childress AR. Retrospective study: Influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tobacco Res. 2004;6:171. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- 10.Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. J Subst Abuse. 1996;8:303. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- 11.Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. J Subst Abuse. 1994;6:227. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 12.Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tobacco Res. 2000;2:231. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- 13.O'Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav. 1989;14:595. doi: 10.1016/0306-4603(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 14.Perkins KA, Levine M, Marcus M, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol. 2000;68:176. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- 15.Craig D, Parrott A, Coomber JA. Smoking cessation in women: Effects of the menstrual cycle. Int J Addict. 1992;27:697. doi: 10.3109/10826089209068761. [DOI] [PubMed] [Google Scholar]
- 16.Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and premenstrual symptomatology during the menstrual cycle in short-term smoking abstinence: Effects of menstrual cycle on smoking abstinence. Nicotine Tobacco Res. 1999;1:129. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- 17.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 18.Zickler P. Gender differences in prevalence of drug abuse traced to opportunities to use, in National Institute for Drub Abuse (NIDA) Notes. 2000;15:4. [Google Scholar]
- 19.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacology. 1999;7:274. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 20.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self administration: Review of human and animal evidence. Nicotine Tobacco Res. 1999;1:301. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 21.Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 22.Gariti P, Alterman AI, Barber W, Bedi N, Luck G, Cnaan A. Cotinine replacement levels for a 21 mg/day transdermal nicotine patch in an outpatient treatment setting. Drug Alcohol Depend. 1999;54:111. doi: 10.1016/s0376-8716(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 23.Gariti P, Alterman AI, Mulvaney FD, Epperson L. The relationship between psychopathology and smoking cessation treatment response. Drug Alcohol Depend. 2000;60:267. doi: 10.1016/s0376-8716(00)00114-9. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. APA; Washington, DC: 1994. [Google Scholar]
- 25.McLellan A, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Mental Dis. 1980;168:26. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Jensvold MF, Hamilton JA, Halbreich U. Future research directions: Methodological considerations for advancing gender-sensitive pharmacology. In: Jensvold MF, editor. Psychopharmacology and women: Sex, gender, and hormones. American Psychiatric Press; Washington, DC: 1996. p. 415. [Google Scholar]
- 27.Heatherton TF, Kozlowski LT, Frecker RC, Fager-strom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]