Abstract
The surge in dopamine in ventral striatal regions in response to drugs of abuse and drug-associated stimuli is a final common pathway of addiction processes. GABA B agonists exert their effects indirectly, by quieting dopaminergic afferents. The ability of the GABA B agonist, baclofen to ameliorate nicotine and drug motivated behavior is established within the animal literature, however its potential to do so in humans is understudied, particularly with respect to its possible utility as a smoking cessation agent.
We conducted a nine-week double-blind placebo-controlled pilot trial of baclofen for smoking reduction (N=30/group) in smokers contemplating, but not quite ready to quit. Baclofen was titrated upwards to 20 mg q.i.d. over a period of twelve days. The primary outcome measure was the number of cigarettes smoked per day (CPD).
A significant group by time effect of medication was observed. Baclofen was superior to placebo in reducing CPD (β=0.01, t=1.97, p<0.05). The most common side effect reported during baclofen treatment is transient drowsiness, however there were no differences between groups in mild, moderate, or severe sedation. Craving was significantly lowered at end of treatment in all smokers (p<0.02). Retention did not differ between groups.
In line with a multitude of preclinical studies examining the effects of baclofen on drug-motivated behavior, baclofen reduced CPD. In agreement with other studies examining craving and drug use, reductions in CPD were accompanied by a reduction in craving, a major motivator underlying continued smoking and relapse. These preliminary results demonstrate provisional evidence of the utility of baclofen to aid in smoking cessation and indicate further investigation.
Keywords: tobacco, treatment, pharmacotherapy, baclofen
1. Introduction
Cigarette dependence is the most common form of chemical dependence in the nation and is as addictive as heroin, cocaine, or alcohol (American Psychiatric Association: DSM IV, 1994). The list of debilitating and/or fatal diseases that are worsened by or attributed to cigarette smoking continues to grow (Foundation for a smoke-free America, 2000; Cancer Facts and Figures, 2004). Quitting greatly reduces the risk of premature death related to lung and other types of cancer, coronary heart disease, stroke and numerous other illnesses (The Health Benefits of Smoking Cessation. A Report of the Surgeon General, 1990). However, quitting is not easily accomplished - 70% of smokers want to quit each year (American Cancer Society, 2004) but less than 7% succeed (Baillie et al., 1995; Hughes et al., 2003).
Based on consistent preclinical studies over the last 30 years it is believed that the dopamine surge in the ventral striatum in response to addictive drugs and stimuli (cues) previously and repeatedly ‘paired’ with the drug leads to drug-motivated behaviors (Cardinal et al., 2002; Di Chiara, 1995; Koob, 1992). Similar findings are now being replicated in humans. At least two studies reported that cocaine cues elicit craving and increased dopamine release in the striatum of cocaine addicted individuals (Volkow et al., 2006; Wong et al., 2006). Boileau and colleagues showed that three administrations of amphetamine within the same environment produced conditioned dopamine release in the ventral striatum accompanied by amphetamine-like behavioral responses (Boileau et al., 2007). Further support for the role of dopamine is provided by Franklin and colleagues wherein both brain and behavioral responses to smoking cues were modulated by genetic variance in the dopamine transporter (Franklin et al., 2008 ) a key component regulating the dopamine system (Jaber et al., 1997).
It is theorized that GABA-ergic agents reduce dopamine availability in the ventral striatum (Ashby et al., 1999; Dewey et al., 1998; Gerasimov et al., 2001), thereby preventing the self-administration of psychoactive drugs (Dewey et al., 1999; Roberts and Brebner, 2000). These effects are reversed by GABA B antagonists demonstrating specificity (Xi and Stein, 1998; Xi and Stein, 1999). The mechanisms are still under investigation but one hypothesis is that activation of GABA B receptors located somato-dendritically on ventral tegmental area (VTA) neurons inhibit the release of dopamine in interconnected regions such as the ventral striatum and prefrontal cortex (Koob, 1992; Mueller and Brodie, 1989; Xi and Stein, 1998).
It is well established in the animal literature that the GABA B agonist, baclofen is effective at reducing dopamine release and in reducing drug-seeking motivated behavior at doses that do not affect responding for food, water, or locomotor activity (Corrigall et al., 2000; Fattore et al., 2002; Markou et al., 2004; Paterson et al., 2004; Roberts and Andrews, 1997; Shoaib et al., 1998; Spano et al., 2007; Xi and Stein, 1999). Most recently, Fattore and colleagues reported that baclofen dose-dependently blocked nicotine-induced reinstatement of self-administration in rats and dose-dependently abolished nicotine conditioned place preference in mice (Fattore et al., In Press)
Although studied much less in clinical settings, the results are consistent with the animal literature showing that baclofen treatment reduces motivation to use addictive drugs. Baclofen reduced drug use and craving in cocaine (Ling et al., 1998; Shoptaw et al., 2003), amphetamine (Heinzerling et al., 2006), and alcohol (Addolorato et al., 2000; Addolorato et al., 2002; Colombo et al., 2004; Johnson et al., 2005) dependent individuals [Review, (Malcolm, 2003)]. In a study examining the effects of baclofen on opiate dependent subjects, baclofen significantly increased treatment retention, decreased withdrawal symptoms, and showed a trend in reducing craving (Assadi et al., 2003). Three case studies in alcoholics report complete remission as indexed by the absence of craving and alcohol use (Agabio et al., 2007; Ameisen, 2005; Bucknam, 2007). A laboratory study conducted by Cousins and colleagues examined the effects of a single dose of baclofen on subjective effects of smoking in non-treatment seeking smokers. Baclofen negatively impacted cigarette enjoyment and increased feelings of relaxation, both of which may facilitate eventual abstinence and aid in relapse prevention (Cousins et al., 2001). To our knowledge, there are no published studies examining the effects of baclofen treatment (chronic dosing) on smoking behavior.
Baclofen is an FDA-approved GABA B agonist used for the treatment of spasticity since the early seventies (Basmajian, 1975; From and Heltberg, 1975; Taricco et al., 2000). It does not carry a significant abuse liability (Addolorato et al., 2000), it is self administered only minimally by rhesus monkeys or baboons (Griffiths et al., 1991; Negus et al., 2000), and other than initial mild sedation, has few side effects (Physicians Desk Reference, 1993). Its safety and tolerability has been confirmed in numerous studies over the years, including studies in alcoholics and cocaine addicts (Addolorato et al., 2002; Aisen et al., 1992; Johnson et al., 2005; Stallings and Schrader, 2007; Taricco et al., 2000).
Based on the pre-clinical and clinical evidence summarized above, we hypothesized that baclofen might reduce the number of cigarettes smoked per day (CPD) in smokers contemplating quitting smoking.
2. Materials and Methods
2.1. Subjects
The study was conducted at the Addiction Treatment Research Center, a University of Pennsylvania School of Medicine-affiliated outpatient treatment center. It was approved and monitored by the University Of Pennsylvania School of Medicine Institutional Review Board, and adhered to the Declaration of Helsinki. Smokers were compensated $6.00 for each appointment, $5.00 for each returned medication card, and $30.00 for study completion. Subjects were recruited by word of mouth and from a radio advertisement that specifically stated the study was for smokers who were interested in quitting but not quite ready. Smokers were encouraged to set a Quit Date after the target dose was attained and maintained for 9 additional days (at three weeks) however, setting a Quit Date was not required to participate in the study.
Subjects were screened, tested on study knowledge, and consented prior to psychological and physical evaluations. The Minnesota International Neuropsychiatric Interview (MINI) was used to determine current DSM-IV diagnosis of psychoactive substance dependence other than nicotine and to diagnose current severe psychiatric symptoms. Individuals with other current psychoactive substance dependence or current DSM-IV psychiatric diagnoses were excluded. Severity of nicotine dependence was determined from a laboratory-developed Smoking History Questionnaire that included the 6 items assessed by the Fagerstrom Test for Nicotine Dependence [FTND (Fagerstrom and Schneider, 1989)].
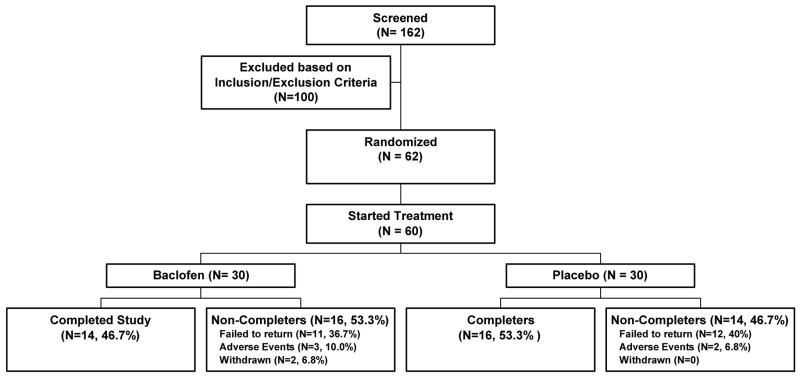
During screening it was ascertained whether smokers were ready to make a quit attempt. Those who were ready to quit were referred to one of two other ongoing smoking cessation trials at the University of Pennsylvania. The sample consisted of 30 subjects per group stratified by sex, contemplating but not quite ready to quit. Subjects were physically healthy and mentally stable smokers between the ages of 18 and 60 who met DSM-IV criteria for nicotine dependence [FTND Mean and SEM: 5.66 (±0.3), scores ranged from 1.5 (minimal dependence) to 9.5 (high dependence)]. Subjects smoked 10–40 cigarettes per day. Characteristics for randomized subjects and for study completers are listed in Table 1. Participant disposition is shown in Figure 1.
Table 1.
General baseline characteristics of all randomized subjects and treatment completers.
| Randomized | COMPLETERS | |||||||
|---|---|---|---|---|---|---|---|---|
| Number (%) | Number (%) | |||||||
| All N = 60 | Bac N= 30 | Plac N = 30 | p | All N=30 | Bac N=14 | Plac N=16 | p | |
| Sex | 32 M (53.3%) | 16 M (53.3%) | 16 M (53.3%) | 0.72 | 16 M (53.3%) | 7 M (50%) | 9 M (56.3%) | 0.62 |
| Race | 28 AA (46.7%) | 17 AA (57.7%) | 11 AA (36.7%) | 0.26 | 17 AA (56.7%) | 10AA (71.4%) | 7 AA (43.8%) | 0.47 |
| 28 EA (46.7%) | 11 EA (36.7%) | 17 EA (57.7%) | 0.26 | 10 EA (33.3%) | 3 EA (21.4%) | 7 EA (43.8%) | 0.21 | |
| 4 Multi (~6%) | 2 Multi (~6%) | 2 Multi (~6%) | 1.0 | 3 Multi (10%) | 1 Multi (7.2%) | 2 Multi(12.4%) | 0.56 | |
| MEANS ± (SEMs) | MEANS ± (SEMs) | |||||||
| Age | 40.6 ± 1.5 | 39.9 ± 2.2 | 41.3 ± 2.0 | 0.63 | 40.7 ± 2.0 | 40.5 ± 3.0 | 40.9 ± 2.7 | 0.92 |
| Education | 14.1 ± 0.3 | 13.6 ± 0.4 | 14.7 ± 0.7 | 0.06 | 14.2 ± 0.4 | 13.2 ± 0.5 | 15.1 ± 0.7 | 0.03* |
| CPD (study start) | 20.7 ± 1.2 | 20.5 ± 2.1 | 20.8 ± 1.4 | 0.90 | 20.8 ± 1.9 | 20.0 ± 1.6 | 21.8 ±1.9 | 0.41 |
| Pack Yearsa | 24.9 ± 2.8 | 25.7 ± 4.6 | 24.0 ± 3.0 | 0.76 | 21.5 ± 3.2 | 20.2 ± 5.4 | 22.8 ± 3.8 | 0.70 |
| FTND Scores | 5.66 ± 0.3 | 5.64 ± 0.4 | 5.68 ± 0.3 | 0.91 | 5.4 ± 1.7 | 5.60 ± 1.7 | 5.2 ± 1.2 | 0.95 |
Abbreviations: Bac, baclofen; Plac, placebo; AA, African American, EA, European American; Multi, reported more than one race.
Pack years calculation: CPD (÷) cigarettes in a pack (X) years smoking. FTND scores ranged from 1.5 – 9.5. T-tests demonstrated no differences between groups in any characteristics other than education, which is greater in the Plac compared to Bac completers
p < 0.05.
Figure 1.
Subject Disposition
2.2. Baclofen/placebo treatment regimen
Study medication was manufactured and donated by Murty Pharmaceuticals Inc., Lexington, Kentucky. Study medication was prepared and maintained by the Institutional Drug Service (IDS) located at the Hospital of the University of Pennsylvania in capsules containing 10 mg baclofen, or matching placebo that consisted of a dextrose matrix. The study physician dispensed the medication.
Approximately 15% of individuals who take baclofen experience drowsiness (Physicians Desk Reference, 1993). To minimize this side effect the dose was titrated upwards over a twelve-day period (see Table 2 for Induction Schedule) to a final dose of 20 mg baclofen four times a day (80 mg total). This is the recommended dose used to treat spasticity provided by the Physician’s Desk Reference (1993) but is a minimum of 20 mg higher than that utilized in other studies examining the effects of baclofen on drug craving and relapse. Side effects, adherence to the dosing schedule, and cigarette smoking behaviors were monitored by the subjects using a ‘Daily Diary’, by study staff during biweekly telephone calls, and by the study physician at weekly medication monitoring appointments. Medication cards were collected at weekly appointments to monitor compliance. Subjects received medication for a total of 9 weeks. During the 9th week, the dose was reduced by half every other day over the course of seven days, tapering to discontinuation. No medication was administered during the 10th week (follow up). Subjects met with study staff for a follow up visit and final evaluation at Week 11. Tapering is necessary as baclofen should not be stopped abruptly after chronic use (the body adjusts to the muscle relaxant effects, and abrupt cessation can result in muscle spasms, or, rarely, seizures in those with a history of seizures) (Physician’s Desk Reference, 1993). Study completion was defined as the date on which data was collected at full dose baclofen (at Week 9).
Table 2.
Induction Schedule.
| Day | 8 AM | 12PM | 4 PM | 8 PM | Total |
|---|---|---|---|---|---|
| 1 – 2 | P | 10 | P | 10 | 20 |
| 3 – 4 | P | 10 | 10 | 10 | 30 |
| 5 – 7 | 10 | 10 | 10 | 10 | 40 |
| 8 – 9 | 10 | 20 | 10 | 20 | 60 |
| 10 – 11 | 10 | 20 | 20 | 20 | 70 |
| 12 – 51 | 20 | 20 | 20 | 20 | 80 |
| 52 | 10 | 20 | 20 | 20 | 70 |
| 53 | 10 | 20 | 10 | 20 | 60 |
| 54 | 10 | 10 | 10 | 10 | 40 |
| 55 | P | 10 | 10 | 10 | 30 |
| 56 | P | 10 | P | 10 | 20 |
| 57 | P | P | P | 10 | 10 |
| 58 | P | P | P | P | P |
| 59–65 | Follow Up | ||||
Abbreviations: P = placebo, 10 = 10 mg baclofen, 20 = 20 mg baclofen
2.3. Daily Diary
A Daily Diary was used to measure cigarette consumption throughout treatment. Smokers were instructed to fill out the diary at the same time each day, surrounding a daily activity such as breakfast, lunch, or dinner. On each day, they were instructed to record the date, time, whether they had taken their medication, the time each pill was taken, whether they were still smoking and how many CPD they had smoked over the last 24 hours.
2.3. Adverse Event Monitoring
Baclofen has been used safely as an anti-spastic for many years (From and Heltberg, 1975), but it can be associated with some sedation or drowsiness, particularly in the early dosing. Its effects are often transient and can be alleviated or eliminated by decreasing the dosage. The most common adverse reactions associated with baclofen in addition to drowsiness are daytime sedation, dizziness, weakness, and fatigue. Less common side effects include skin rash and itching, shortness of breath, problems in urinating, constipation or diarrhea, dizziness, headache, nausea, irregular heartbeat or chest pain (Physician’s Desk Reference, 1993; RxList The internet Drug Index, Kemstro, Side Effects and Drug Interactions, 2008). To monitor adverse events subjects met with the study physician at weekly appointments to discuss medication issues and side effects. The physician-administered Side Effects Inventory lists 11 adverse events specifically associated with the use of baclofen and a line for ‘other’. Subjects were asked to rate the severity of each of their symptoms as mild, moderate or severe.
2.4 Statistical Analyses
Intention-to-treat analysis was performed. The main outcome measure was the number of cigarettes per day (CPD) acquired at study start and at weekly appointments. As there was a great deal of variability in CPD across subjects at baseline (ranging from 10–40), CPD was analyzed using a full generalized estimating equation (GEE) model of the log odds of CPD [sqrt(1+cpd)] with Group × Quadratic Time effects, which allows patterns of changes in CPD to be observed in different groups. The secondary outcome measure was an assessment of craving as measured by a visual analog scale (VAS). The VAS has been used to measure craving ‘at the moment’ and craving occurring over longer periods of time. As the time since subjects smoked their last cigarette was unknown, and smoking alleviates craving for twenty minutes or longer, craving was assessed over the last 24 hours. At study start (baseline) and at Week 9, before initiating the medication taper, smokers were asked to rate their craving for a cigarette over the last 24 hours by making a vertical mark on a 100mm visual analog scale (VAS) with 0 corresponding to No Craving and 100 corresponding to Intense Craving. Craving, and other continuous demographic variables were summarized by calculating means, standard error measurements and ranges. Analysis of variance was used to assess differences across time or groups. Nominal demographic variables were summarized by calculating proportions and compared across groups using chi square analyses. Statistical analyses were conducted in SPSS version 11.0.
3. Results
Treatment attrition was defined as failing to appear for the next scheduled weekly appointment. Survival analysis demonstrated that there were no differences in retention rate between baclofen and placebo groups (β2 = 0.70, df = 1, p = 0.40).
3.1 Demographics
There were no significant baseline differences in age, sex, race, CPD, pack years (a measure to quantify intensity of chronic cigarette exposure since smoking initiation: CPD divided by number of cigarettes in a pack multiplied by the number of years smoking), FTND scores or other general characteristics in study participants. There were no differences in the above characteristics in study completers with the exception of education (See Table 1).
3.2. Primary Outcome Measure, CPD
At study start baclofen-treated subjects smoked 20.5 ± 2.1 CPD versus 8.1 ± 1.2 at Week 9. Placebo-treated subjects smoked 20.8 ± 1.4 CPD at study start compared to 11.7± 2.7 at Week 9. Figure 1 illustrates a significant medication by time effect (β= 0.01, t= 1.97, p < 0.05). Note that there is a steeper descent in CPD in the baclofen group followed by a flattening out, which occurs at approximately three weeks into the study when the full dose had been maintained for over one week. Table 3 provides the coefficients, standard errors, t scores and p values from the GEE model rounded to 2 decimal places. Smokers were tapered off medication during the 10th week. Week 11 was follow-up.
Table 3.
Coefficients, standard errors, t statistics and p values from the GEE model analysis on CPD
| Model term | Coefficient (SE) | t | p |
|---|---|---|---|
| Intercept | 4.70 (0.22) | 21.08 | <.0001 |
| Baclofen | −0.04 (0.31) | −0.12 | 0.90 |
| Week | −0.17 (0.06) | −2.88 | 0.006 |
| Baclofen × Week | −0.17 (0.08) | −2.02 | 0.04 |
| Week × Week | 0.005 (.004) | 1.03 | 0.31 |
| Baclofen × Week × Week | 0.01 (0.01) | 1.97 | 0.05 |
3.3 Craving
Paired t-tests of the change in craving scores measured by the VAS within groups administered at baseline and at Week 9 showed that craving for cigarettes was significantly reduced in both treatment conditions (data not shown). Means and SEMS: baclofen (baseline, 52.5. ± 8.9 versus Week 9, 30.3 ± 7.0, p < 0.007); and placebo (baseline, 53.1 ± 6.9 versus Week 9, 34.0 ± 6.0, p < 0.008). Craving scores at baseline and at Week 9 between baclofen and placebo groups were not different (p < 0.34 and 0.48 respectively).
3.4. Side Effects
A 2-tailed t-test demonstrated a lack of differences between groups in side effects possibly study-related (p < 0.07), unlikely study-related (p < 0.3), or not study-related (p < 0.09). As sedation or drowsiness is the side effect most often reported, sedation was analyzed separately. There were no differences in sedation possibly study-related (p < 0.10), unlikely related (p < 0.17), or not related (none were reported in either group). In the baclofen group there were 20 mild, 4 moderate and 0 severe reports of sedation. In the placebo group there were 4 reports of mild sedation and 0 reports of either moderate or severe sedation.
4. Discussion
The primary objective of this study was to systematically assess the ability of baclofen to reduce cigarette consumption in smokers, prior to conducting further clinical trials. Here, we report that baclofen, administered at higher doses than those used in prior studies examining its anti-craving or anti-relapse potential, significantly reduced the number of cigarettes smoked per day (CPD) in a smoking reduction trial. This is consistent with the human literature examining the effects of baclofen on drug-motivated behavior. Chronic administration of baclofen has shown potential for reducing drug-motivated behaviors including craving and relapse in opiate (Assadi et al., 2003), cocaine (Gudeman et al., 1997; Ling et al., 1998), and alcohol (Addolorato et al., 2000; Addolorato et al., 2007; Agabio et al., 2007; Ameisen, 2005; Colombo et al., 2004; Johnson et al., 2005) addicted individuals. One human laboratory study examining the effects of baclofen on smoking behavior has been published. In this study, an acute dose (20 mg) of baclofen dose changed the taste of the cigarette, making it less palatable (Cousins et al., 2001). The present report supports previous studies and suggests that this readily available, inexpensive and easily tolerated medication might be a valuable smoking cessation aid. As at least one other trial of baclofen for smoking cessation (Tennegi, Squassante, Milleri, and Bye, personal communication, 2004) using 30 mg/day showed no effects on smoking, craving or withdrawal symptoms, our data suggest that higher doses may be more beneficial for smoking cessation. In agreement with Aisen and colleagues who confirmed the safety and efficacy of high dose baclofen (Aisen et al., 1992), there were no differences in side effects between groups.
The results reported here are also consistent with the animal literature. At least 16 preclinical studies have examined the effects of baclofen on drug-seeking motivated behavior and dopamine release, several of which were nicotine studies (Corrigall et al., 2000; Fadda et al., 2003; Fattore et al., 2002; Markou et al., 2004; Paterson et al., 2004; Xi and Stein, 1998). Most recently it was shown that nicotine-induced reinstatement was dose-dependently attenuated by baclofen (Fattore et al., In Press). Replicating other preclinical studies, Fattore and colleagues demonstrated that baclofen reduced nicotine-reinforced behavior at doses that do not affect responding for food, water, or locomotor activity, suggesting that it is not sedating and does not disturb normal activities (Roberts and Andrews, 1997; Roberts et al., 1996; Spano et al., 2007).
Despite the small sample size, most of the demographic variables are equivalent between groups. At study start the composition of smokers was 50% European American, which is lower than that found in other smoking cessation trials. This is most likely a function of the location of the Addiction Treatment Research Center where the study was conducted. We are located in West Philadelphia, which is predominantly composed of an African American population. Interestingly, there was higher attrition in European Americans compared to African Americans. This may also be a function of location as more European Americans had to travel to the center compared to African Americans who lived within walking distance.
Both baclofen- and placebo- treated groups reported a reduction in craving for cigarettes without significant differences between groups. As baclofen demonstrates anti-craving properties in alcohol and other studies it might be expected that baclofen-treated smokers would crave less. However, we, and others have shown that self-reported craving is an unreliable predictor of behavior (Robbins et al., 1997; Tiffany and Carter, 1998) and evidence suggests genetic variability in the ability to correctly identify craving may underlie this apparent contradiction. We recently demonstrated that genetic variance in the dopamine transporter (DAT) SLC6A3 gene has a strong influence on subjective craving responses and brain activity in reward-related brain circuitry (ventral striatum/amygdala/orbitofrontal cortex). Craving in response to smoking cue exposure in individuals carrying a 9-variable number of tandem repeats (VNTRs) polymorphism of the DAT gene did not correlate with brain activity while robust correlations were observed in smokers homozygous for the 10-VNTR (Franklin et al., 2009). Although untested thus far, we suggest that carriers of a 9-VNTR are unable to correctly label craving. This genetic variability may underlie the inconsistencies in the literature regarding medication effectiveness and its ability to reduce craving. For example, clinical trials of bupropion, a front-line smoking cessation agent, are mixed with regards to its ability to reduce craving (Durcan et al., 2002; Shiffman et al., 2000; Review, Mooney and Sofuoglu, 2006). Alternatively, the absence of differences between groups could be related to a placebo effect. It has been shown in randomized placebo controlled smoking cessation trials that craving is significantly reduced in smokers receiving placebo as well as those receiving medication. For example, craving was significantly reduced in placebo-, bupropion- and varenicline-treated smokers while abstinence rates were approximately twice as high as placebo in bupropion-treated smokers and 3 times as high in varenicline-treated smokers (Gonzales et al., 2006; Jorenby et al., 2006; West et al., 2008).
The question may arise as to why we chose to conduct a smoking reduction study rather than a cessation study, even though complete abstinence is preferred. First, although smokers who seek treatment are initially strongly motivated to quit smoking, their motivation is later compromised by the desire to smoke. Attrition is high in smoking studies, especially as the Quit Date approaches. Thus the original sample is biased by the loss of this subgroup who may be a harder-to-treat group with higher dependence, have a higher probability of smoking-related illnesses, and have more anxiety related to quitting. In an attempt to minimize attrition, and maintain an unbiased study, patients were asked to set a tentative Quit Date. As the Quit Date became imminent, patients were allowed to re-evaluate their intentions and choose either to continue to try and quit or to minimize their use as much as possible.
Smoking reduction is gaining ground as a desirable treatment outcome for at least three reasons. First, some studies show that reducing smoking derives significant health benefits (Godtfredsen et al., 2005; Pisinger and Godtfredsen, 2007; Song et al., 2008). In particular, one study showed that patients who were able to reduce CPD reduced their respiratory and pulmonary symptoms compared to those who did not reduce (Jimenez-Ruiz et al., 2002). Further, it was shown that even a 1% reduction in smoking could prevent up to 1000 cancer-related deaths in the European Union (Groman et al., 1999). Second, a reduction in cigarette consumption may increase the probability of subsequent successful quit attempts as light smokers have a higher success rate than heavy smokers (Lennox and Taylor, 1994; Tonnesen et al., 1996; Zhu et al.). Third, smokers who are initially unwilling to quit may become motivated by their ability to reduce (Riggs et al., 2001).
The field is not in agreement as to whether cutting down CPD is a beneficial method of harm reduction. A meta-analysis pooled the results of thirteen controlled trials of smoking reduction, as assessed by reductions in CPD and/or reductions in biomarkers such as carbon monoxide (CO) and/or cotinine levels (Stead and Lancaster, 2007). Changes in these biomarkers did not always distinguish treatment groups. Although several of the studies acquired measures of health status, they varied considerably across studies and there was no objective way for these to be analyzed in the meta-analysis. The results suggest that there is insufficient evidence that smoking reduction provides any long-term benefit. Even so, in light of the studies showing that reduction can lead to increased confidence in the ability to quit, and that light smokers have greater success in quitting, it may be an effective intermediary to the eventual goal of abstinence.
It is reasonable that reductions in smoking were not always accompanied by reductions in CO or cotinine levels in several of the studies included in the Stead and Lancaster, (2007) meta-analysis. CO levels only provide information on recent smoking and can have limited value in a ‘reduction’ study. Cotinines measure the primary metabolite of nicotine and are unreliable markers of reductions because of the changes in smoking topography that may occur as smokers cut down. Smokers may decrease puff interval, increase puff volume, increase puff duration, and use vent blocking (change the way in which the filter is held) in an effort to compensate for nicotine reductions (Hammond et al., 2005; Hofer et al., 1991). Indeed, it takes reductions in the number of CPD that are greater than half before changes in cotinine levels can be realized in some smokers. Because of the unreliability of CO and cotinine biomarkers in smoking reduction, neither of these measures was collected in this study.
One limitation regarding the clinical utility of baclofen is that it must be taken every 4–6 hours. In this study smokers were compensated and monitored to increase compliance. In reality such rigorous medication management and supervision is inconceivable and it is likely adherence would be difficult. Encouragingly, at least three pharmaceutical companies have recently developed extended-release formulations of baclofen, which has shown efficacy in reducing spasticity in multiple sclerosis patients (its present indication). It is expected that this formulation will receive FDA-approval and become available in the near future for use in clinical smoking cessation trials.
A common criticism of the use of baclofen for the treatment of smoking cessation or other addictions is its reputation for possessing undesirable side effects. The most common adverse reaction during treatment with baclofen is transient drowsiness. In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen tablets compared to 36% of those in the placebo group (RxList The internet Drug Index, Kemstro, Side Effects and Drug Interactions, 2008). Here, we observed no differences in sedation or any other side effects, which we attribute to the slow induction period during which baclofen was titrated to full dose over a period of twelve days. The practice of slowly introducing a psychiatric medication for smoking cessation or other illness, is gaining recognition as it eases the occurrence and intensity of side effects (Jorenby et al., 2006).
Despite the abundant evidence supporting the potential utility of baclofen as an anti-relapse/anti-craving agent and the confirmed safety profile and tolerability of this FDA-approved medication (Addolorato et al., 2007; Aisen et al., 1992; Colombo et al., 2000; Griffiths et al., 1991; Johnson et al., 2005; Negus et al., 2000; Stallings and Schrader, 2007; Taricco et al., 2000; Physicians Desk Reference, 1993), this is the first published clinical treatment trial of its effects on smoking behavior. Reducing cigarette smoking rates is crucial to the health of our nation and may be hastened by exploiting existing medications such as baclofen that prove efficacious in smoking cessation treatment trials. Although these results are preliminary, they indicate the importance of further investigation into the potential utility of baclofen as a smoking cessation agent.
Figure 2.
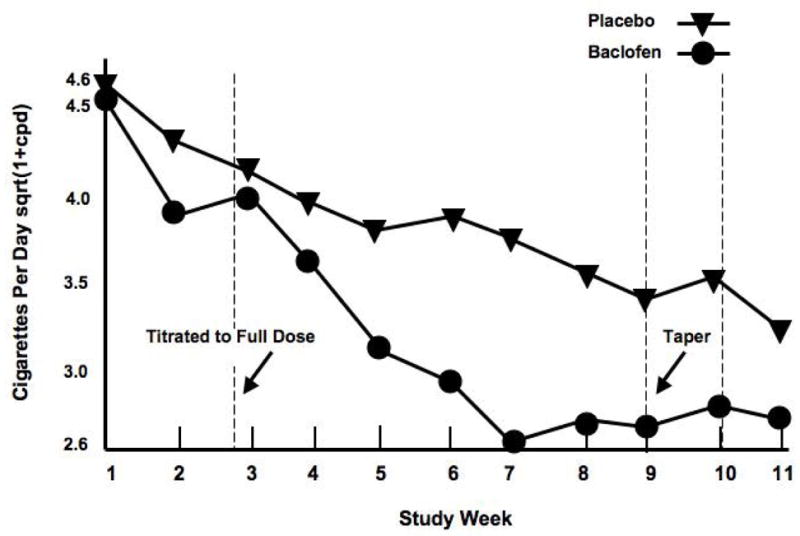
Graph depicts the sqrt(1+CPD) over the nine-week trial. Target dose of medication (20 mg q.i.d.) was achieved by Day 12. Smokers were tapered off of medication during the 9th Week. The 10th week was a follow-up visit. CPD were significantly reduced in baclofen- versus placebo-treated smokers (p < 0.05).
Acknowledgments
Funding Sources
Work supported by NIH grants DA015149, K01 DA 015426-011A1, 5-P60-DA-005186-18, and NS045839, BCS-0224007, RR02305, Alexander Foundation and the GCRC of the University of Pennsylvania School of Medicine. None of the sponsors had any role in the design, collection, analysis, or preparation of this manuscript.
The authors wish to acknowledge the nursing staff at the University of Pennsylvania Addiction Treatment Research for conducting physical evaluations and medication monitoring. We also would like to thank our clinicians Anita Hole Ph.D., Jesse Suh, Psy. D., and Marta MacDougal, Psy. D for conducting the psychological evaluations. Also, we wish to thank Moo Park, Ph.D., Chemist and Nora Chiang, Ph.D. Chief of Chemistry & Pharmaceutics Branch, both at NIH/NIDA/DPMC, and Murty Pharmaceuticals for providing baclofen and matching placebo tablets.
Footnotes
Role of Authors
Teresa R. Franklin: Primary Investigator, wrote protocol, designed experiments. Derek Harper: Study coordinator, participated in collection, analyses of data, manuscript preparation. Kyle Kampman: Study physician, participated in manuscript preparation. Susan Kildea: Participated in collection, analyses of data, manuscript preparation. Will Jens: Participated in collection, analyses of data, manuscript preparation. Kevin Lynch: Statistician, manuscript preparation. Charles P. O’Brien: Critique and participate in writing manuscript. Anna Rose Childress:Critique and participate in writing manuscript, aided in developing experimental design.
Conflict of Interest
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Agabio R, Marras P, Addolorato G, Carpiniello B, Gessa GL. Baclofen suppresses alcohol intake and craving for alcohol in a schizophrenic alcohol-dependent patient: a case report. J Clin Psychopharmacol. 2007;27:319–320. doi: 10.1097/01.jcp.0000270079.84758.fe. [DOI] [PubMed] [Google Scholar]
- Aisen ML, Dietz MA, Rossi P, Cedarbaum JM, Kutt H. Clinical and pharmacokinetic aspects of high dose oral baclofen therapy. J Am Paraplegia Soc. 1992;15:211–216. doi: 10.1080/01952307.1992.11761520. [DOI] [PubMed] [Google Scholar]
- Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40:147–150. doi: 10.1093/alcalc/agh130. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Ashby CR, Jr, Rohatgi R, Ngosuwan J, Borda T, Gerasimov MR, Morgan AE, Kushner S, Brodie JD, Dewey SL. Implication of the GABA(B) receptor in gamma vinyl-GABA’s inhibition of cocaine-induced increases in nucleus accumbens dopamine. Synapse. 1999;31:151–153. doi: 10.1002/(SICI)1098-2396(199902)31:2<151::AID-SYN8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Assadi SM, Radgoodarzi R, Ahmadi-Abhari SA. Baclofen for maintenance treatment of opioid dependence: a randomized double-blind placebo-controlled clinical trial [ISRCTN32121581] BMC Psychiatry. 2003;3:16. doi: 10.1186/1471-244X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baclofen. Physician’s Desk Reference. Montvale: Medical Economics Data; 1993. p. 1063. [Google Scholar]
- Baillie AJ, Mattick RP, Hall W. Quitting smoking: estimation by meta-analysis of the rate of unaided smoking cessation. Aust J Public Health. 1995;19:129–131. doi: 10.1111/j.1753-6405.1995.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Lioresal (baclofen) treatment of spasticity in multiple sclerosis. Am J Phys Med. 1975;54:175–177. [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007;42:158–160. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- Cancer Facts and Figures. American Cancer Society; Atlanta, GA: 2004. [Accessed on March 23, 2009]. http://www.cancer.org/docroot/PRO/content/PRO_1_1_Cancer_Statistics_2007_Presentation.asp. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–414. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I--Preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res. 2001;3:123–129. doi: 10.1080/14622200110042624. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alexoff DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Morgan AE, Ashby CR, Jr, Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–129. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, Rigotti N, Sachs DP. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin Ther. 2002;24:540–551. doi: 10.1016/s0149-2918(02)85130-x. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano SM, Cossu G, Scherma M, Fratta W. Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. European Neuropsychopharmacology. doi: 10.1016/j.euroneuro.2009.01.007. In Press. [DOI] [PubMed] [Google Scholar]
- Foundation for a smoke-free America, Anti-smoking links and resources. 2000 http://www.anti-smoking.org/quitting.htm.accessed.
- Franklin TR, Lohoff F, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrittini W, Detre J, O’Brien C, Childress A. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2008:1–12. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- From A, Heltberg A. A double-blind trial with baclofen (Lioresal) and diazepam in spasticity due to multiple sclerosis. Acta Neurol Scand. 1975;51:158–166. doi: 10.1111/j.1600-0404.1975.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Schiffer WK, Gardner EL, Marsteller DA, Lennon IC, Taylor SJ, Brodie JD, Ashby CR, Jr, Dewey SL. GABAergic blockade of cocaine-associated cue-induced increases in nucleus accumbens dopamine. Eur J Pharmacol. 2001;414:205–209. doi: 10.1016/s0014-2999(01)00800-7. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. Jama. 2005;294:1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. Self-injection of barbiturates, benzodiazepines and other sedative-anxiolytics in baboons. Psychopharmacology (Berl) 1991;103:154–161. doi: 10.1007/BF02244196. [DOI] [PubMed] [Google Scholar]
- Groman E, Kunze U, Schmeiser-Rieder A, Schoberberger R. [Reduced smoking--a possible strategy for control of tobacco-associated illnesses?] Versicherungsmedizin. 1999;51:180–185. [PubMed] [Google Scholar]
- Gudeman D, Shoptaw S, Majewska D, Scherf S, Yeats D, Ling W. Preliminary report of baclofen as a cocaine craving medication. In: Harris L, editor. Problems of Drug Dependence 1996. NIH; San Juan, Puerto Rico: 1997. p. 183. [Google Scholar]
- Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14:1370–1375. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:177–184. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hofer I, Nil R, Battig K. Nicotine yield as determinant of smoke exposure indicators and puffing behavior. Pharmacol Biochem Behav. 1991;40:139–149. doi: 10.1016/0091-3057(91)90335-y. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–633. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- Jimenez-Ruiz C, Solano S, Viteri SA, Ferrero MB, Torrecilla M, Mezquita MH. Harm reduction-- a treatment approach for resistant smokers with tobacco-related symptoms. Respiration. 2002;69:452–455. doi: 10.1159/000064015. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248–254. doi: 10.1097/01.alc.0000153542.10188.b0. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Lennox AS, Taylor RJ. Factors associated with outcome in unaided smoking cessation, and a comparison of those who have never tried to stop with those who have. British Journal of General Practictioners. 1994;44:245–250. [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–404. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry 64 Suppl. 2003;3:36–40. [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci. 2004;1025:491–503. doi: 10.1196/annals.1316.061. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev Neurother. 2006;6:965–981. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- Morgan AE, Dewey SL. Effects of pharmacologic increases in brain GABA levels on cocaine-induced changes in extracellular dopamine. Synapse. 1998;28:60–65. doi: 10.1002/(SICI)1098-2396(199801)28:1<60::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS. Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. Journal of Neuroscience Methods. 1989;28:15–22. doi: 10.1016/0165-0270(89)90005-8. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Fivel PA. Effects of GABA agonists and GABA-A receptor modulators on cocaine discrimination in rhesus monkeys. Psychopharmacology (Berl) 2000;152:398–407. doi: 10.1007/s002130000543. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9:631–646. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- Riggs RL, Hughes JR, Pillitteri JL. Two behavioral treatments for smoking reduction: a pilot study. Nicotine Tob Res. 2001;3:71–76. doi: 10.1080/14622200020032114. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology (Berl) 1997;131:271–277. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K. GABA modulation of cocaine self-administration. Ann N Y Acad Sci. 2000;909:145–158. doi: 10.1111/j.1749-6632.2000.tb06680.x. [DOI] [PubMed] [Google Scholar]
- [Accessed on April 2, 2009];RxList The internet Drug Index, Kemstro, Side Effects and Drug Interactions. 2008 http://www.rxlist.com/cgi/generic/baclofen_ad.htm.
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol. 1998;9:195–206. [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Song YM, Sung J, Cho HJ. Reduction and cessation of cigarette smoking and risk of cancer: a cohort study of Korean men. Journal of CLinical Oncology. 2008;26:5101–5106. doi: 10.1200/JCO.2008.17.0498. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Fratta W, Fadda P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology. 2007;52:1555–1562. doi: 10.1016/j.neuropharm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Stallings W, Schrader S. Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review. J Okla State Med Assoc. 2007;100:354–360. [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007:CD005231. doi: 10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- Taricco M, Adone R, Pagliacci C, Telaro E. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000:CD001131. doi: 10.1002/14651858.CD001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, Mikkelsen K, Markholst C. Nurse-conducted smoking cessation with minimal intervention in a lung clinic: a randomized controlled study. European Respiratory Journal. 1996;9:2351–2355. doi: 10.1183/09031936.96.09112351. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Research. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290:1369–1374. [PubMed] [Google Scholar]
- Zhu S, Melcer T, Sun J, Rosbrook B, JPierce JP. Smoking cessation with and without assistance: a population-based analysis. American Journal of Preventative Medicine. 2000;18:305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]