Abstract
Using a small resonant loop to produce a longitudinal traveling wave on a human 7-T system allows MR to be performed over the entire volume of the human leg. We have used this capability to perform localized proton MR spectroscopy of the lipid composition of muscle in volunteers with a coil placed ~30 cm away from the region of interest. Spectra with a reasonable signal-to-noise ratio can be acquired in a clinically relevant data acquisition time of less than 5 min using the loop in transmit/receive mode, maintaining the full flexibility to acquire spectra from any part of the calf and/or thigh. If a local receive coil is used in combination with the remote transmit coil, then the signal-to-noise improves significantly, as expected.
Keywords: remote transmission, traveling waves, muscle spectroscopy, intracellular and extracellular lipids, high field imaging
The push toward higher static magnetic fields for human MR applications is driven primarily by the increases in signal-to-noise (S/N), spatial resolution, and magnetic susceptibility contrast for MRI in morphologic (1) and functional (2) brain imaging, improved spatial resolution and background tissue suppression in magnetic resonance angiography (3), and increased S/N and spectral resolution in localized MR spectroscopy (4–8). Clinical research studies are currently being performed at 7 T in both neurologic (9–11) and musculoskeletal (12–14) areas. In addition, both cardiac and abdominal imaging has recently been shown to be feasible at 7 T in humans (15–17). Receive radiofrequency (RF) coil design at 7 T have largely mirrored that at lower field strengths, with multielement phased arrays being standard on both commercial and research systems. Creating a spatially homogeneous RF field (B1+) during transmission, however, is much more challenging due to the short wavelength in tissue and associated spatial B1 distortions, as well as the high power and corresponding high specific absorption rate required. The use of transmit arrays (18) has become increasingly important at high fields, particularly when trying to acquire images over a large field of view (FOV) with good penetration depth. Images acquired using up to 16 separate transmit array elements in combination with B1-shimming (19) have shown impressive results, for example, in prostate.
A potential alternative strategy for imaging a large FOV has recently been proposed, in which energy is introduced into the patient via a remote RF antenna designed to produce a “traveling” wave that can propagate through the bore of the magnet, provided that the bore of the magnet is large enough that the cutoff frequency of the TE11 dominant circular mode (with the human subject inside the bore) is below the Larmor frequency (20–23). The cutoff frequency of a waveguide is defined as the frequency above which propagating waves are very rapidly attenuated as a function of distance traveled down the waveguide. Since only a certain fraction of the energy is deposited at any surface position along the body, this allows large FOV imaging, as described more fully later in this paper. This approach has the advantage of being extremely simple to implement from a technological point of view, without requiring a sophisticated multiple-channel transmit system architecture, which is currently only available on a handful of commercial and academic platforms.
In this paper, we apply the traveling wave concept to large FOV imaging and localized MR spectroscopy in human leg muscle. Rather than using a large patch antenna, as previously shown (20), we use a small loop source placed close to one leg of the subject. Two modes of data acquisition were used: transmit and receive, both using the remote coil, and remote coil transmit with local surface coil receive. Using both approaches, we were able to acquire localized spectra from a voxel of 20 mL far away from the transmit coil in a clinically acceptable time of ~4 min.
MATERIALS AND METHODS
All in vivo experiments (n = 3 volunteers) were performed on a 7-T whole-body MRI system (Philips Health-care, Best, NL). The inner RF shield has a diameter of 58cm, corresponding to a cutoff frequency of ~303 MHz for the lowest TE waveguide mode. Since the cutoff frequency of this mode is inversely proportional to the dielectric constant of the material filling the waveguide, when the human body is positioned in the magnet bore, its dielectric properties reduce this frequency to well below the Larmor frequency of 298.1 MHz (20,24). The RF coil consisted of a loop of wire of diameter 8 cm, segmented into four equal lengths by four equally valued nonmagnetic capacitors. The coil was impedance matched to 50 Ω using a conventional balanced capacitive π-network. For the spectra acquired with a local receive coil, a PIN-diode decoupled surface coil of diameter 10 cm was constructed.
Mapping of the extent of RF coverage was performed using a series of three-dimensional fast low angle shot sequences. The FOV for each image was 250 × 202 × 225 mm3 in the foot/head, anterior/posterior, and right/left directions, respectively. The spatial resolution was 2 × 2 × 2.5 mm3, with pulse repetition time/echo time 20/2.37 ms and an acquisition time of 3.2 min. After each image, the table was manually moved ~20 cm in the head/foot direction. Images were imported into MATLAB (The Mathworks Inc., Natick, MA) and “stitched together” manually, with no further image processing or smoothing.
Localized proton spectroscopy was performed by choosing a voxel within the thigh muscle, followed by an image-based shimming method to calculate the optimum first- and second-order shim values: shimming was performed using the loop antenna in transmit and receive mode. Stimulated echo acquisition mode spectra were acquired, with an echo time of 144 ms to refocus scalar-coupled metabolites and a mixing time of 25 ms. No water suppression was necessary due to the short T2 of water in muscle (25). The spectral volume had dimensions 20 × 20 × 50 mm3.
To gain insight into the propagation of fields in this situation, we carried out full-Maxwell numerical calculations of the electromagnetic fields, as produced by a similar circular coil near a model of an average-sized male (26) inside a cylindrical conductor with a diameter equal to that used in experiment. All simulation work was performed using commercially available finite-difference time-domain software (xFDTD; Remcom, Inc., State College, PA), with the coil driven by four voltage sources spaced equally around the coil and an isometric resolution of 5 × 5 × 5 mm3. After completion of the calculation, results were scaled so that the input power was 1 kW. Analysis of the results was performed in MATLAB.
RESULTS
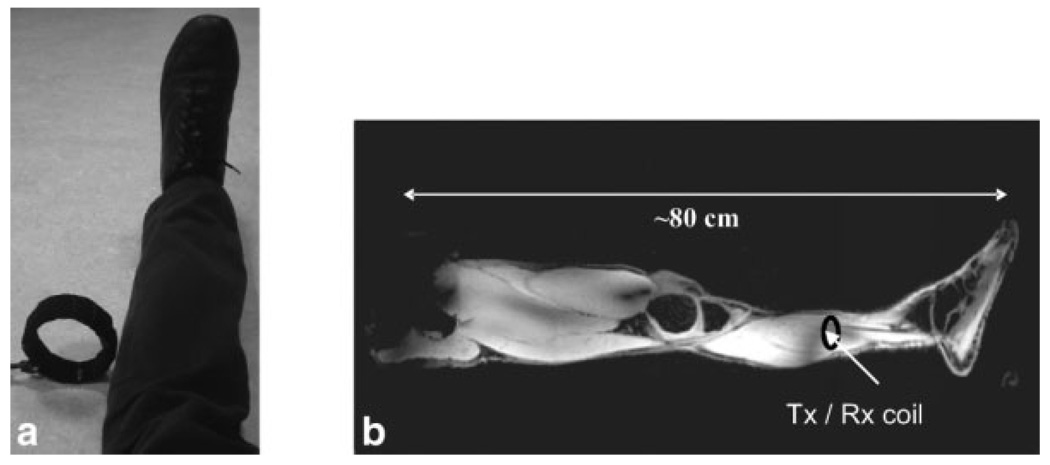
Figure 1a shows the orientation of the RF coil with respect to the left leg of the volunteer. It is placed approximately halfway up the calf muscle and is oriented with its major axis parallel to the amplitude of static field, although it can in fact be oriented in any direction. The exact position with respect to the calf is not critical, and typically it was placed ~5 cm away from the leg. The power delivered to the coil was ~1 kW and was limited to this value by the amplifier output and cable losses. In order to determine the RF distribution within the entire length of the leg, a series of five separate three-dimensional scans was acquired, each with an FOV in the z-direction of 25 cm. The table was then moved ~20 cm and the scan repeated. The RF coil moved with the table and therefore maintained the same position relative to the leg. Figure 1b shows a slice through the composite image produced from the five separate sections: although there are clear nonuniformities in the composite image, the FOV is more than 10 times larger than the linear dimensions of the RF coil. The signal intensity along the length of the leg does not decrease significantly until the hip/pelvis area is reached. These scans are acquired at a relatively coarse resolution and were not intended to be of clinical quality, but rather to illustrate the RF coverage of the coil setup. The effect is particularly striking when considering the physical setup shown in Fig. 1a, in which the coil geometry runs counter to the most basic principles of signal transmission and reception in that the directions of the B1 and the static field is parallel. The effect of the table movement has only a minor effect on the magnitude of the field distribution. There will be an effect on the phase of the RF at a given location for two different positions of the patient table, but since magnitude images were simply stitched together, this effect is not seen in the current implementation. The same power was applied to the loop at each “station”, with a target low flip angle of ~5° for the pulse repetition time of 20 ms.
FIG. 1.
a: RF coil oriented along the amplitude of static field axis and placed ~5 cm from the calf muscle. b: A composite image formed from several three-dimensional data sets that must be obtained separately due to the limited gradient linearity in the head-foot direction. The table position within the magnet changes, but the RF coil is not moved. The volume excited is several hundred times the dimensions of the coil. The coil is used in both transmit and receive mode.
Useful insights into the factors causing the large RF coverage can be obtained using electromagnetic simulations. Perhaps most useful is a map of the time-average Poynting vector, Sav, which is a representation of the direction of energy propagation. This is calculated as:
| [1] |
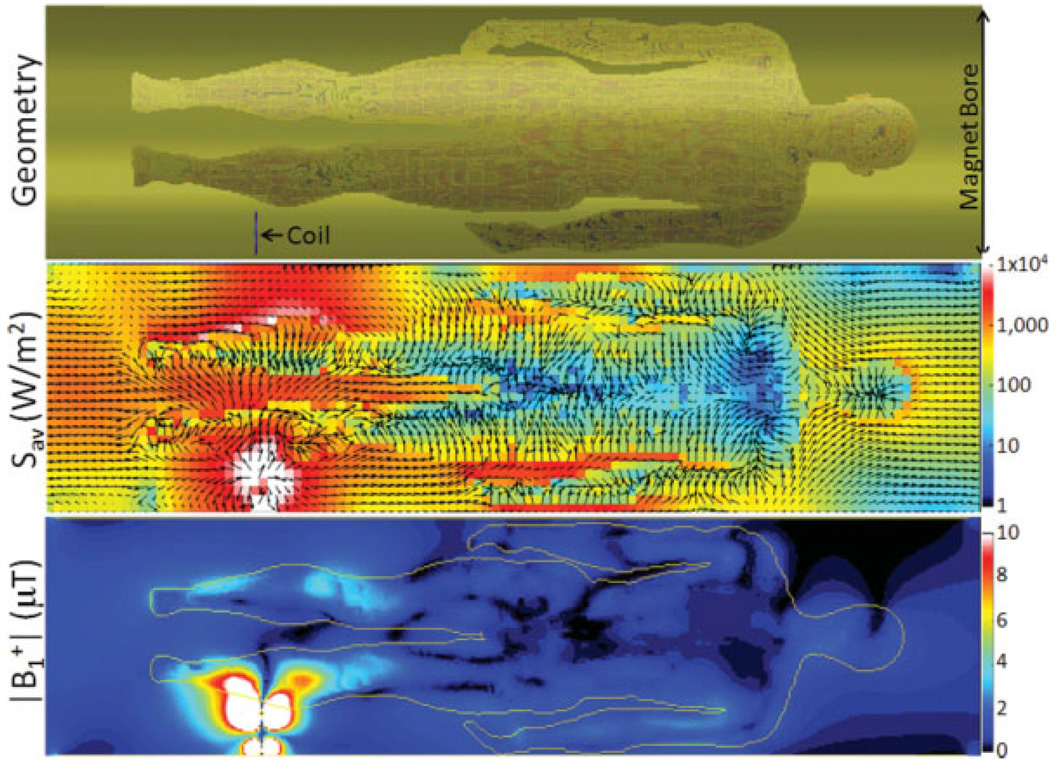
where E and H are the electric and magnetic fields respectively, the asterisk indicates a complex conjugate, and “Re” indicates the real part. The Poynting vector is calculated on a pixel-by-pixel basis in MATLAB from the H and E field maps produced from the electromagnetic simulation software. The middle of Fig. 2 shows a Poynting vector plot of the subject placed within the 58 cm-diameter RF shield of the magnet. To reduce the number of arrows and produce an intelligible figure, Poynting vector data were resampled at 2-cm intervals. A plot of the distribution of one pertinent circularly polarized component of the B1+ is also shown at the bottom of Fig. 2. As expected, the intensity of the RF energy and fields is highest in the calf local to the coil. At the ends of the magnet bore, the Poynting vector lies parallel to the axis of the magnet bore and indicates the RF energy flowing out of the bore. In areas where the RF encounters the surface of the tissue, the direction of the vector is refracted to be more perpendicular to the tissue surface. In some places, the strength of B1+ increases due to the high dielectric constant of the tissue. Energy then travels through the body in this perpendicular direction while being attenuated due to conductive losses. One can see that areas with a high surface area–to-volume ratio such as the legs and arms have a high B1 field strength with a relatively uniform distribution. In contrast, in the torso, where the ratio is smallest, there is very little energy in the center due to high attenuation as the energy and fields travel from the surface.
FIG. 2.
Geometry (top), time-average Poynting vector (middle), and magnitude of one circularly polarized component of the RF magnetic field (bottom) from numerical calculations. Values of watts per meter squared for the Poynting vector and microtesla for the B1 field are derived with 1-kW power supplied to the coil. Arrows in Poynting vector plot are all of the same length in three dimensions: background color indicates magnitude and arrows that appear shorter have a significant anterior-posterior component. Images are shown in a coronal orientation.
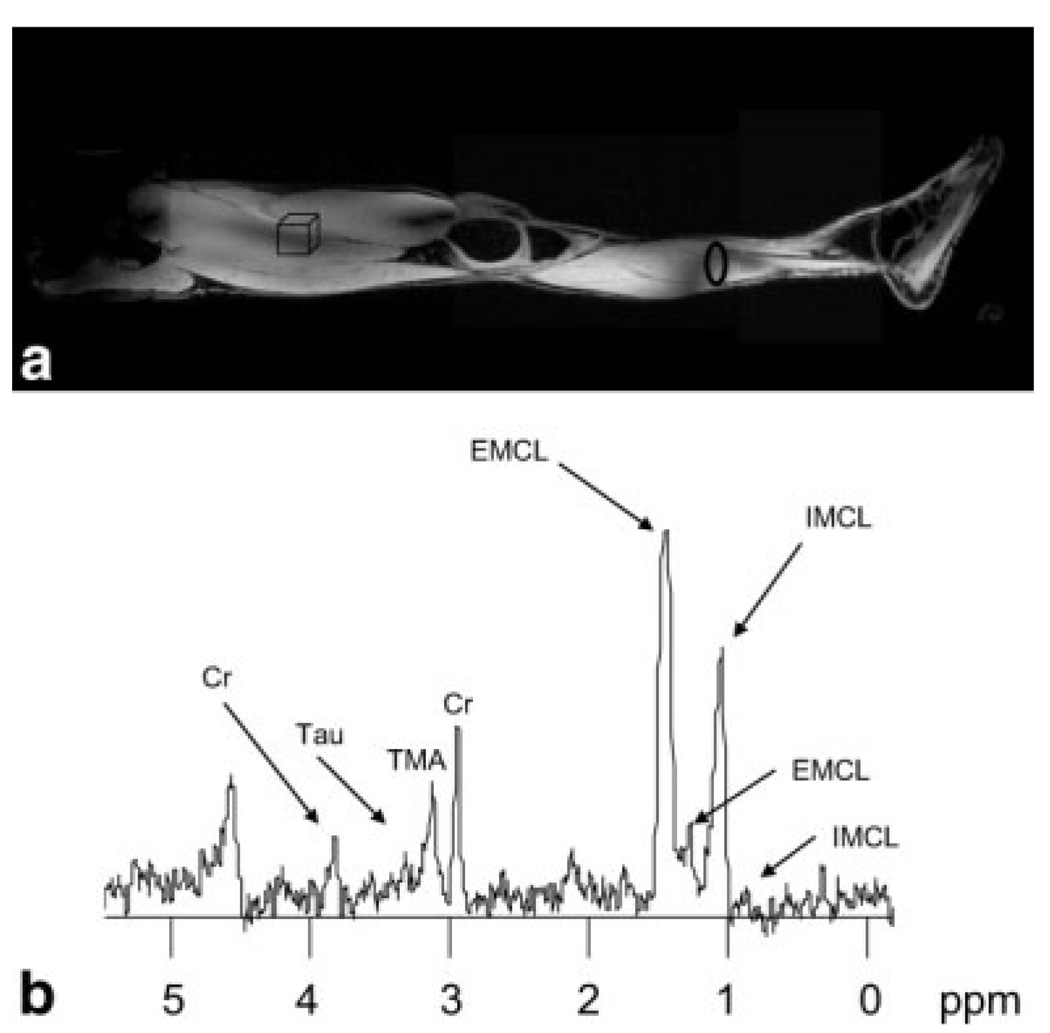
Figure 3a shows the location of the loop antenna close to the calf muscle and the localized voxel placed ~30 cm distant from the coil. Although superior results could be obtained with the voxel positioned closer to the coil, we were interested in investigating the limits of this approach by placing the voxel as far away as possible. Figure 3b shows the localized stimulated echo acquisition mode spectrum acquired in a data acquisition time of 4.5 min from a volume of 20 mL, with the voxel placed ~3 cm from the surface of the thigh in order to eliminate the contribution from subcutaneous fat signals and acquire only the lipid signals from the muscle. Signals from the intramyocellular lipid, extramyocellular lipid, taurine, trimethylammonium and creatine are visible, with the S/N of the intramyocellular lipid and extramyocellular lipid peaks high enough for reliable quantitation.
FIG. 3.
a: Position of the localized voxel (20-mL volume) superimposed on the composite image of the leg. The distance between the transmit loop and voxel is ~30 cm. Localized proton spectra were acquired using the loop coil in transmit and receive mode and a stimulated echo acquisition mode sequence with echo time 144 ms and total data acquisition time 4.5 min. A line-broadening factor of 5 Hz was applied before Fourier transformation. Spectral assignment: Cr, creatine; TMA, trimethylammonium; EMCL, extramyocellular lipid; IMCL, intramyocellular lipid; Tau, taurine. The peak at 4.7 ppm is from residual water.
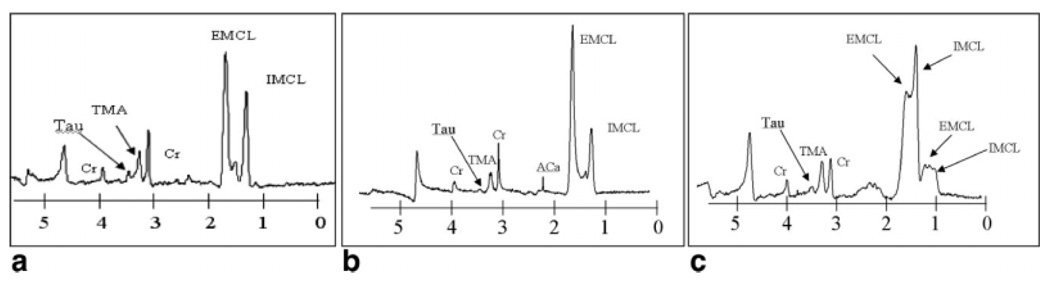
As suggested and shown previously (20,22,23), the S/N can be improved, at the expense of versatility, by using a separate receiver coil, but still using the loop as the transmitter. Figure 4 shows proton spectra using this arrangement for three different volunteers, with metabolic differences seen between the three in terms of the intramyocellular lipid/extramyocellular lipid ratio. Figure 4a is the same subject as represented in Fig. 3b, and this shows that the S/N was increased by approximately a factor of 8 by using a local RF coil. This is unsurprising, given the fact that by using the loop antenna as a receive coil, it picks up noise from the entire body, as well as the surroundings, including the RF shield.
FIG. 4.
Localized proton spectra acquired using the loop coil to transmit and a separate receiver coil placed over a volume of interest (VOI) in the thigh muscle. a: Spectrum corresponding to the same volunteer (female) as in Fig. 3b, with identical data acquisition parameters and localized volume. b: Spectrum from a 27-year-old male, and (c) spectrum from a veteran marathon runner.
DISCUSSION
In this paper, we have shown the first in vivo localized proton MR spectra to be acquired using a remote transmitter placed ~30 cm away from the voxel from which the signal was received. Using this coil in transmit/receive mode resulted in spectra that gave useable data within a clinically relevant acquisition time of less than 5 min. If a separate decoupled surface coil is used as the receiver, then the S/N of the spectra increase by approximately a factor of 8, although this number is dependent upon the exact position of the localized voxel. One of the major applications of high-field MR is to localized MR spectroscopy, taking advantage not only of the higher S/N but also the increased spectral resolution since the absolute difference in hertz in individual peaks is linearly proportional to the magnetic field. Studies in muscle at 7 T have shown metabolic profiles of lipid, for example, in unprecedented detail (7,8). Conventional approaches to RF coils for muscle studies are extremely limited, typically only able to cover a small volume in one of the calves. Given both the temporal restrictions of clinical spectroscopy (spectra must typically be acquired in only a few minutes), as well as acquiring spectra from the smallest possible volume to avoid partial-volume effects, we performed localized proton MR spectroscopy experiments to determine the feasibility of using the loop antenna in both transmit and receive mode (thus maintaining the full flexibility in terms of voxel location) and the antenna in transmit-only mode with a local receive coil. As mentioned previously, the alternative approach of constructing a very large and long volume coil to span both the thigh and calf muscles is extremely challenging at these high fields.
In this study, a small loop was used, which allows it to be placed close to the region of interest, therefore reducing the specific absorption rate somewhat in remote areas of the body and increasing the local sensitivity compared to an approach using a “global” patch antenna. Due to its large size, the patch antenna has to be placed outside the bore of the magnet and for these experiments would have to be placed at the back of the magnet, which introduces additional power losses in transmission for the particular setup of the Philips 7T Achieva. The approach of using a small antenna could also be very useful in imaging regions of the torso since the patch antenna approach leads to significant signal attenuation by the time the traveling wave reaches this area. The RF coil used is extremely simple, and certainly one can envision significant improvements. Quadrature excitation and reception is an obvious next step, as well as appropriate shielding of the resonator on one side in order to avoid RF signal propagating “backwards” toward the feet. Energy transmitted into the far field rather than stored in the near field is traditionally optimized by using a loop antenna with an electrical length of one-half wavelength (or integer multiples thereof), and therefore this type of design could also be investigated.
There are also many opportunities to manipulate the fundamental propagation of energy to different places in the body. Materials with high dielectric properties can be placed around the leg to concentrate the flux in specific places; for example, by using dielectric rings of ceramics with dielectric constants of many hundreds. Such materials have already been used in high-frequency resonators for MR microscopy (27). Wiggins et al. (22) have shown that effective dielectric matching can produce more uniform B1 fields in the head, and energy “lost” in parts of the body that are not of interest can be reduced by shielding the body in a conducting material (28). In our particular application, this type of shield could cover the torso and pelvic areas, for example. Such shielding could be expected to reduce the noise received by the coil from regions of the body irrelevant to the experiment and also reduce the whole-body average specific absorption rate.
Ultimately, we are interested in the feasibility of applying such remote transmission methods to large FOV imaging of muscle in spectroscopy of the human calf and thigh muscle, with the aim of studying debilitating pathologies such as Duchenne and Becker muscular dystrophies. In these but also in other muscular dystrophies, the pathology shows a pronounced spatial heterogeneity between and within muscles (29), but it can also differ between limbs (30). In Duchenne muscular dystrophy, the pattern of involvement is stereotypical; it progresses with age and often the proximal part of a limb is affected before the distal part (31). Therefore, large FOV imaging would be beneficial in determining the appropriate tissue areas to study. The lack of a close-fitting/spatially restrictive RF coil is also an important advantage in patients for which long periods of immobility are highly challenging. This type of study in which a degree of sensitivity loss can be traded for increased versatility and improved patient comfort unattainable using conventional RF coil designs is an area in which we envision that this new type of remote transmission will play an important role.
ACKNOWLEDGMENTS
This research was funded in part by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO).
Grant sponsor: National Institutes of Health; Grant number: R01 EB000454.
REFERENCES
- 1.Duyn JH, van GP, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U|S|A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn spin echo BOLD functional MRI at 7 tesla. Neuroimage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang CK, Park CW, Han JY, Kim SH, Park CA, Kim KN, Hong SM, Kim YB, Lee KH, Cho ZH. Imaging and analysis of lenticulostriate arteries using 7.0-tesla magnetic resonance angiography. Magn Reson Med. 2009;61:136–144. doi: 10.1002/mrm.21786. [DOI] [PubMed] [Google Scholar]
- 4.Gambarota G, Mekle R, Xin L, Hergt M, van der ZW, Krueger G, Gruetter R. In vivo measurement of glycine with short echo-time 1H MRS in human brain at 7 T. MAGMA. 2009;22:1–4. doi: 10.1007/s10334-008-0152-0. [DOI] [PubMed] [Google Scholar]
- 5.Mangia S, Tkac I, Gruetter R, van de Moortele PF, Giove F, Maraviglia B, Ugurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging. 2006;24:343–348. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 7.Khuu A, Ren J, Dimitrov I, Woessner D, Murdoch J, Sherry AD, Malloy CR. Orientation of lipid strands in the extracellular compartment of muscle: effect on quantitation of intramyocellular lipids. Magn Reson Med. 2009;61:16–21. doi: 10.1002/mrm.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 tesla. J Lipid Res. 2008;49:2055–2062. doi: 10.1194/jlr.D800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond KE, Metcalf M, Carvajal L, Okuda DT, Srinivasan R, Vigneron D, Nelson SJ, Pelletier D. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 10.Lupo JM, Banerjee S, Hammond KE, Kelley DA, Xu D, Chang SM, Vigneron DB, Majumdar S, Nelson SJ. GRAPPA-based susceptibility-weighted imaging of normal volunteers and patients with brain tumor at 7 T. Magn Reson Imaging. 2009;27:480–488. doi: 10.1016/j.mri.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metcalf M, Xu D, Okuda DT, Carvajal L, Srinivasan R, Kelley DA, Mukherjee P, Nelson SJ, Vigneron DB, Pelletier D. High-resolution phased-array MRI of the human brain at 7 tesla: initial experience in multiple sclerosis patients. J Neuroimaging. doi: 10.1111/j.1552-6569.2008.00338.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee S, Krug R, Carballido-Gamio J, Kelley DA, Xu D, Vigneron DB, Majumdar S. Rapid in vivo musculoskeletal MR with parallel imaging at 7T. Magn Reson Med. 2008;59:655–660. doi: 10.1002/mrm.21455. [DOI] [PubMed] [Google Scholar]
- 13.Krug R, Carballido-Gamio J, Banerjee S, Burghardt AJ, Link TM, Majumdar S. In vivo ultra-high-field magnetic resonance imaging of trabecular bone microarchitecture at 7 T. J Magn Reson Imaging. 2008;27:854–859. doi: 10.1002/jmri.21325. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich KM, Chang G, Vieira RL, Wang L, Wiggins GC, Schweitzer ME, Regatte RR. In vivo 7.0-tesla magnetic resonance imaging of the wrist and hand: technical aspects and applications. Semin Musculoskel Radiol. 2009;13:74–84. doi: 10.1055/s-0029-1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele PF, Akgun C, Ugurbil K. Initial results of cardiac imaging at 7 tesla. Magn Reson Med. 2008;61:517–524. doi: 10.1002/mrm.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole-body imaging at 7T: preliminary results. Magn Reson Med. 2009;61:244–248. doi: 10.1002/mrm.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Elderen S, Webb AG, Versluis M, Westenberg J, Doornbos J, Smith NB, de Roos A, Stuber M. In vivo human coronary magnetic resonance angiography at 7 tesla. J Cardiol Magn Reson. 2009;11:O46. doi: 10.1002/mrm.22168. [DOI] [PubMed] [Google Scholar]
- 18.Adriany G, van de Moortele PF, Ritter J, Moeller S, Auerbach EJ, Akgun C, Snyder CJ, Vaughan T, Ugurbil K. A geometrically adjustable 16-channel transmit/receive transmission line array for improved RF efficiency and parallel imaging performance at 7 tesla. Magn Reson Med. 2008;59:590–597. doi: 10.1002/mrm.21488. [DOI] [PubMed] [Google Scholar]
- 19.Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, van de Moortele PF. Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn Reson Med. 2008;59:396–409. doi: 10.1002/mrm.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner DO, De Zanche N, Frohlich J, Paska J, Pruessmann KP. Travelling-wave nuclear magnetic resonance. Nature. 2009;457:994–998. doi: 10.1038/nature07752. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg CA, van den Bergen B, Bartels LW, Lagendijk JJ. Proceedings of the International Society of Magnetic Resonance in Medicine Workshop on Advances in High Field MR. Asilomar, CA: 2007. Using the natural resonant modes of the RF cavity for whole body excitation at 7 T; p. 39. [Google Scholar]
- 22.Wiggins G, Zhang B, Duan Q, Sodickson DK. Proceedings of the International Society of Magnetic Resonance in Medicine. Honolulu, HI: 2009. Traveling wave imaging of the human head at 7 tesla: assessment of SNR, homogeneity and B1+ efficiency; p. 2942. [Google Scholar]
- 23.Smith NB, Haines K, Versluis M, Webb AG. Proceedings of the International Society of Magnetic Resonance Workshop on Advances in High Field Systems and Applications. Florence, Italy: 2008. Human imaging using traveling waves at 7 tesla. p. 18. [Google Scholar]
- 24.Mahmoud SF. Institution of Engineering and Technology. Stevenage, UK: 1991. Electromagnetic waveguides: theory and application (IEEE Electromagnetic Waves Series) [Google Scholar]
- 25.Ren J, Sherry AD, Malloy CR. Proton MRS acquisition scheme with long echo time and without water suppression simplifying IMCL evaluation. Proc Int Soc Magn Reson Med. 2009:683. [Google Scholar]
- 26.Christ A, Kainz W, Hahn E, Honegger K, Rascher W. Development of CAD based anatomical human body models of two adults and two children; 8th International Congr Eur Bioelectromag Assoc; 2007. p S-4–2. [Google Scholar]
- 27.Neuberger T, Tyagi V, Seminouchkina E, Lanagan M, Baker A, Haines K, Webb AG. Design of a ceramic dielectric resonator for NMR microimaging at 14.1 tesla. Concepts Magn Reson Part B Magn Reson Eng. 2008;33B:109–114. [Google Scholar]
- 28.Andreychenko A, Klomp DW, van den Bergen B, van de Bank BL, Kroeze H, Lagendijk JJ, Luijten P, van den Berg CA. Effective delivery of the traveling wave to distant locations in the body at 7T. Proc Int Soc Magn Reson Med. 2009:500. [Google Scholar]
- 29.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–W12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- 30.Liu GC, Jong YJ, Chiang CH, Jaw TS. Duchenne muscular dystrophy: MR grading system with functional correlation. Radiology. 1993;186:475–480. doi: 10.1148/radiology.186.2.8421754. [DOI] [PubMed] [Google Scholar]
- 31.Dubovitz V, Sewry CA. Muscle biopsy: a practical approach. 3rd. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]